Abstract
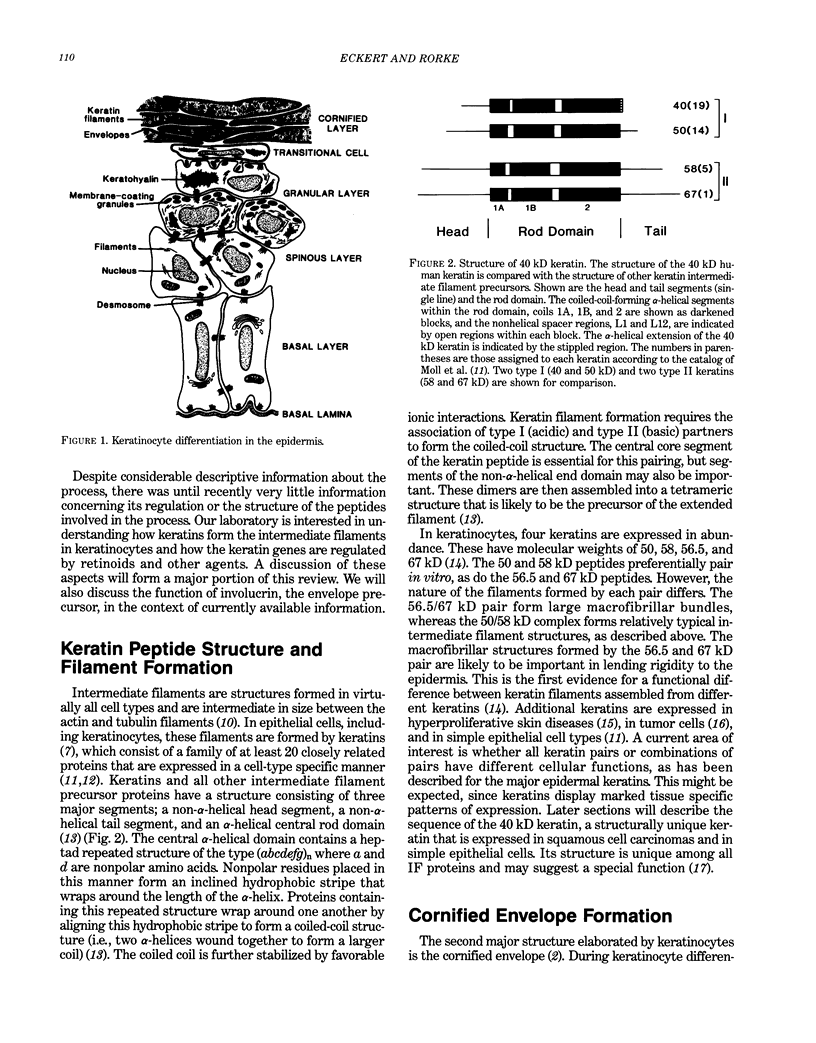
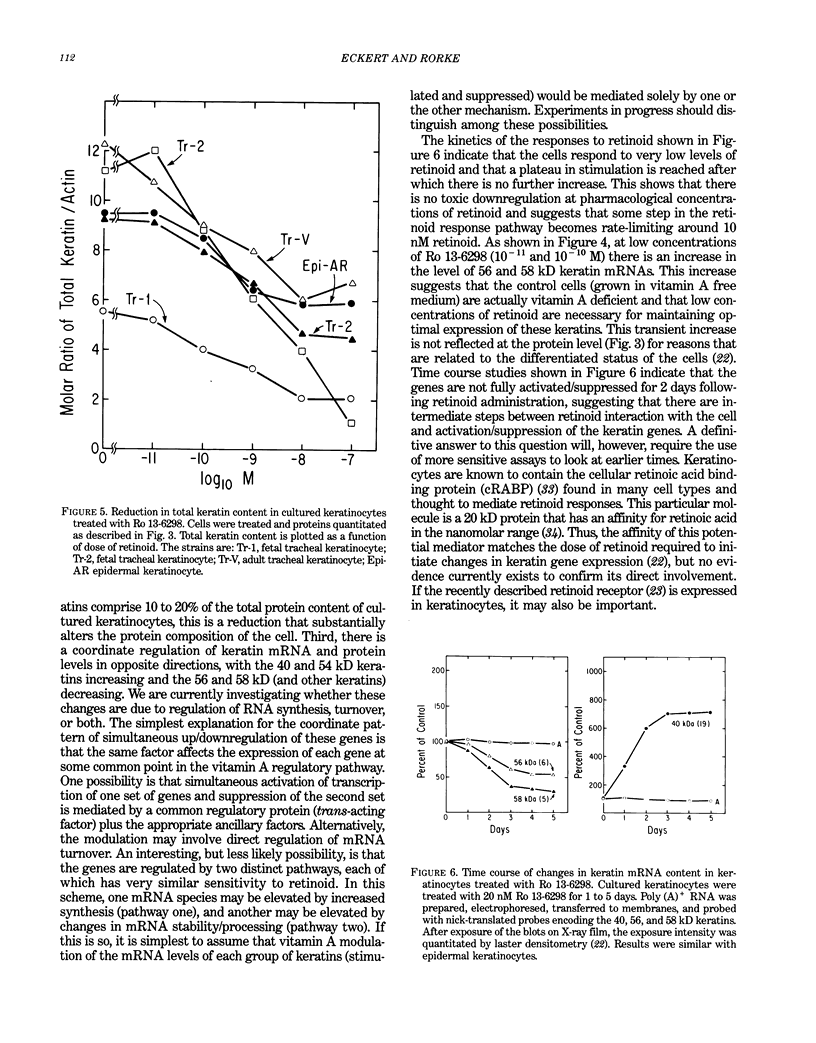
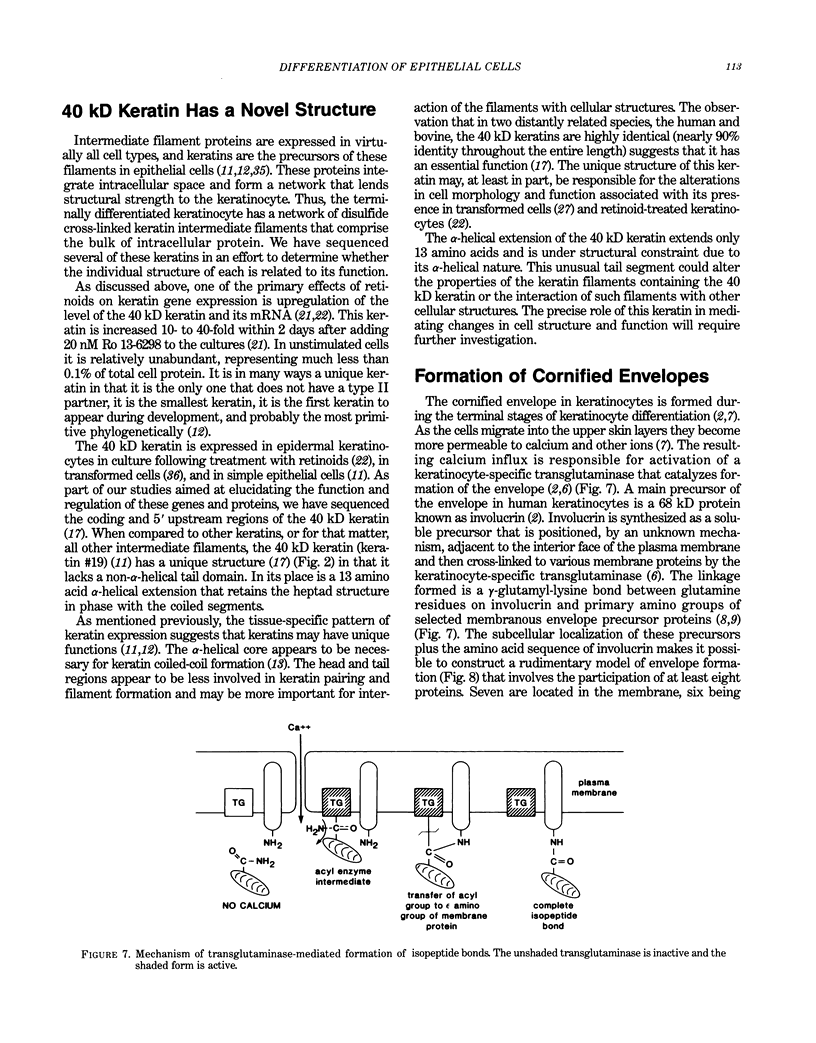
Epidermal keratinocytes (skin cells) are highly specialized epithelial cells designed to perform a very specific function, separation of the organism from its environment. To accomplish this the cells synthesize precursors and assemble them into two distinct structures, the cornified envelope and keratin intermediate filaments. The intermediate filaments are assembled from keratin monomers and the cornified envelope is assembled from a protein called involucrin and several other proteins. Expression of involucrin and the keratins genes are regulated as a function of the stage of keratinocyte differentiation and by various external agents such as calcium and vitamin A. To study the function of these structures and the regulation of precursor production we have cloned cDNA and genomic clones encoding involucrin and four of the keratin polypeptides. Retinoids profoundly alter the differentiation pattern of human epidermal keratinocytes, but the underlying biochemical basis of this change is not known. In this report we describe retinoid-promoted changes in keratin gene expression that may, in part, be responsible for the alteration in cellular phenotype in the presence of the vitamin. We also describe the novel structure of the human 40 kD keratin, a member of the keratin family that is retinoid responsive and is likely to be important during epidermal development. Finally, we describe the structure of the envelope precursor protein, involucrin, as determined from its DNA sequence and speculate on its role in cornified envelope formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- Cline P. R., Rice R. H. Modulation of involucrin and envelope competence in human keratinocytes by hydrocortisone, retinyl acetate, and growth arrest. Cancer Res. 1983 Jul;43(7):3203–3207. [PubMed] [Google Scholar]
- Cline P. R., Rice R. H. Modulation of involucrin and envelope competence in human keratinocytes by hydrocortisone, retinyl acetate, and growth arrest. Cancer Res. 1983 Jul;43(7):3203–3207. [PubMed] [Google Scholar]
- Dale B. A., Resing K. A., Lonsdale-Eccles J. D. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. The direct involvement of vitamin A in glycosyl transfer reactions of mammalian membranes. Vitam Horm. 1977;35:1–57. doi: 10.1016/s0083-6729(08)60520-8. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Cloning of cDNAs specifying vitamin A-responsive human keratins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4321–4325. doi: 10.1073/pnas.81.14.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986 Aug 15;46(4):583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
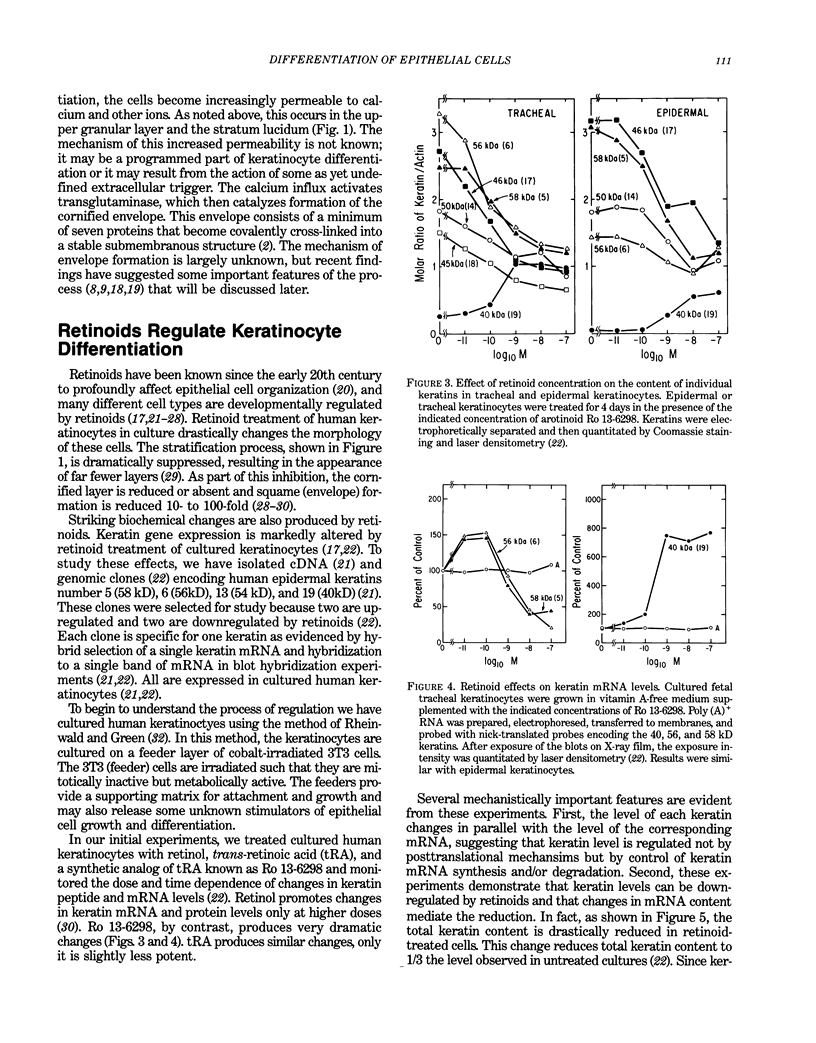
- Eckert R. L. Sequence of the human 40-kDa keratin reveals an unusual structure with very high sequence identity to the corresponding bovine keratin. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1114–1118. doi: 10.1073/pnas.85.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Grayson S., Caldwell T. M., McNutt N. S. Gap junction proliferation in retinoic acid-treated human basal cell carcinoma. Lab Invest. 1980 Apr;42(4):469–474. [PubMed] [Google Scholar]
- Etoh Y., Simon M., Green H. Involucrin acts as a transglutaminase substrate at multiple sites. Biochem Biophys Res Commun. 1986 Apr 14;136(1):51–56. doi: 10.1016/0006-291x(86)90875-2. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969 Jul 10;244(13):3707–3713. [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Gilfix B. M., Eckert R. L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985 Nov 15;260(26):14026–14029. [PubMed] [Google Scholar]
- Gilfix B. M., Green H. Bioassay of retinoids using cultured human conjunctival keratinocytes. J Cell Physiol. 1984 May;119(2):172–174. doi: 10.1002/jcp.1041190205. [DOI] [PubMed] [Google Scholar]
- Green H., Fuchs E., Watt F. Differentiated structural components of the keratinocyte. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):293–301. doi: 10.1101/sqb.1982.046.01.031. [DOI] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- Green H., Watt F. M. Regulation by vitamin A of envelope cross-linking in cultured keratinocytes derived from different human epithelia. Mol Cell Biol. 1982 Sep;2(9):1115–1117. doi: 10.1128/mcb.2.9.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Yamada Y., Hassell J. R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev Biol. 1987 Oct;123(2):508–516. doi: 10.1016/0012-1606(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Roop D. R., De Luca L. M. Vitamin A deficiency and keratin biosynthesis in cultured hamster trachea. In Vitro Cell Dev Biol. 1986 Apr;22(4):223–230. doi: 10.1007/BF02623307. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Schwartz F., Fuchs E. Differences in keratin synthesis between normal epithelial cells and squamous cell carcinomas are mediated by vitamin A. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4280–4284. doi: 10.1073/pnas.81.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Ma A. S., Sun T. T. Differentiation-dependent changes in the solubility of a 195-kD protein in human epidermal keratinocytes. J Cell Biol. 1986 Jul;103(1):41–48. doi: 10.1083/jcb.103.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinoic acid-binding protein from rat testis. Purification and characterization. J Biol Chem. 1978 Jul 10;253(13):4551–4554. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Simon M., Green H. Enzymatic cross-linking of involucrin and other proteins by keratinocyte particulates in vitro. Cell. 1985 Mar;40(3):677–683. doi: 10.1016/0092-8674(85)90216-8. [DOI] [PubMed] [Google Scholar]
- Simon M., Green H. Participation of membrane-associated proteins in the formation of the cross-linked envelope of the keratinocyte. Cell. 1984 Apr;36(4):827–834. doi: 10.1016/0092-8674(84)90032-1. [DOI] [PubMed] [Google Scholar]
- Smits H. L., Floyd E. E., Jetten A. M. Molecular cloning of gene sequences regulated during squamous differentiation of tracheal epithelial cells and controlled by retinoic acid. Mol Cell Biol. 1987 Nov;7(11):4017–4023. doi: 10.1128/mcb.7.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Tseng S. C., Huang A. J., Cooper D., Schermer A., Lynch M. H., Weiss R., Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann N Y Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Coe E. L., Rice R. H. Retinoid suppression of transglutaminase activity and envelope competence in cultured human epidermal carcinoma cells. Hydrocortisone is a potent antagonist or retinyl acetate but not retinoic acid. Differentiation. 1985;29(1):82–87. doi: 10.1111/j.1432-0436.1985.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Rice R. H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985 Mar;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982 Feb 4;295(5848):434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Guillet G. Y., Freedberg I. M., Farmer E. R., Small E. A., Weiss M. M., Sun T. T. The use of monoclonal antibody to keratin in human epidermal disease: alterations in immunohistochemical staining pattern. J Invest Dermatol. 1983 Sep;81(3):224–230. doi: 10.1111/1523-1747.ep12518198. [DOI] [PubMed] [Google Scholar]
- Wu R., Wu M. M. Effects of retinoids on human bronchial epithelial cells: differential regulation of hyaluronate synthesis and keratin protein synthesis. J Cell Physiol. 1986 Apr;127(1):73–82. doi: 10.1002/jcp.1041270110. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]


