Abstract
Reactive oxygen species (ROS) have been proposed to function as second messengers in abscisic acid (ABA) signaling in guard cells. However, the question whether ROS production is indeed required for ABA signal transduction in vivo has not yet been addressed, and the molecular mechanisms mediating ROS production during ABA signaling remain unknown. Here, we report identification of two partially redundant Arabidopsis guard cell-expressed NADPH oxidase catalytic subunit genes, AtrbohD and AtrbohF, in which gene disruption impairs ABA signaling. atrbohD/F double mutations impair ABA-induced stomatal closing, ABA promotion of ROS production, ABA-induced cytosolic Ca2+ increases and ABA- activation of plasma membrane Ca2+-permeable channels in guard cells. Exogenous H2O2 rescues both Ca2+ channel activation and stomatal closing in atrbohD/F. ABA inhibition of seed germination and root elongation are impaired in atrbohD/F, suggesting more general roles for ROS and NADPH oxidases in ABA signaling. These data provide direct molecular genetic and cell biological evidence that ROS are rate-limiting second messengers in ABA signaling, and that the AtrbohD and AtrbohF NADPH oxidases function in guard cell ABA signal transduction.
Keywords: abscisic acid/calcium channels/guard cell/reactive oxygen species/stomata
Introduction
The plant hormone abscisic acid (ABA) controls important cellular processes including regulation of seed dormancy, seed maturation and vegetative growth during plant development (Koornneef et al., 1998; Finkelstein et al., 2002). Furthermore, ABA plays a protective role in response to abiotic stresses including drought, salinity and cold (Schroeder et al., 2001; Finkelstein et al., 2002; Zhu, 2002). ABA regulates the expression levels of a range of genes, and several transcription factors mediating ABA responses have been isolated by analyses of ABA-insensitive mutants (Giraudat et al., 1992; Finkelstein et al., 1998, 2002). However, surprisingly few signal transduction components have been identified as recessive ABA-insensitive disruption mutants that likely function upstream of transcription during early ABA signal transduction. Cloned signaling genes in which disruption mutations cause recessive ABA insensitivity include the GPA1 Gα subunit, the RCN1 protein phosphatase type 2A subunit and the OST1 protein kinase (Wang et al., 2001; Kwak et al., 2002; Mustilli et al., 2002). In contrast, several negative regulators of ABA signaling have been identified from characterization of ABA hypersensitive mutants (Cutler et al., 1996; Pei et al., 1998; Lu and Fedoroff, 2000; Hugouvieux et al., 2001; Lee et al., 2001;Lemichez et al., 2001; Xiong et al., 2001a,b). The limited number of genetically identified positive ABA transducers is most likely due to redundancy in genes encoding ABA signaling components (Arabidopsis Genome Initiative, 2000).
Under drought conditions, ABA causes closing of stomatal pores that are formed by pairs of guard cells located in the leaf epidermis, which in turn reduces transpirational water loss from plants. The second messenger cytosolic calcium ([Ca2+]cyt) mediates ABA signaling in guard cells (McAinsh et al., 1990; Schroeder and Hagiwara, 1990). ABA-induced [Ca2+]cyt increases are mediated by Ca2+ influx through plasma membrane Ca2+-permeable (ICa) channels and Ca2+ release from internal stores (Wu et al., 1997; Leckie et al., 1998; Staxen et al., 1999; MacRobbie, 2000; Pei et al., 2000).
Recently, ABA-regulated hyperpolarization-activated plasma membrane ICa channels were identified in guard cells of Vicia and Arabidopsis (Hamilton et al., 2000; Pei et al., 2000). These ICa channels were demonstrated to be stimulated by reactive oxygen species (ROS) in Arabidopsis and Vicia guard cells (Pei et al., 2000; Köhler et al., 2003). Furthermore, in Arabidopsis guard cells, ABA was shown to enhance cellular ROS levels (Pei et al., 2000). In addition, the ABA-insensitive mutations gca2 (Himmelbach et al., 1998) and abi2-1 impaired ROS activation of ICa channels, providing genetic evidence that ICa channels are central components of ABA signaling (Pei et al., 2000; Murata et al., 2001). ABA is capable of increasing H2O2 levels in maize embryos and seedlings and in Vicia guard cells, further supporting roles of ROS in ABA signaling (Guan et al., 2000; Zhang et al., 2001; Jiang and Zhang, 2002). Protein phosphatase type 1/2A pharmacological inhibitors activate ICa channels in Vicia faba guard cells (Köhler and Blatt, 2002), and the ost1 protein kinase mutant (Mustilli et al., 2002) and the dominant abi1-1 mutant (Murata et al., 2001) disrupt ABA-induced ROS production, suggesting that protein phosphorylation functions in ABA-induced ROS production and ICa channel activation.
ICa channels have been characterized in tomato suspension cells, Arabidopsis root apex cells and in root hair cells, indicating that ICa-like channels function in various cell types (Gelli and Blumwald, 1997; Kiegle et al., 2000; Véry and Davies, 2000). Interestingly, a recent study in Fucus rhizoid cells showed that during polar growth, tip-localized ROS production precedes activation of tip-focused Ca2+ influx, and that H2O2 activates plasma membrane Ca2+ channels (Coelho et al., 2002). Together with guard cell studies, this indicates that ROS activation of ICa channels could be of broad significance in plant biology.
Hydrogen peroxide was recently shown to inactivate ABI1 and ABI2 PP2C enzyme activity in vitro (Meinhard and Grill, 2001; Meinhard et al., 2002). The question whether ROS production is rate limiting for ABA signal transduction has not yet been addressed in vivo. Therefore, genetic disruption of ROS-producing enzymes would provide a direct approach to unequivocally test the proposed functions and relative importance of ROS for ABA signal transduction and ABA activation of ICa channels. However, at least nine different classes of well-characterized cellular proteins can mediate ROS production in plant cells (Lamb and Dixon, 1997; Mittler, 2002; see Discussion), and it remains unknown which enzymatic mechanisms are responsible for ABA-triggered ROS generation in guard cells at the molecular level.
In this report, we identify two NADPH oxidase catalytic subunit genes, AtrbohD and AtrbohF, which function in ABA-induced ROS production in guard cells, and demonstrate that ROS production is rate-limiting for ABA signal transduction in vivo.
Results
AtrbohD and AtrbohF are guard cell-expressed NADPH oxidase genes
NADPH oxidases are plasma membrane proteins (Keller et al., 1998) that may produce ROS in the vicinity of plasma membrane ion channels. However, 10 NADPH oxidase catalytic subunit genes exist in the Arabidopsis genome. Assuming redundancy in these 10 genes, 45 unique double mutant combinations could be analyzed. To test the hypothesis that NADPH oxidases may function in ICa channel regulation and ABA-induced stomatal closing, we first pursued isolation of ROS-producing NADPH oxidase genes that are expressed in guard cells. To identify guard cell-expressed NADPH oxidase catalytic subunit genes, gp91phox homologous sequences (Torres et al., 1998; Bánfi et al., 2000) were aligned. Degenerate oligomers were used to amplify guard cell-expressed Atrboh genes using enriched (>95% purified) Arabidopsis guard cell cDNA libraries (Kwak et al., 2002). A guard cell-expressed gp91phox homologous gene, AtrbohD, was identified from guard cell cDNA libraries. In addition, Genechip experiments were performed with RNA that was independently prepared from highly purified guard cells (>98% pure) and mesophyll cells (>96% pure), and also led to identification of AtrbohD and another guard cell-expressed gp91phox homolog, AtrbohF. Furthermore, microarray results suggested that AtrbohA, AtrbohC, AtrbohE, AtrbohG and AtrbohI genes are not expressed in guard cells, irrespective of ABA treatment. AtrbohB was potentially expressed in guard cells at low levels but ABA treatment abrogated gene expression in guard cells. AtrbohH and AtrbohJ are not present on the Genechip we used.
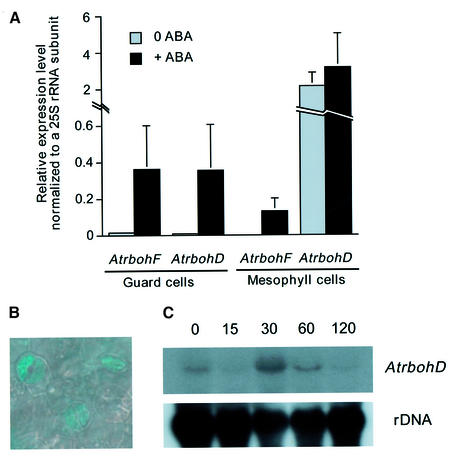
Genechip microarray expression analyses showed that expression of both AtrbohD and AtrbohF mRNAs is upregulated by ABA in guard cells (Figure 1A). Without ABA treatments, these two genes were expressed in guard cells based on chip hybridization signals (Figure 1A; see Materials and methods). AtrbohD was highly expressed in mesophyll cells with and without ABA treatment (Figure 1A). In contrast, AtrbohF was not detectable in mesophyll cells before ABA treatment, and ABA treatment led to a low expression level of AtrbohF in mesophyll cells (Figure 1A). Transgenic plants expressing the β-glucuronidase (GUS) reporter under the control of a 1536 bp AtrbohD promoter fragment also showed that AtrbohD is expressed in guard cells (Figure 1B) and mesophyll cells (data not shown). To further examine the expression of AtrbohD and AtrbohF, we carried out RNA blot analyses with total RNA extracted from leaves treated with ABA before RNA isolation. Interestingly, AtrbohD expression reached the highest induction by ABA at 30 min and then decreased back to basal levels after 120 min (Figure 1C). Under the same conditions, AtrbohF was not detectable on RNA blots containing total leaf RNA, which correlates with the low level of AtrbohF expression in mesophyll cells (Figure 1A; Keller et al., 1998).
Fig. 1. AtrbohD and AtrbohF genes are upregulated by ABA in guard cells. (A) Genechip experiments show expression levels of AtrbohD and AtrbohF mRNA in guard cells and mesophyll cells. Gene chip experiments were performed with guard cell and mesophyll cell RNA that was extracted from WT plants sprayed with 100 µM ABA or water 4 h prior to RNA isolation. (B) AtrbohD promoter drives GUS expression in guard cells of WT plants expressing the AtrbohD::GUS fusion construct. (C) WT plants were sprayed with 100 µM ABA for 15, 30, 60 or 120 min prior to RNA isolation. Total RNA (25 µg) extracted from rosette leaves was separated in a denaturing gel and then transferred onto a nylon membrane. The blot was hybridized with 32P-labeled AtrbohD or AtrbohF cDNA. The same blot was probed with 32P-labeled 18S rDNA to show relative amounts of RNA samples.
ABA-induced stomatal closing is partially impaired in the atrbohF single mutant and more strongly impaired in atrbohD/F double mutants
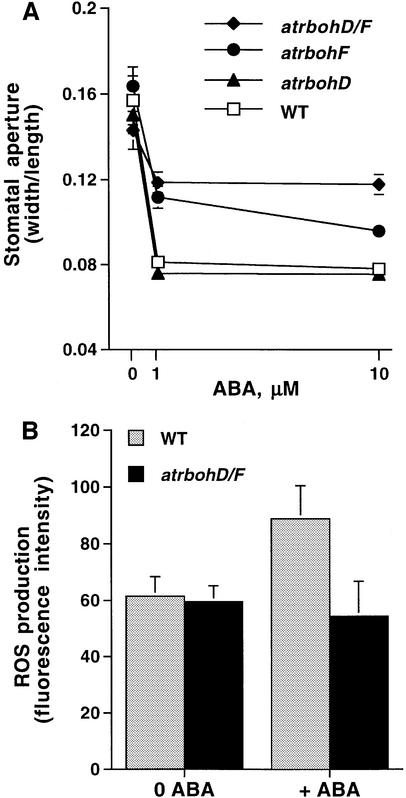
To analyze whether NADPH oxidases function in ABA signaling and more specifically whether the AtrbohD and AtrbohF genes contribute to ABA responses, we isolated mutants carrying dSpm insertions in each gene using PCR-based screening of a dSpm insertion mutant population (Tissier et al., 1999). The atrbohD and atrbohF alleles used here show lack of expression in these genes (Torres et al., 2002). Moreover, an atrbohE mutant carrying a dSpm insertion was also isolated for control experiments. Because NADPH oxidases are only one of several possible enzymatic sources of ROS production in plant cells, we subsequently performed ABA-induced stomatal closing analyses with Atrboh single mutants. As shown in Figure 2A, stomatal closure in response to ABA in the atrbohD single mutant did not show any difference from the response in wild type (WT; P > 0.29 at 10 µM ABA). However, ABA-induced stomatal closure in the atrbohF single mutant was partially reduced compared with WT (Figure 2A; P < 0.0005 at 10 µM ABA). atrbohD/F double mutant lines were obtained from crosses between homozygous atrbohD and atrbohF mutants. Interestingly, in the atrbohD/F double mutant, ABA-induced stomatal closing was clearly impaired compared with both WT and atrbohF (P < 0.0001 at 10 µM ABA; for atrbohD/F versus atrbohF). In controls, neither atrbohE nor atrbohD/E mutants showed any altered ABA responses in stomatal closing assays compared with WT plants (data not shown). These results show that the atrbohD mutation contributes to increased ABA insensitivity of the atrbohD/F double mutant, and suggest that AtrbohD and AtrbohF show partial overlap in their functions in the guard cell ABA response.
Fig. 2. ABA-induced stomatal closing and ABA-induced ROS generation are disrupted in the atrbohD/F double mutant. (A) Stomatal aperture measurements show that ABA-induced stomatal closing is partially reduced in atrbohF and atrbohD/F double mutants. (B) ABA (50 µM) induces ROS increases in guard cells of WT (three experiments; n = 44 guard cells before ABA treatment, n = 41 guard cells after ABA treatment). ABA failed to induce an increase in ROS levels in guard cells of atrbohD/F double mutant (three experiments; n = 59 guard cells before ABA treatment, n = 39 guard cells after ABA treatment). In (A), n = 6 experiments (120 stomatal apertures per data point) for WT; n = four experiments (80 stomatal apertures) for atrbohD/F; n = 3 experiments each (60 stomatal apertures each) for atrbohD and for atrbohF. Starting stomatal apertures were: 3.46 ± 0.63 µm (WT), 3.88 ± 0.37 µm (atrbohD), 3.36 ± 0.48 µm (atrbohF), 3.16 ± 0.44 µm (atrbohD/F). Error bars represent ± SEM relative to number of experiments. Error bars are smaller than symbols when not visible.
ABA-induced ROS production is impaired in atrbohD/F double mutants
We carried out ABA-induced ROS production measurements using the fluorescent dye 2′,7′-dichlorofluorescin diacetate (H2DCF-DA) in guard cells of atrbohD/F double mutants in order to determine whether NADPH oxidases are the major enzymatic source of ABA-induced ROS generation in guard cells. As previously reported, ABA treatment produced increases in fluorescence in WT guard cells (Figure 2B; n = 41 guard cells, P < 0.005). In contrast, 50 µM ABA treatment failed to induce increases in ROS levels in atrbohD/F guard cells (Figure 2B), suggesting that NADPH oxidases mediate ABA-induced ROS generation in guard cells and that AtrbohD and AtrbohF are the major catalytic subunits in this response.
Exogenous ROS rescue stomatal closing in atrbohD/F double mutants
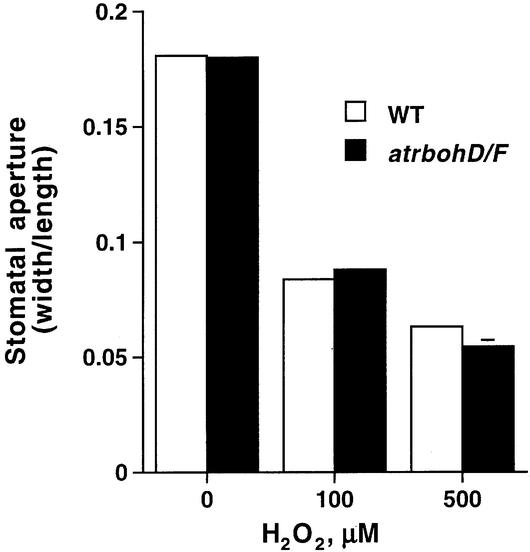
To further test whether lack of ABA-induced ROS (Figure 2B) can be linked to ABA insensitivity of the atrbohD/F double mutant, we examined whether exogenously applied ROS can induce stomatal closure in atrboh mutants. Stomatal apertures were measured at two different concentrations of exogenously applied H2O2. Figure 3 shows that ROS-induced stomatal closing in the atrbohD/F double mutant exhibited no significant difference to WT (P > 0.28 at 100 µM H2O2). Furthermore, in atrbohD and atrbohF single mutants, ROS-induced stomatal closure showed no difference to WT (data not shown). These results indicate that H2O2 can rescue WT stomatal responses in the atrbohD/F double mutant.
Fig. 3. Exogenous H2O2 rescues stomatal closing in atrbohD/F double mutant. H2O2 induces stomatal closing both in atrbohD/F and WT. Stomatal apertures were measured 3 h after addition of 100 or 500 µM H2O2. n = 3 experiments (60 stomatal apertures) for WT; n = 3 experiments (60 stomatal apertures) for atrbohD/F. Starting stomatal apertures: 3.81 ± 0.20 µm (WT), 3.40 ± 0.04 µm (atrbohD/F). Error bars represent ± SEM relative to three independent experiments. Error bars are smaller than symbols when not visible.
ABA-induced cytosolic calcium increases are significantly reduced in atrbohD/F
To analyze the relative contributions of NADPH oxidases and ABA-induced ROS production to [Ca2+]cyt elevations, we carried out calcium imaging analysis on guard cells in intact epidermal strips using WT and atrbohD/F plants expressing the cytosolic Ca2+ reporter yellow cameleon 2.1 (Miyawaki et al., 1997; Allen et al., 1999). Two independent homozygous WT and atrbohD/F plant lines expressing yellow cameleon 2.1 were used for ratiometric calcium imaging experiments.
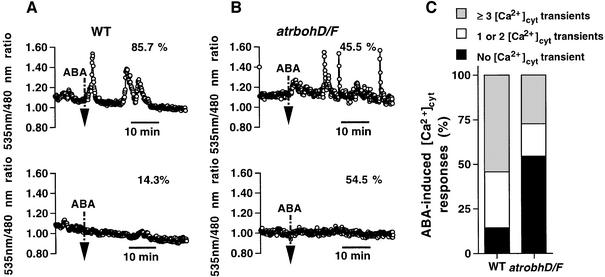
ABA was applied to guard cells that showed stable Ca2+ baselines to analyze ABA-dependent [Ca2+]cyt changes. When treated with 5 µM ABA, 85.7% of WT guard cells showed ABA-induced [Ca2+]cyt elevations during recordings (Figure 4A, top trace and C). Among them, 54.3% of WT guard cells showed three or more [Ca2+]cyt transients (n = 19 of 35 cells; Figure 4C) and 31.4% showed one or two [Ca2+]cyt transients (n = 11 of 35 cells; Figure 4C) within the recording period. In contrast, in atrbohD/F only 45.5% of guard cells exhibited ABA responsiveness in [Ca2+]cyt elevations (Figure 4B, top trace and C). Among them, 27.3% of atrbohD/F guard cells showed three or more [Ca2+]cyt transients (n = 9 of 33 cells; Figure 4C) and 18.2% showed a single or two [Ca2+]cyt transients (n = 6 of 33 cells; Figure 4C). Moreover, 54.5% of atrbohD/F guard cells showed no measurable ABA-induced [Ca2+]cyt elevations (n = 18 of 33 cells; Figure 4B, bottom trace and C), whereas only 14.3% of WT guard cells showed no response to 5 µM ABA (n = 5 of 35 cells; Figure 4A, bottom trace and C). Statistical analyses indicate that the number of cells showing ABA-induced [Ca2+]cyt increases was significantly reduced in atrbohD/F guard cells (χ2 = 12.16, P < 0.0005), suggesting that the atrbohD/F mutation partially decreases the responsiveness of guard cells with respect to ABA-induced cytosolic calcium elevations.
Fig. 4. atrbohD/F mutation impairs ABA-induced [Ca2+]cyt elevations in guard cells. (A) Fluorescence emission ratio (535/480 nm) shows responsiveness of ABA-induced [Ca2+]cyt elevations at 5 µM ABA in WT guard cells. Thirty of 35 cells (85.7%) showed ABA-mediated [Ca2+]cyt elevations whereas five of 35 cells (14.3%) showed no response. (B) Fluorescence emission ratio (535/480 nm) shows examples of ABA-induced [Ca2+]cyt elevations at 5 µM ABA in atrbohD/F guard cells (15 of 33 cells = 45.5%). Traces showing ABA-induced [Ca2+]cyt transients are shown in the top panels of (A) and (B), and those with no clear ABA-induced [Ca2+]cyt elevations are shown in the bottom panels of (A) and (B). Vertical arrow lines indicate when guard cells were challenged with 5 µM ABA. [Ca2+]cyt transients were counted when changes in [Ca2+]cyt ratios were ≥0.1 U. (C) Stack column representation of number of ABA-induced [Ca2+]cyt transients recorded in WT (n = 35) and atrbohD/F (n = 33) guard cells at 5 µM ABA.
atrbohD/F mutation abolishes ABA but not H2O2 activation of plasma membrane ICa channels
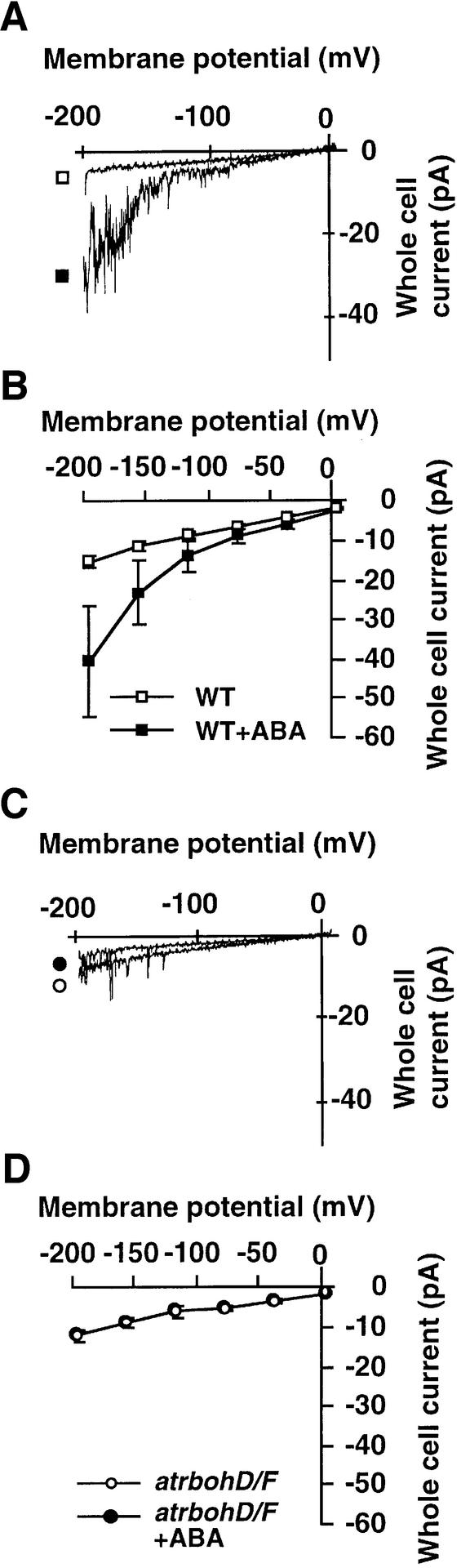
To determine whether the AtrbohD and AtrbohF NADPH oxidase subunits function in ABA activation of plasma membrane ICa channels, we tested ABA activation of ICa channels in atrbohD/F by patch–clamping guard cells. ABA clearly activated ICa channels in 10 of 18 WT guard cells (Figure 5A and B). The average response of all 18 cells, including non-responsive cells, showed a significant ABA activation of ICa channels in WT (Figure 5B; P < 0.05 at –196 mV). In contrast, as shown in Figure 5C and D, ABA activation of ICa channels was completely abolished in atrbohD/F guard cells (n = 10 of 10 cells, P > 0.99 at –196 mV).
Fig. 5. ABA-activation of ICa channels is abolished in atrbohD/F guard cells. (A and B) ABA (50 µM) activated ICa channel currents in WT guard cells. Current traces before and after ABA activation of ICa channels are shown in a representative cell in (A) and average current– voltage curves of 18 cells are shown in (B). (C and D) ABA failed to activate ICa channel currents in atrbohD/F guard cells. A response in a representative cell is shown in (C) and average current–voltage curves (n = 10 cells) are shown in (D). Symbols of the atrbohD/F guard cells perfused with ABA overlap with atrbohD/F control guard cell symbols when not visible.
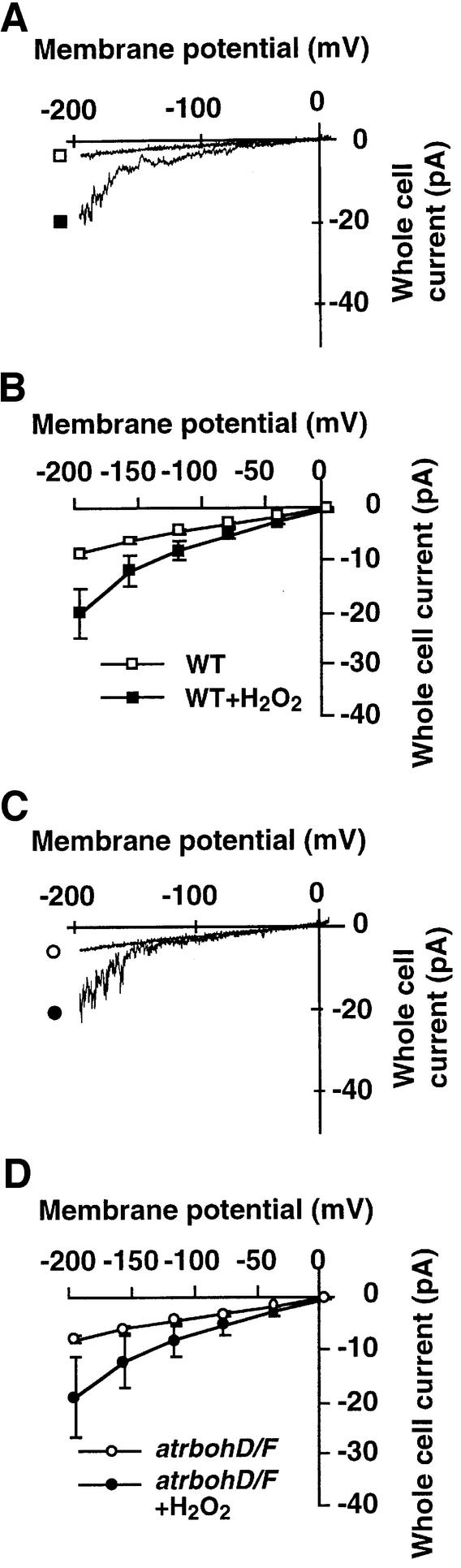
We further tested whether H2O2, a rapid turnover product of superoxide produced by NADPH oxidases, can bypass the atrbohD/F double mutation and activate ICa channels in atrbohD/F guard cells. ROS activation of ICa channels occurred both in WT (Figure 6A and B; n = 10) and atrbohD/F guard cells (Figure 6C and D; n = 7). Impairment in ABA- but not in ROS-activation of ICa channels (Figures 5 and 6) is consistent with the stomatal phenotype of atrbohD/F that showed ABA-insensitive but H2O2-responsive stomatal movements (Figures 2 and 3). These data suggest that the AtrbohD and AtrbohF catalytic subunits of NADPH oxidases function in the signaling pathway that mediates ABA activation of ICa channels, and provide direct genetic evidence that ROS function as rate-limiting second messengers in ABA signaling.
Fig. 6. ROS activate plasma membrane ICa channels both in atrbohD/F and WT guard cells. (A and C) H2O2 activation of ICa channels in a representative cell of WT and atrbohD/F, respectively. (B and D) Average current–voltage curves (WT, n = 10; atrbohD/F, n = 7). Error bars represent SEM. Error bars are smaller than symbols when not visible.
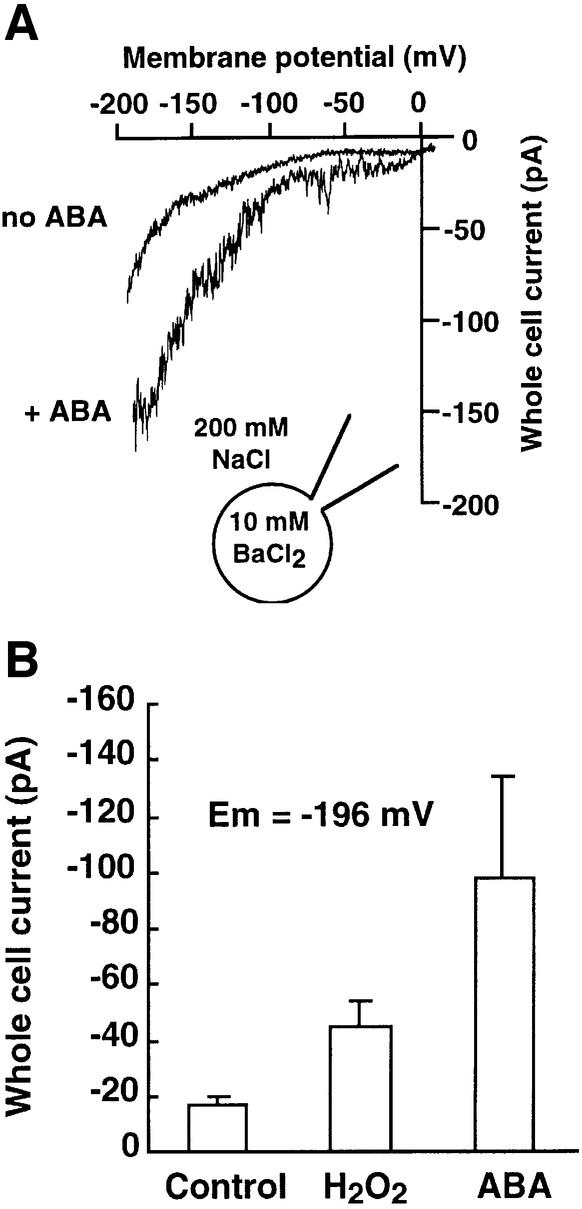
To further examine whether ABA-activated hyperpolarization-induced currents in WT were carried by the same channels as ROS-activated currents, we analyzed the cation selectivity of both currents. As reported previously, both ABA- and ROS-activated currents were Ba2+ permeable (Figures 5 and 6). In further experiments, we analyzed the Na+ conductance through both ABA- and ROS-regulated currents with 200 mM extracellular Na+. Both ABA (Figure 7A; n = 6 cells) and H2O2 (n = 7 cells) activated hyperpolarization-induced currents (Figure 7B). In the absence of ABA and ROS, a constitutive background inward Na+ current was also observed in guard cells, indicating the presence of an additional type of plasma membrane Na+ current in Arabidopsis guard cells (Figure 7A, no ABA). These data show that ICa currents in guard cells are permeable to the monovalent cation Na+, as well as the divalent cations Ca2+, Mg2+, Cd2+ and Ba2+ (Pei et al., 2000; Perfus-Barbeoch et al., 2002).
Fig. 7. H2O2- and ABA-activated ICa currents are Na+ permeable in guard cells. (A) Whole-cell current recordings without (no ABA) and with 50 µM ABA (+ ABA) in the same guard cell bathed in 200 mM NaCl. (B) Average Na+ currents at –196 mV show that both ABA and H2O2 activated inward Na+ currents in Arabidopsis guard cells (H2O2, n = 7 cells; ABA, n = 6 cells).
ABA inhibition of root growth and seed germination is impaired in atrbohD/F
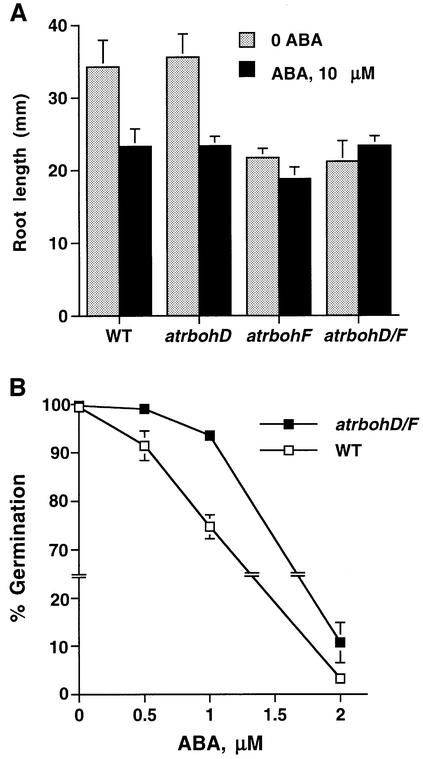
To test whether disruption mutations in atrboh genes affect other ABA responses, we examined ABA inhibition of root growth and seed germination. Root elongation was measured over a 5 day period (7- to 12-day-old seedlings) in WT, atrbohD, atrbohF and atrbohD/F grown on medium containing 0 and 10 µM ABA. Root lengths of atrbohF and atrbohD/F mutant seedlings grown on Murashige and Skoog (MS) medium containing no ABA were smaller than those of WT and atrbohD seedlings (Figure 8; P < 0.02). Roots of atrbohF and atrbohD/F were insensitive to ABA compared with WT (Figure 8; P < 0.006, atrbohF; P < 0.001, atrbohD/F), whereas the roots of atrbohD showed a similar ABA sensitivity compared with WT (P > 0.93), suggesting that NADPH oxidases may contribute to ABA regulation of root elongation. Despite differences in root lengths of seedlings, root elongation rates during 5 days (from 7- to 12-day-old seedlings) on ABA-free medium did not show significant differences between WT, atrbohF and atrbohD/F mutants (190% growth for WT, 201% growth for atrbohF, 172% growth for atrbohD/F), further indicating that mutants may be less ABA sensitive.
Fig. 8. Root growth and seed germination in atrbohD/F show reduced ABA sensitivity. (A) Seven-day-old WT, atrbohD, atrbohF and atrbohD/F seedlings were placed on MS medium supplemented with 0 and 10 µM ABA. Root elongation was measured after 5 days. Each data point represents the mean value of 12–15 seedlings. Error bars represent ±SEM. (B) The atrbohD/F mutant shows partial reduction in ABA sensitivity of seed germination inhibition. Error bars are smaller than symbols when not visible. Error bars represent ±SE of n = 3 independent experiments; >360 seeds at each data point.
To determine whether NADPH oxidase mutation affects ABA regulation of seed germination in Arabidopsis, we examined seed germination of atrbohD/F double mutants. ABA inhibition of seed germination shows a partial ABA insensitivity in the atrbohD/F mutant compared with WT (Figure 8B; P < 0.02 at 0.5 µM ABA, P < 0.0005 at 1 µM ABA). These results show that NADPH oxidases affect ABA inhibition of seed germination in Arabidopsis as well as ABA inhibition of root elongation.
Discussion
Here, we directly demonstrate through molecular genetic, cell biological and biophysical analyses that ROS production is required for ABA signal transduction in guard cells, and that AtrbohD and AtrbohF are major NADPH oxidase catalytic subunits that mediate ABA-induced ROS production, ABA activation of ICa channels and ABA-induced stomatal closure. Furthermore, H2O2 activates ICa channels and rescues stomatal closing in atrbohD/F double mutants, indicating that AtrbohD and AtrbohF NADPH oxidase catalytic subunits act upstream of plasma membrane ICa channels in this recently identified ABA signaling branch. These results unequivocally demonstrate that ROS function as rate-limiting second messengers and that NADPH oxidases function as a central positive transducer in ABA signal transduction in guard cells.
Redundancy in ABA signaling and cell-type specific function
Given that many gene families in the Arabidopsis genome have large numbers of homologs relative to other sequenced genomes (Arabidopsis Genome Initiative, 2000), the relatively low number of recessive ABA-insensitive mutants (see Introduction) is most likely due to redundancy in genes encoding ABA transducers. This explains why conventional genetic screens have not identified many recessive ABA-insensitive mutants. Ten Atrboh genes are present in the Arabidopsis genome (Arabidopsis Genome Initiative, 2000). Therefore, determination of which two Atrboh genes are highly expressed in a single cell type (guard cells) was pivotal for functional analyses (Figures 1 and 2). By pursuing single-cell functional genomics, two of the NADPH oxidase catalytic subunit genes were identified as positive ABA signal transducers.
ROS-mediated Ca2+ channel activation may be of general importance in plant signal transduction
ABA inhibition of root elongation is reduced in atrbohD/F and atrbohF (Figure 8), suggesting that ROS production by the NADPH oxidase AtrbohF may be of broader significance for ABA signal transduction. In addition, ABA inhibition of seed germination is reduced in atrbohD/F double mutants (Figure 8B). A recent study in Fucus rhizoid cells showed that tip-localized ROS production and plasma membrane ICa channel activation precede polar growth (Coelho et al., 2002). Additionally, ROS were shown to activate plasma membrane Ca2+ channels in plant root cells (Demidchik et al., 2003). Therefore, it appears that the ROS-mediated plasma membrane Ca2+ channel activation pathway may be of more general importance in plant signal transduction and development. It is conceivable that different NADPH oxidase genes or combinations may contribute to this signaling branch in distinct responses and cell types.
NADPH oxidase-mediated ROS production, a rate-limiting ABA signal transduction branch
ROS were shown to induce cytosolic calcium elevations in tobacco seedlings and guard cells (Price et al., 1994; McAinsh et al., 1996; Pei et al., 2000). A recent study has led to the suggestion that ROS may not be a critical second messenger for ABA signaling in guard cells (Köhler et al., 2003). However, this study focuses on exogenous H2O2 regulation of K+ channels including the Ca2+-independent outward K+ channels, which show differences compared with ABA regulation. Note that previous studies have shown that exogenous H2O2 application causes partial stomatal closing responses compared with ABA (Pei et al., 2000), and that responses to exogenous H2O2 or ozone application are not equivalent to ABA responses (Torsethaugen et al., 1999; Allen et al., 2000; Pei et al., 2000). Differences between (i) biological localized enzymatic ROS production and exogenous global H2O2 application, (ii) ion channel micro-environments, (iii) temporal ROS production and (iv) a model in which parallel ROS-independent ABA signaling mechanisms were proposed (see figure 5F in Pei et al., 2000) may contribute to differences in exogenous H2O2 application and multiple roles for ROS in biological signaling.
Our results show that ABA-induced cytosolic Ca2+ elevations, ABA activation of ICa channels as well as ABA-induced stomatal closing are impaired in atrbohD/F mutants (Figures 2A, 4 and 5). However, 45% of atrbohD/F guard cells still show ABA-induced cytosolic Ca2+ elevations (Figure 4), suggesting that other ROS-independent parallel pathways as proposed by Pei et al. (2000) are functional in atrbohD/F and contribute to ABA-induced cytosolic Ca2+ increases. This result supports the current model for guard cells in which ABA signaling is mediated by parallel Ca2+ influx and Ca2+ release pathways (MacRobbie, 2000), and correlates with the partial ABA-induced stomatal closing response observed in atrbohD/F double mutants (Figure 2A). Note that our data do not exclude the possibility that one of the parallel ABA signaling branches may be able to partially activate ICa channels via a parallel ROS-independent branch in intact Arabidopsis guard cells. Ion channels often function as integrating targets of multiple signaling branches (Hille, 2001). Despite these possibilities, our findings directly demonstrate that ABA-induced ROS are rate limiting for functional ABA signaling in vivo, and illustrate the relative contribution of AtrbohD and AtrbohF to ROS-mediated ABA signaling.
Although the atrbohD/F double mutation impairs ABA-induced ROS production, there is a background level of cellular ROS in guard cells before ABA treatment, which suggests the existence of other cellular mechanisms generating ROS in guard cells (Figure 2B). Multiple enzymes and reactions are known to cause ROS production, including the mitochondrial respiration electron transport chain, chloroplast photosynthetic electron transport, oxalate oxidases, glycolate oxidases, xanthine oxidases, fatty acid β-oxidation, amine oxidases and cell wall-bound peroxidases (Mittler, 2002). On the other hand, ROS scavenging enzymes including catalases, superoxide dismutases, glutathione peroxidases, thioredoxin peroxidases and ascorbate peroxidases remove cellular ROS and thus contribute to ROS homeostasis in plant cells (Mittler, 2002) and may also be ABA regulated. It is interesting that in light of these diverse activities, extracellular ROS production by AtrbohD and AtrbohF is a requirement for ABA-induced ROS production and ABA activation of plasma membrane Ca2+ channels. Our data do not exclude additional contributions of further Atrboh or ROS producing and scavenging genes to ABA responses.
NADPH oxidase regulation
NADPH oxidases in mammals are composed of two plasma membrane proteins, gp91phox and p22phox, which form a heterodimeric flavocytochrome_558 (Bokoch, 1994). During activation, two cytosolic proteins, p47 and p67, and the small G protein Rac translocate to the plasma membrane, resulting in the formation of the active NADPH oxidase complex in neutrophils (Bokoch, 1994; Diekmann et al., 1994). In plants, AtrbohF was shown to be a plasma membrane protein (Keller et al., 1998), and a plasma membrane NADPH oxidase was shown to produce superoxide (Sagi and Fluhr, 2001). Therefore, plant NADPH oxidases may produce ROS in the vicinity of plasma membrane ICa channels, thus regulating ICa channels. Moreover, plant NADPH oxidases do not require additional cytosolic factors for enzymatic activity (Sagi and Fluhr, 2001), suggesting that plant NADPH oxidases differ from the mammalian NADPH oxidases in their functional composition. This is further supported by the finding that no p47 and p67 homologs of the mammalian NADPH oxidase are found in the Arabidopsis genome (Torres et al., 2002). It will be of interest to investigate AtrbohD- and AtrbohF-interacting proteins in vivo.
Dual functions of NADPH oxidases in ABA signal transduction and in plant defense response
ROS are known to control programmed cell death and pathogen defense in plants (Lamb and Dixon, 1997; Mittler, 2002). Cell wall-bound peroxidases and plasma membrane NADPH oxidases have been proposed to be the main ROS sources in plant defense responses (Lamb and Dixon, 1997). A recent study showed that the AtrbohD and AtrbohF NADPH oxidase catalytic subunits contribute to pathogen-induced ROS production in Arabidopsis. ROS production induced by bacterial and fungal infection was reduced in atrbohD/F double mutants (Torres et al., 2002). However, unexpectedly, despite reduction in ROS generation, enhanced cell death was observed in atrbohD/F mutants in response to oomycete pathogen, whereas bacteria-induced cell death was reduced in atrbohD/F mutants (Torres et al., 2002). These data indicate that other genes may mediate fungal pathogen-induced cell death. Together with this study, our results indicate that these NADPH oxidases have a dual function in mediating ABA signal transduction and in contributing to pathogen-associated ROS production.
Materials and methods
Identification of Atrboh genes in guard cells
To identify guard cell-expressed Atrboh genes, we carried out degenerate oligomer-based RT–PCR using enriched Arabidopsis guard cell cDNA libraries (Kwak et al., 2002). Six sequences of Atrboh genes, which were available at the outset of this project, were aligned to design degenerate oligomers, and then two highly conserved regions were selected from the protein sequences: VCRNTITW (for sense primer) and GLGIGATP (for antisense primer). The degenerate oligomers from these sequences were 5′-GTITGYMGIAAYACIATHACITGG-3′ (sense primer) and 5′-GGIGTIGCICCDATICCIARICC-3′ (antisense primer). Total RNA was extracted from guard cell-enriched epidermal strips as described previously (Kwak et al., 2002), and then 2 µg of total RNA were converted to cDNA using the First-strand cDNA Synthesis Kit (Amersham-Pharmacia Biotech). PCR reactions were performed as described previously (Kwak et al., 2002). PCR products were purified, cloned into the pGEM-T Easy vector (Promega) and subjected to sequencing reactions to obtain the insert sequence as described previously (Kwak et al., 1997). Arabidopsis Genome Initiative numbers for Atrboh genes are AtrbohA (At5g07390), AtrbohB (At1g09090), AtrbohC (At5g51060), AtrbohD (At5g47910), AtrbohE (At1g19230), AtrbohF (At1g64060), AtrbohG (At4g25090), AtrbohH (At5g60010), AtrbohI (At4g11230), and AtrbohJ (At3g45810).
Genechip experiments and GUS activity analysis
Total RNA (10 µg) from two independently extracted guard cell and two mesophyll cell protoplast preparations (Kwak et al., 2002) was pooled and used for experiments with DNA chip (Affymetrix), which represents ∼8000 Arabidopsis genes. Probe labeling and hybridization of the chips were performed at the UC San Diego and UC Irvine Gene Chip Cores. Data normalization and analysis were carried out using Affymetrix GeneChip Suite 4.0 software. After performing standard normalization of scanned chip images relative to whole-chip intensities (Zhu et al., 2001), expression levels of AtrbohD and AtrbohF in guard cells and mesophyll cells were further normalized relative to the 25S rRNA expression levels in each cell type. GUS activity was assayed in epidermal strips of 7-day-old seedlings as described previously (Hugouvieux et al., 2001).
Isolation of atrbohD and atrbohF null mutants
Homozygous knockout plants carrying a single dSpm transposon insertion in AtrbohD and AtrbohF genes were isolated in which no full-length transcript was detected, as described previously (Torres et al., 2002). atrbohD/F double mutant plants were obtained by crossing atrbohF and atrbohD single mutants and confirmed by PCR.
Stomatal aperture measurements
Stomatal apertures were measured as described previously (Kwak et al., 2002) in 5 mM KCl, 50 µM CaCl2 and 10 mM MES–Tris pH 6.15. Stomatal apertures were measured 3 h after ABA (1 and 10 µM) or H2O2 (100 and 500 µM) was added. Student’s t-test (two-tailed distribution, two-sample assuming equal variance) was used to determine the statistical significance of the data.
RNA isolation and RNA blot analyses
Total RNA was extracted using Trizol reagent (Invitrogen Life Technologies) from rosette leaves of 5-week-old WT plants sprayed with 100 µM ABA 0, 15, 30, 60 or 120 min prior to RNA extraction. Total RNA samples were separated as described previously (Kwak et al., 2002). The blots were hybridized with 32P-labeled AtrbohD, AtrbohF cDNA or 18S rDNA and washed as described previously (Kwak et al., 2001).
ROS production measurements in guard cells
H2DCF-DA was used to analyze ABA-induced ROS production in guard cells as described previously (Murata et al., 2001), with a slight modification. Epidermal strips were prepared from 4- to 5-week-old WT and atrbohD/F plants using a blender. The epidermal strips were incubated in 5 mM KCl, 10 mM MES–Tris pH 6.15 in the light with a fluence rate of 95 µE m–2 s–1 for 2 h. Fifty micromolar H2DCF-DA was added to the solution containing the epidermal strips for 30 min on an orbital shaker (70 r.p.m.). Then 50 µM ABA or 0.1% ethanol (control) was added to the incubation medium after dye loading. Guard cell images were taken using Adobe Photoshop 5.5 (Mountain View, CA) during short 2 s UV exposures (one UV exposure per sample) under a fluorescence microscope equipped with a digital camera (Murata et al., 2001). Fluorescence emission of guard cells was analyzed using Adobe Photoshop 5.5.
Calcium imaging analyses
To perform calcium imaging analyses, WT and atrbohD/F plants were transformed with the pH-insensitive yellow cameleon construct p35SYC2.1 (Allen et al., 1999). Homozygous WT (two independent lines) and atrbohD/F (two independent lines) expressing yellow cameleon were used to measure [Ca2+]cyt changes as described previously (Allen et al., 1999; Hugouvieux et al., 2001; Kwak et al., 2002). Guard cells that showed spontaneous [Ca2+]cyt transients (Allen et al., 1999; Staxen et al., 1999) were excluded from analyses. [Ca2+]cyt transients were counted when the change in [Ca2+]cyt ratios was ≥0.1 U above the baseline. Background fluorescence was measured in guard cell-less epidermal domains and was subtracted from the epidermal field prior to ABA application (Allen et al., 1999; Hugouvieux et al., 2001; Kwak et al., 2002). Statistical analyses showed that the average of raw data fluorescence baseline ratios prior to ABA application was not significantly different in WT (1.13 ± 0.19, n = 35) and atrbohD/F double mutant (1.05 ± 0.20, n = 33; P > 0.086). The fluorescence baseline of the top trace in Figure 4B was reduced by 0.05 U subtraction. Cells showing stable [Ca2+]cyt ratios during the first 10 min of recordings (continuous bath perfusion) were perfused with the stomatal opening solution containing 5 µM ABA. The recording continued for 50 min. The P value was calculated from the χ2-test for a 2 × 2 contingency table containing ABA-responsive cells versus non-responsive cells of WT and atrbohD/F obtained from calcium imaging analyses.
Patch–clamp analyses
Guard cell protoplasts were enzymatically isolated from Arabidopsis leaf epidermal strips of 4- to 6-week-old WT and atrbohD/F double mutant plants as described previously (Murata et al., 2001). Whole-cell patch–clamp recordings of guard cells were performed as described previously (Murata et al., 2001). The pipette solution was composed of 10 mM BaCl2, 0.1 mM DTT, 4 mM EGTA and 10 mM HEPES–Tris pH 7.1. The bath solution contained 100 mM BaCl2 and 10 mM MES–Tris pH 5.6. To measure Na+ currents, 100 mM BaCl2 in the bath solution was replaced with 200 mM NaCl. When ABA activation of plasma membrane ICa channels was measured, 1 mM NADPH was supplemented in the pipette solution, whereas 0.1 mM DTT was additionally added in the bath solution when H2O2 activation of plasma membrane ICa channels was measured. Osmolalities were adjusted to 500 mmol/kg (pipette solution) and 485 mmol/kg (bath solution). Seal resistance was >10 GΩ and liquid junction potentials were corrected (Ward and Schroeder, 1994). Whole-cell leak currents were not subtracted. The standard voltage protocol ramped from +14 to –196 mV (ramp speed 210 mV/1.5 s). ABA and H2O2 were applied by continuous bath perfusion during whole-cell recordings.
Root growth and seed germination measurements
To measure root growth in the presence of ABA, seeds of WT, atrbohD, atrbohF and atrbohD/F were plated on one-fourth-strength MS medium. Plates containing seeds were stratified at 4°C for 4 days and then transferred to a growth chamber (22°C under a 16 h light/8 h dark regime) in a vertical orientation. Seedlings grown for 7 days were transferred to plates containing one-fourth-strength MS medium with 0 or 10 µM ABA. Seedlings were further incubated in the growth chamber for another 5 days and then root length was measured. To measure seed germination rate, seeds of WT and atrbohD/F were plated on one-fourth-strength MS medium containing 0, 0.5, 1 or 2 µM ABA. Plates containing seeds were stratified at 4°C for 4 days and then transferred to a growth chamber (22°C under a 16 h light/8 h dark regime). Seed germination rates were scored after 5 days in the growth chamber.
Acknowledgments
Acknowledgements
We thank Jihye Moon and Yoshiyuki Murata for help with stomatal aperture measurements and patch–clamp controls, respectively. This research was supported by NIH (R01GM60396-01) and NSF (MCB 0077791) grants, and in part by grants from the DOE (FG03-94-ER20148) to J.I.S., NSF (MCB 0132894) to Z.-M.P., NSF (IBN-0077887) to J.L.D., the Gatsby Foundation to J.D.G.J., and by fellowships to J.M.K. and N.L. from the Human Frontier Science Program Organization, and to M.A.T. from the EEC.
Note added in proof
Foreman et al. (2003) recently reported a requirement for an NADPH oxidase in root hair growth (Nature, 422, 442–446).
References
- Allen G.J., Kwak,J.M., Chu,S.P., Llopis,J., Tsien,R.Y., Harper,J.F. and Schroeder,J.I. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J., 19, 735–747. [DOI] [PubMed] [Google Scholar]
- Allen G.J. et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science, 289, 2338–2342. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Bánfi B., Maturana,A., Jaconi,S., Arnaudeau,S., Laforge,T., Sinha,B., Ligeti,E., Demaurex,N. and Krause,K.-H. (2000) A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science, 287, 138–142. [DOI] [PubMed] [Google Scholar]
- Bokoch G.M. (1994) Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr. Opin. Cell Biol., 6, 212–218. [DOI] [PubMed] [Google Scholar]
- Coelho S.M., Taylor,A.R., Ryan,K.P., Sousa-Pinto,I., Brown,M.T. and Brownlee,C. (2002) Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell, 14, 2369–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S., Ghassemian,M., Bonetta,D., Cooney,S. and McCourt,P. (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science, 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shabala,S.N., Coutts,K.B., Tester,M.A. and Davies,J. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci., 116, 81–88. [DOI] [PubMed] [Google Scholar]
- Diekmann D., Abo,A., Johnston,C., Segal,A.W. and Hall,A. (1994) Interaction of Rac with p67-phox and regulation of phagocytic NADPH oxidase activity. Science, 265, 531–533. [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R., Wang,M.L., Lynch,T.L., Rao,S. and Goodman,H.M. (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell, 10, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R., Gampala,S.S.L. and Rock,C.D. (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell, 14, S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli A. and Blumwald,E. (1997) Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J. Membr. Biol., 155, 35–45. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Hauge,B.M., Valon,C., Smalle,J., Parcy,F. and Goodman,H.M. (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell, 4, 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L.M., Zhao,J. and Scandalios,J.G. (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J., 22, 87–95. [DOI] [PubMed] [Google Scholar]
- Hamilton D.W.A., Hills,A., Kohler,B. and Blatt,M.R. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl Acad. Sci. USA, 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (2001) Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA.
- Himmelbach A., Iten,M. and Grill,E. (1998) Signalling of abscisic acid to regulate plant growth. Philos. Trans. R. Soc. Lond. B Biol. Sci., 353, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V., Kwak,J.M. and Schroeder,J.I. (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell, 106, 477–487. [DOI] [PubMed] [Google Scholar]
- Jiang M. and Zhang,J. (2002) Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta, 215, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Keller T., Damude,H.G., Werner,D., Doerner,P., Dixon,R.A. and Lamb,C. (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell, 10, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E., Gilliham,M., Haseloff,J. and Tester,M. (2000) Hyper polarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J., 21, 225–229. [DOI] [PubMed] [Google Scholar]
- Köhler B. and Blatt,M.R. (2002) Protein phosphorylation activates the guard cell Ca2+ channel and its a prerequiste for gating by abscisic acid. Plant J., 32, 185–194. [DOI] [PubMed] [Google Scholar]
- Köhler B., Hills,A. and Blatt,M.R. (2003) Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate singaling pathways. Plant Physiol., 131, 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Leon-Kloosterziel,K.M., Schwartz,S.H. and Zeevaart,J.A.D. (1998) The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem., 36, 83–89. [Google Scholar]
- Kwak J.M., Kim,S.A., Hong,S.W. and Nam,H.G. (1997) Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris. Planta, 202, 9–17. [DOI] [PubMed] [Google Scholar]
- Kwak J.M., Murata,Y., Baizabal-Aguirre,V.M., Merrill,J., Wang,M., Kemper,A., Hawke,S.D., Tallman,G. and Schroeder,J.I. (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol., 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Moon,J.-H., Murata,Y., Kuchitsu,K., Leonhardt,N., DeLong,A. and Schroeder,J.I. (2002) Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell, 14, 2849–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. and Dixon,R.A. (1997) The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol., 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Leckie C.P., McAinsh,M.R., Allen,G.J., Sanders,D. and Hetherington,A.M. (1998) Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl Acad. Sci. USA, 95, 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Xiong,L., Gong,Z., Ishitani,M., Stevenson,B. and Zhu,J.-K. (2001) The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev., 15, 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E., Wu,Y., Sanchez,J.-P., Mettouchi,A., Mathur,J. and Chua,N.-H. (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev., 15, 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. and Fedoroff,N. (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell, 12, 2351–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A.C. (2000) ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc. Natl Acad. Sci. USA, 97, 12361–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh M.R., Brownlee,C. and Hetherington,A.M. (1990) Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature, 343, 186–188. [Google Scholar]
- McAinsh M.R., Clayton,H., Mansfield,T.A. and Hetherington,A.M. (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol., 111, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard M. and Grill,E. (2001) Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett., 508, 443–446. [DOI] [PubMed] [Google Scholar]
- Meinhard M., Rodriguez,P.L. and Grill,E. (2002) The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta, 214, 775–782. [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci., 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Llopis,J., Heim,R., McCaffery,J.M., Adams,J.A., Ikura,J.A. and Tsien,R.Y. (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature, 388, 882–887. [DOI] [PubMed] [Google Scholar]
- Murata Y., Pei,Z.-M., Mori,I.C. and Schroeder,J.I. (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell, 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A.-C., Merlot,S., Vavasseur,A., Fenzi,F. and Giraudat,J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell, 14, 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.-M., Ghassemian,M., Kwak,C.M., McCourt,P. and Schroeder,J.I. (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science, 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei Z.-M., Murata,Y., Benning,G., Thomine,S., Klusener,B., Allen,G.J., Grill,E. and Schroeder,J.I. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature, 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L., Leonhardt,N., Vavasseur,A. and Forestier,C. (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J., 32, 539–548. [DOI] [PubMed] [Google Scholar]
- Price A.H., Taylor,S., Ripley,S.J., Griffiths,A., Trewavas,A.J. and Knight,M.R. (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell, 6, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M. and Fluhr,R. (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol., 126, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.I. and Hagiwara,S. (1990) Repetitive increases in cytosolic calcium of guard cells by abscisic acid activation of nonselective calcium permeable channels. Proc. Natl Acad. Sci. USA, 87, 9305–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.I., Kwak,J.M. and Allen,G.J. (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature, 410, 327–330. [DOI] [PubMed] [Google Scholar]
- Staxen I., Pical,C., Montgomery,L.T., Gray,J.E., Hetherington,A.M. and McAinsh,M.R. (1999) Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl Acad. Sci. USA, 96, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier A.F., Marillonnet,S., Klimyuk,V., Patel,K., Torres,M.A., Murphy,G. and Jones,J.D.G. (1999) Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell, 11, 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A., Onouchi,H., Hamada,S., Machida,C., Hammond-Kosack,K.E. and Jones,J.D.G. (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J., 14, 365–370. [DOI] [PubMed] [Google Scholar]
- Torres M.A., Dangl,J.L. and Jones,J.D.G. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl Acad. Sci. USA, 99, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsethaugen G., Pell,E.J. and Assmann,S.M. (1999) Ozone inhibits guard cell K+ channels implicated in stomatal opening. Proc. Natl Acad. Sci. USA, 96, 13577–13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A.-A. and Davies,J.M. (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl Acad. Sci. USA, 97, 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-Q., Ullah,H., Jones,A.M. and Assmann,S.M. (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science, 292, 2070–2072. [DOI] [PubMed] [Google Scholar]
- Ward J.M. and Schroeder,J.I. (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell, 6, 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kuzma,J., Marechal,E., Graeff,R., Lee,H.C., Foster,R. and Chua,N.-H. (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science, 278, 2126–2130. [DOI] [PubMed] [Google Scholar]
- Xiong L., Gong,Z., Rock,C.D., Subramanian,S., Guo,Y., Xu,W., Galbraith,D. and Zhu,J.-K. (2001a) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev. Cell, 1, 771–781. [DOI] [PubMed] [Google Scholar]
- Xiong L., Lee,B.-h., Ishitani,M., Lee,H., Zhang,C. and Zhu,J.-K. (2001b) FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev., 15, 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang,L., Dong,F., Gao,J., Galbraith,D.W. and Song,C.-P. (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol., 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-K. (2002) Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol., 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Budworth,P., Han,B., Brown,D., Chang,H.-S., Zou,G. and Wang,X. (2001) Toward elucidating the global gene expression patterns of developing Arabidopsis: Parallel analysis of 8300 genes by a high-density oligonucleotide probe array. Plant Physiol. Biochem., 39, 221–242. [Google Scholar]