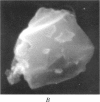
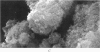
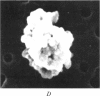
Abstract
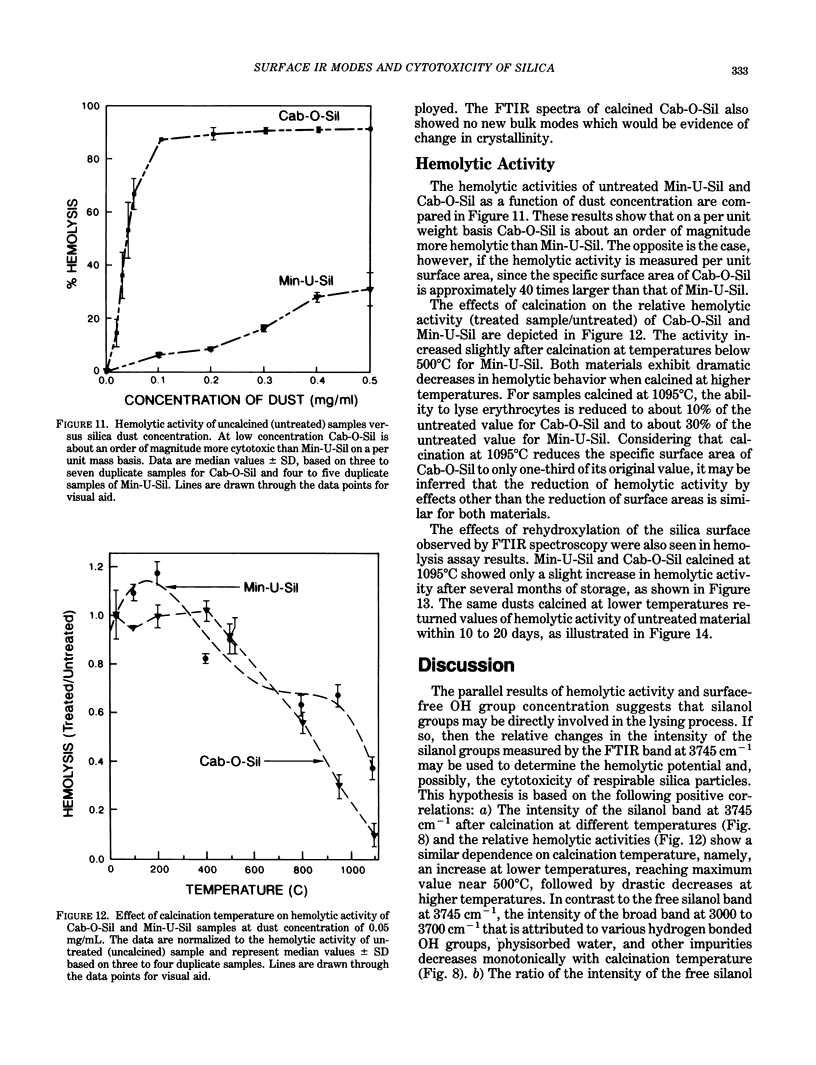
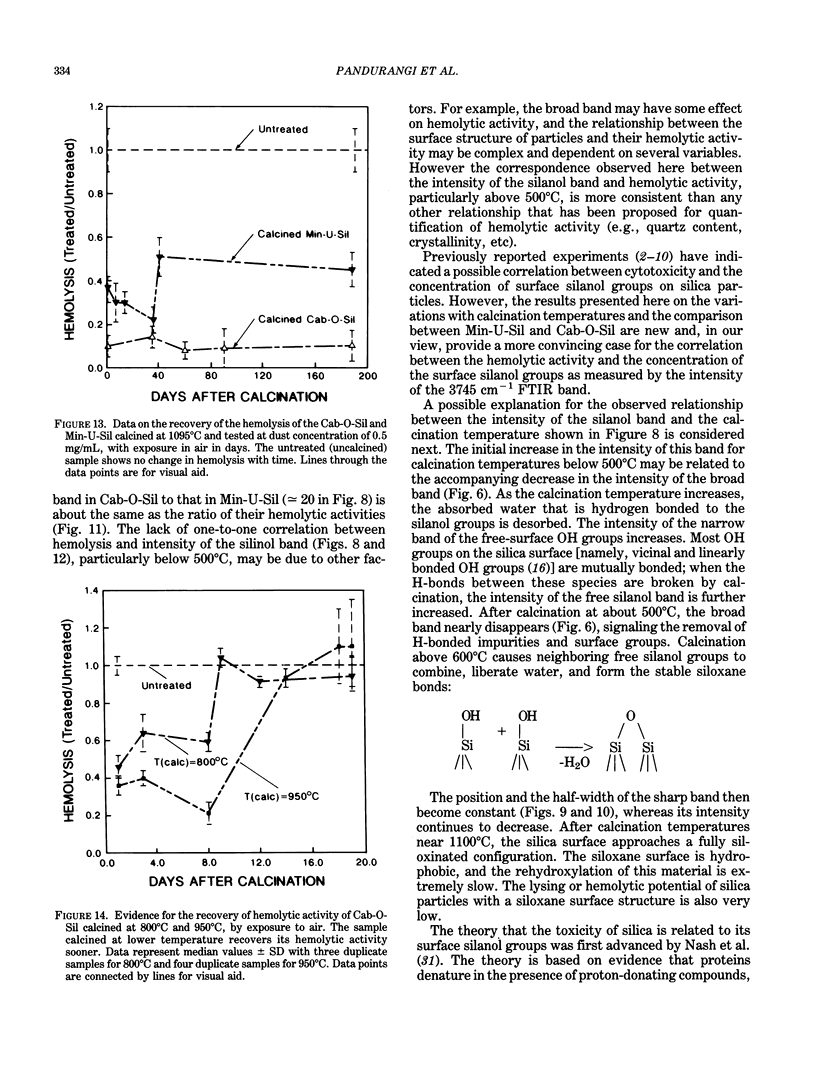
Surface IR (infrared) modes of crystalline and fumed (amorphous) silica particles, calcined at temperatures up to 1095 degrees C, have been studied by Fourier transform infrared spectroscopy. The ability of these same particles to lyse cells has been measured by a hemolysis protocol. The untreated crystalline and amorphous materials differ by a factor of 40 in specific surface area, and the intensity per unit mass of the sharp surface silanol band near 3745 cm-1 in the amorphous material is an order of magnitude larger than in the crystalline material. A similar difference is observed in the lysing potential of the two materials. The intensity of the silanol band increases after calcination for both materials, reaching peak values near 500 degrees C, followed by a dramatic drop at higher calcination temperatures, and reaching negligible values for materials calcined near 1100 degrees C. The lysing potential data follow essentially the same pattern for both crystalline and fumed silica. These results are consistent with the hypothesis that the surface silanol groups are involved in cell lysis. Further experiments are suggested to evaluate the relationship between the surface structure of silica particles and their potential cytotoxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HARLEY J. D., MARGOLIS J. Haemolytic activity of colloidal silica. Nature. 1961 Mar 25;189:1010–1011. doi: 10.1038/1891010a0. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Miller K., Macnab G. Hemolysis by asbestos. Environ Res. 1971 Apr;4(2):95–117. doi: 10.1016/0013-9351(71)90038-7. [DOI] [PubMed] [Google Scholar]
- Langer A. M. Crystal faces and cleavage planes in quartz as templates in biological processes. Q Rev Biophys. 1978 Nov;11(4):543–575. doi: 10.1017/s0033583500005667. [DOI] [PubMed] [Google Scholar]
- Light W. G., Wei E. T. Surface charge and asbestos toxicity. Nature. 1977 Feb 10;265(5594):537–539. doi: 10.1038/265537a0. [DOI] [PubMed] [Google Scholar]
- Nolan R. P., Langer A. M., Harington J. S., Oster G., Selikoff I. J. Quartz hemolysis as related to its surface functionalities. Environ Res. 1981 Dec;26(2):503–520. doi: 10.1016/0013-9351(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Stalder K., Stöber W. Haemolytic activity of suspensions of different silica modifications and inert dusts. Nature. 1965 Aug 21;207(999):874–875. doi: 10.1038/207874a0. [DOI] [PubMed] [Google Scholar]
- Summerton J., Hoenig S. The mechanism of hemolysis by silica and its bearing on silicosis. Exp Mol Pathol. 1977 Feb;26(1):113–128. doi: 10.1016/0014-4800(77)90071-5. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]