Abstract
Current treatment protocols for exposure to nerve and vesicant agents found in the U.S. stockpile of unitary chemical weapons are summarized, and the toxicities of available antidotes are evaluated. The status of the most promising of the new nerve agent antidotes is reviewed. In the U.S. atropine and pralidoxime compose the only approved antidote regimen for organophosphate nerve agent poisoning. Diazepam may also be used if necessary to control convulsions. To avoid death, administration must occur within minutes of substantial exposure together with immediate decontamination. Continuous observation and repeated administration of antidotes are necessary as symptoms warrant. Available antidotes do not necessarily prevent respiratory failure or incapacitation. The toxicity of the antidotes themselves and the individualized nature of medical care preclude recommending that autoinjectors be distributed to the general public. In addition, precautionary administration of protective drugs to the general population would not be feasible or desirable. No antidote exists for poisoning by the vesicant sulfur mustard (H, HD, HT); effective intervention can only be accomplished by rapid decontamination followed by palliative treatment of symptoms. British anti-Lewisite (BAL) (2,3-dimercapto-1-propanolol) is the antidote of choice for treatment of exposure to Lewisite, another potent vesicant. Experimental water-soluble BAL analogues have been developed that are less toxic than BAL. Treatment protocols for each antidote are summarized in tabular form for use by health care providers.
Full text
PDF
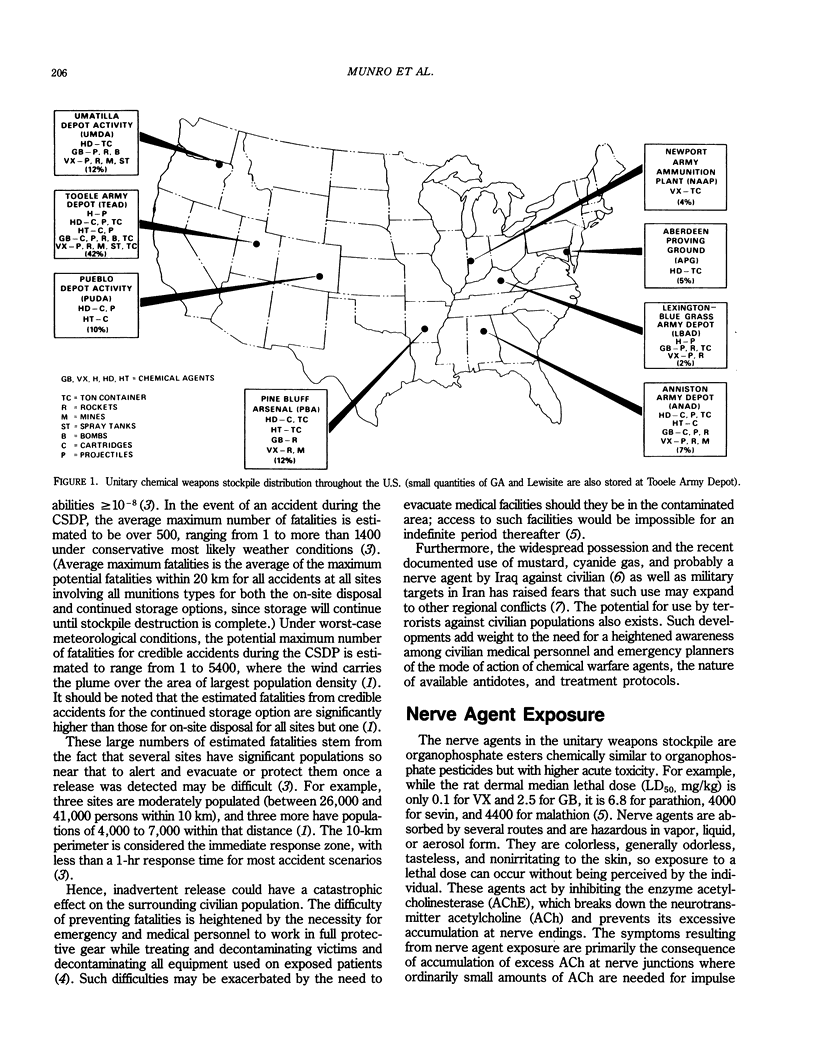



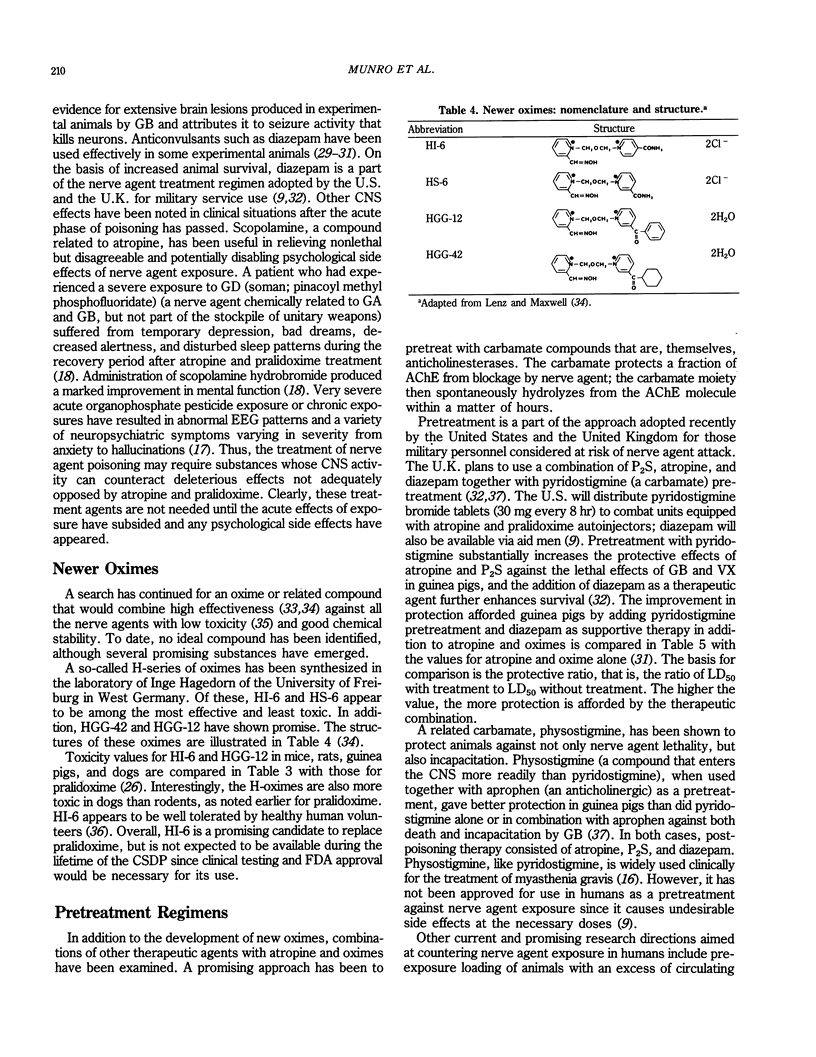
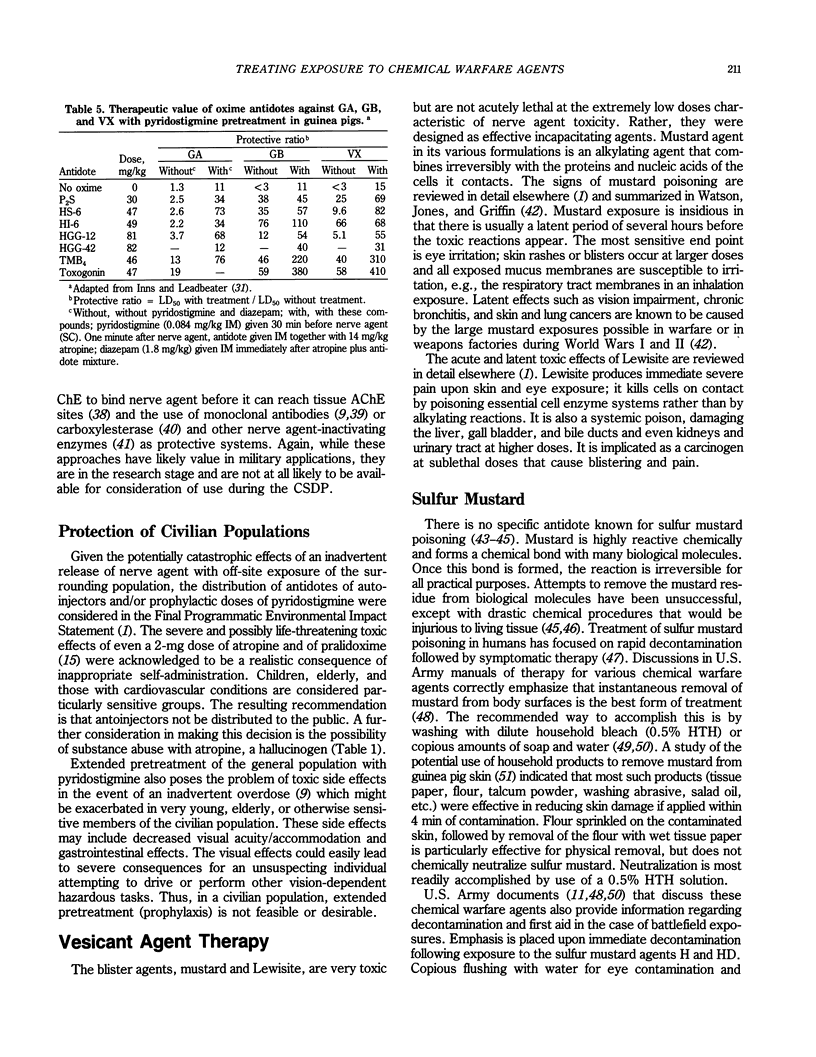




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosković B., Kovacević V., Jovanović D. PAM-2 Cl, HI-6, and HGG-12 in soman and tabun poisoning. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S106–S115. doi: 10.1093/toxsci/4.2part2.106. [DOI] [PubMed] [Google Scholar]
- Bosković B. The treatment of Soman poisoning and its perspectives. Fundam Appl Toxicol. 1981 Mar-Apr;1(2):203–213. doi: 10.1016/s0272-0590(81)80059-0. [DOI] [PubMed] [Google Scholar]
- Calesnick B., Christensen, Richter M. Human toxicity of various oximes. 2-Pyridine aldoxime methyl chloride, its methane sulfonate salt, and 1,1'-trimethylenebis-(4-formylpyridinium chloride). Arch Environ Health. 1967 Nov;15(5):599–608. doi: 10.1080/00039896.1967.10664975. [DOI] [PubMed] [Google Scholar]
- Carnes S. A., Watson A. P. Disposing of the US chemical weapons stockpile. An approaching reality. JAMA. 1989 Aug 4;262(5):653–659. [PubMed] [Google Scholar]
- Clarkson T. W., Magos L., Cox C., Greenwood M. R., Amin-Zaki L., Majeed M. A., Al-Damluji S. F. Tests of efficacy of antidotes for removal of methylmercury in human poisoning during the Iraq outbreak. J Pharmacol Exp Ther. 1981 Jul;218(1):74–83. [PubMed] [Google Scholar]
- Dahl H., Gluud B., Vangsted P., Norn M. Eye lesions induced by mustard gas. Acta Ophthalmol Suppl. 1985;173:30–31. doi: 10.1111/j.1755-3768.1985.tb06833.x. [DOI] [PubMed] [Google Scholar]
- Danielli J. F., Danielli M., Fraser J. B., Mitchell P. D., Owen L. N., Shaw G. BAL-INTRAV: a new non-toxic thiol for intravenous injection in arsenical poisoning: 1. Biological observations. 2. Chemical observations. Biochem J. 1947;41(3):325–333. [PMC free article] [PubMed] [Google Scholar]
- Dunn M. A., Sidell F. R. Progress in medical defense against nerve agents. JAMA. 1989 Aug 4;262(5):649–652. [PubMed] [Google Scholar]
- Friedheim E., Graziano J. H., Popovac D., Dragovic D., Kaul B. Treatment of lead poisoning by 2,3-dimercaptosuccinic acid. Lancet. 1978 Dec 9;2(8102):1234–1236. doi: 10.1016/s0140-6736(78)92103-7. [DOI] [PubMed] [Google Scholar]
- GROB D., HARVEY A. M. The effects and treatment of nerve gas poisoning. Am J Med. 1953 Jan;14(1):52–63. doi: 10.1016/0002-9343(53)90358-1. [DOI] [PubMed] [Google Scholar]
- Gall D. The use of therapeutic mixtures in the treatment of cholinesterase inhibition. Fundam Appl Toxicol. 1981 Mar-Apr;1(2):214–216. doi: 10.1016/s0272-0590(81)80060-7. [DOI] [PubMed] [Google Scholar]
- Heldman E., Ashani Y., Raveh L., Rachaman E. S. Sugar conjugates of pyridinium aldoximes as antidotes against organophosphate poisoning. Carbohydr Res. 1986 Aug 15;151:337–347. doi: 10.1016/s0008-6215(00)90353-7. [DOI] [PubMed] [Google Scholar]
- Hu H., Cook-Deegan R., Shukri A. The use of chemical weapons. Conducting an investigation using survey epidemiology. JAMA. 1989 Aug 4;262(5):640–643. [PubMed] [Google Scholar]
- Inns R. H., Leadbeater L. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J Pharm Pharmacol. 1983 Jul;35(7):427–433. doi: 10.1111/j.2042-7158.1983.tb04316.x. [DOI] [PubMed] [Google Scholar]
- KLIMOVA L. K. K farmakologii novogo antidota unitiola. Farmakol Toksikol. 1958 May-Jun;21(3):53–59. [PubMed] [Google Scholar]
- KROP S., KUNKEL A. M. Observations on pharmacology of the anticholinesterases sarin and tabun. Proc Soc Exp Biol Med. 1954 Jul;86(3):530–533. doi: 10.3181/00379727-86-21155. [DOI] [PubMed] [Google Scholar]
- Kiffer D., Minard P. Reactivation by imidazo-pyridinium oximes of acetylcholinesterase inhibited by organophosphates. A study with an immobilized enzyme method. Biochem Pharmacol. 1986 Aug 1;35(15):2527–2533. doi: 10.1016/0006-2952(86)90050-x. [DOI] [PubMed] [Google Scholar]
- Leadbeater L., Inns R. H., Rylands J. M. Treatment of poisoning by soman. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S225–S231. doi: 10.1016/0272-0590(85)90132-0. [DOI] [PubMed] [Google Scholar]
- Lenz D. E., Brimfield A. A., Hunter K. W., Jr, Benschop H. P., de Jong L. P., van Dijk C., Clow T. R. Studies using a monoclonal antibody against soman. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S156–S164. doi: 10.1016/0272-0590(84)90148-9. [DOI] [PubMed] [Google Scholar]
- Lenz K., Hruby K., Druml W., Eder A., Gaszner A., Kleinberger G., Pichler M., Weiser M. 2,3-Dimercaptosuccinic acid in human arsenic poisoning. Arch Toxicol. 1981 Jun;47(3):241–243. doi: 10.1007/BF00368684. [DOI] [PubMed] [Google Scholar]
- Little J. S., Broomfield C. A., Fox-Talbot M. K., Boucher L. J., MacIver B., Lenz D. E. Partial characterization of an enzyme that hydrolyzes sarin, soman, tabun, and diisopropyl phosphorofluoridate (DFP). Biochem Pharmacol. 1989 Jan 1;38(1):23–29. doi: 10.1016/0006-2952(89)90144-5. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Doebler J. A., Shih T. M., Anthony A. Protective effect of diazepam pretreatment on soman-induced brain lesion formation. Brain Res. 1985 Jan 28;325(1-2):287–289. doi: 10.1016/0006-8993(85)90324-5. [DOI] [PubMed] [Google Scholar]
- Maxwell D. M., Brecht K. M., O'Neill B. L. The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett. 1987 Nov;39(1):35–42. doi: 10.1016/0378-4274(87)90254-2. [DOI] [PubMed] [Google Scholar]
- McLeod C. G., Jr Pathology of nerve agents: perspectives on medical management. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S10–S16. doi: 10.1016/0272-0590(85)90110-1. [DOI] [PubMed] [Google Scholar]
- Meeter E., Wolthuis O. L. The effects of cholinesterase inhibitors on the body temperature of the rat. Eur J Pharmacol. 1968 Aug;4(1):18–24. doi: 10.1016/0014-2999(68)90004-6. [DOI] [PubMed] [Google Scholar]
- Orient J. M. Chemical and biological warfare. Should defenses be researched and deployed? JAMA. 1989 Aug 4;262(5):644–648. [PubMed] [Google Scholar]
- Sidell F. R., Groff W. A. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol Appl Pharmacol. 1974 Feb;27(2):241–252. doi: 10.1016/0041-008x(74)90195-1. [DOI] [PubMed] [Google Scholar]
- Sidell F. R. Soman and sarin: clinical manifestations and treatment of accidental poisoning by organophosphates. Clin Toxicol. 1974;7(1):1–17. doi: 10.3109/15563657408987971. [DOI] [PubMed] [Google Scholar]
- Slighter R. G., Montenaro M. J., Fabian R. J., Bhandari J. C., Donikian M. R., Drobeck H. P. Comparative nephrotoxicity of hydroxygentamicin and other aminoglycosides in rats. Fundam Appl Toxicol. 1984 Aug;4(4):558–567. doi: 10.1016/0272-0590(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Vojvodić V., Milosavljević Z., Bosković B., Bojanić N. The protective effect of different drugs in rats poisoned by sulfur and nitrogen mustards. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S160–S168. [PubMed] [Google Scholar]
- Watson A. P., Jones T. D., Griffin G. D. Sulfur mustard as a carcinogen: application of relative potency analysis to the chemical warfare agents H, HD, and HT. Regul Toxicol Pharmacol. 1989 Aug;10(1):1–25. doi: 10.1016/0273-2300(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Wolfe A. D., Rush R. S., Doctor B. P., Koplovitz I., Jones D. Acetylcholinesterase prophylaxis against organophosphate toxicity. Fundam Appl Toxicol. 1987 Aug;9(2):266–270. doi: 10.1016/0272-0590(87)90048-0. [DOI] [PubMed] [Google Scholar]


