Abstract
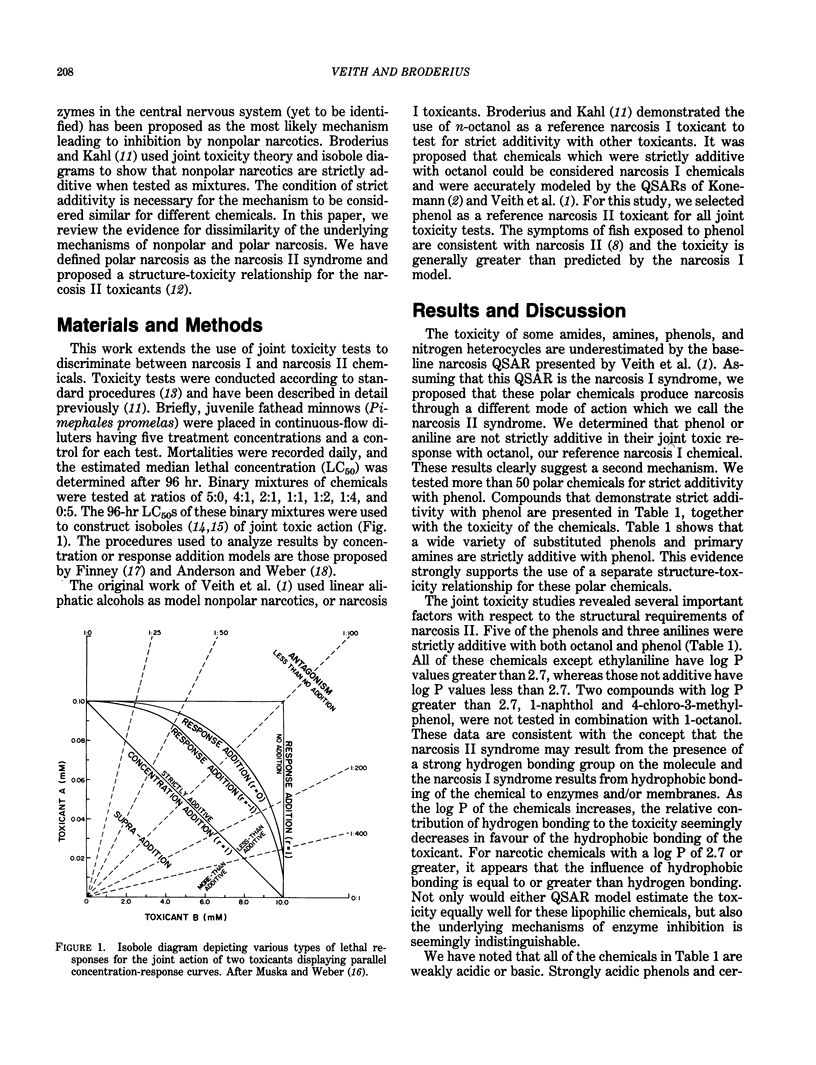
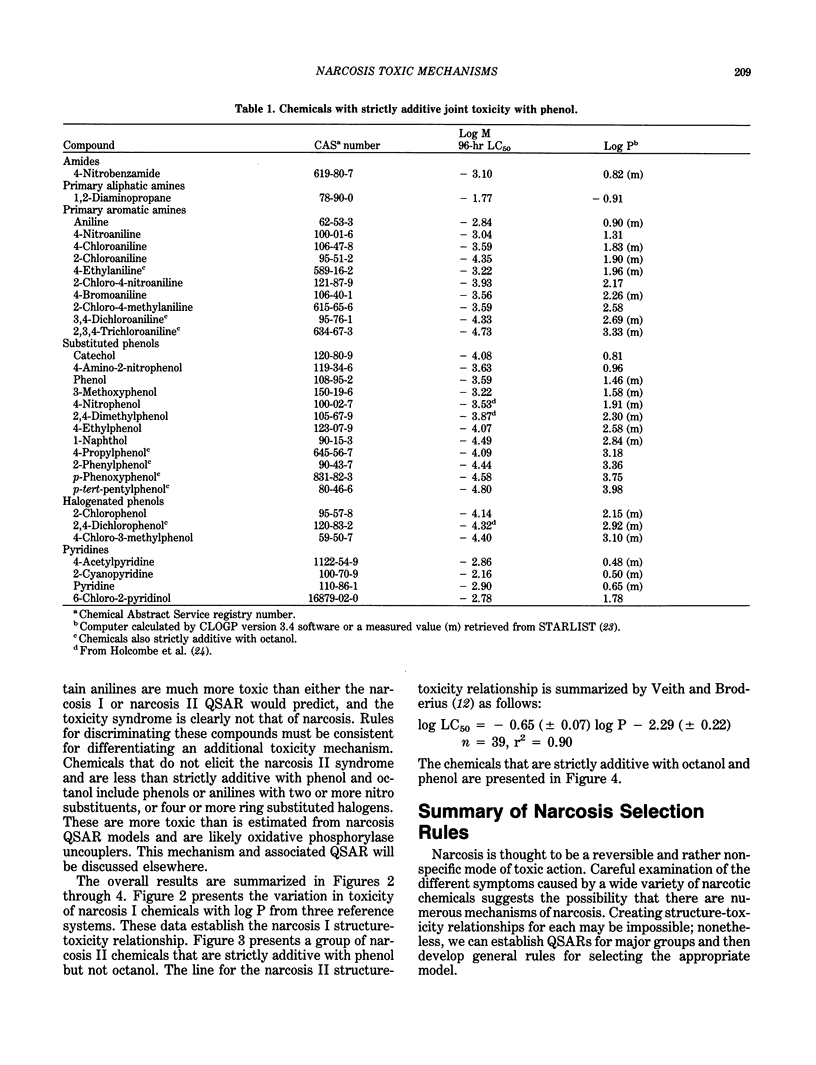
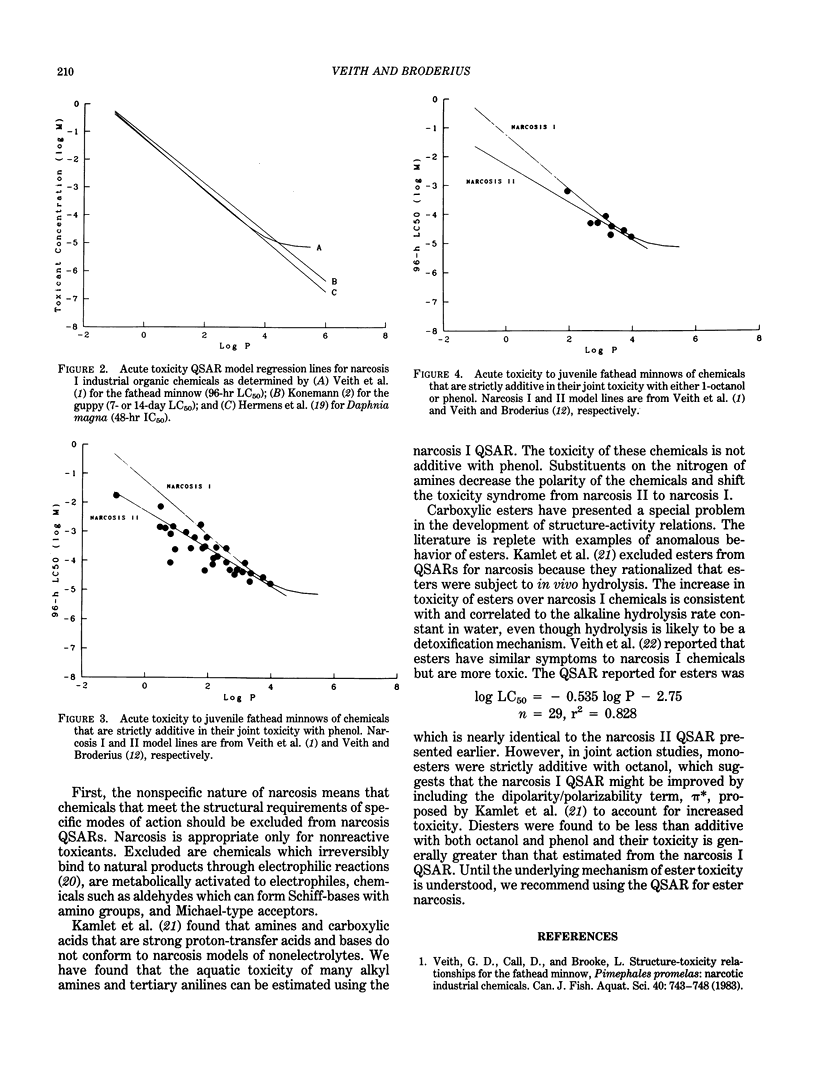
Narcosis is a nonspecific reversible state of arrested activity of protoplasmic structures caused by a wide variety of organic chemicals. The vast majority of industrial organic chemicals can be characterized by a baseline structure-toxicity relationship as developed for diverse aquatic organisms, using only the n-octanol/water partition coefficient as a descriptor. There are, however, many apparent narcotic chemicals that are more toxic than baseline narcosis predicts. Some of these chemicals have been distinguished as polar narcotics. Joint toxic theory and isobole diagrams were used to show that chemicals strictly additive with phenol were generally more toxic than predicted by narcosis I models and characterized by a different mode of action called narcosis II syndrome. This type of toxicity is exemplified by certain amides, amines, phenols, and nitrogen heterocycles. Evidence is provided that suggests that narcosis II syndrome may result from the presence of a strong hydrogen bonding group on the molecule, and narcosis I syndrome results from hydrophobic bonding of the chemical to enzymes and/or membranes. This shift in toxic action is apparently indistinguishable for narcotic chemicals with log P greater than about 2.7. General rules for selecting the appropriate models are proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mechanisms of general anesthesia. Environ Health Perspect. 1990 Jul;87:199–205. doi: 10.1289/ehp.9087199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens J. L. Electrophiles and acute toxicity to fish. Environ Health Perspect. 1990 Jul;87:219–225. doi: 10.1289/ehp.9087219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könemann H. Quantitative structure-activity relationships in fish toxicity studies. Part 1: relationship for 50 industrial pollutants. Toxicology. 1981;19(3):209–221. doi: 10.1016/0300-483x(81)90130-x. [DOI] [PubMed] [Google Scholar]
- LOEWE S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953 Jun;3(6):285–290. [PubMed] [Google Scholar]
- McKim J. M., Bradbury S. P., Niemi G. J. Fish acute toxicity syndromes and their use in the QSAR approach to hazard assessment. Environ Health Perspect. 1987 Apr;71:171–186. doi: 10.1289/ehp.8771171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith G. D., De Foe D., Knuth M. Structure-activity relationships for screening organic chemicals for potential ecotoxicity effects. Drug Metab Rev. 1984;15(7):1295–1303. doi: 10.3109/03602538409029961. [DOI] [PubMed] [Google Scholar]


