Abstract
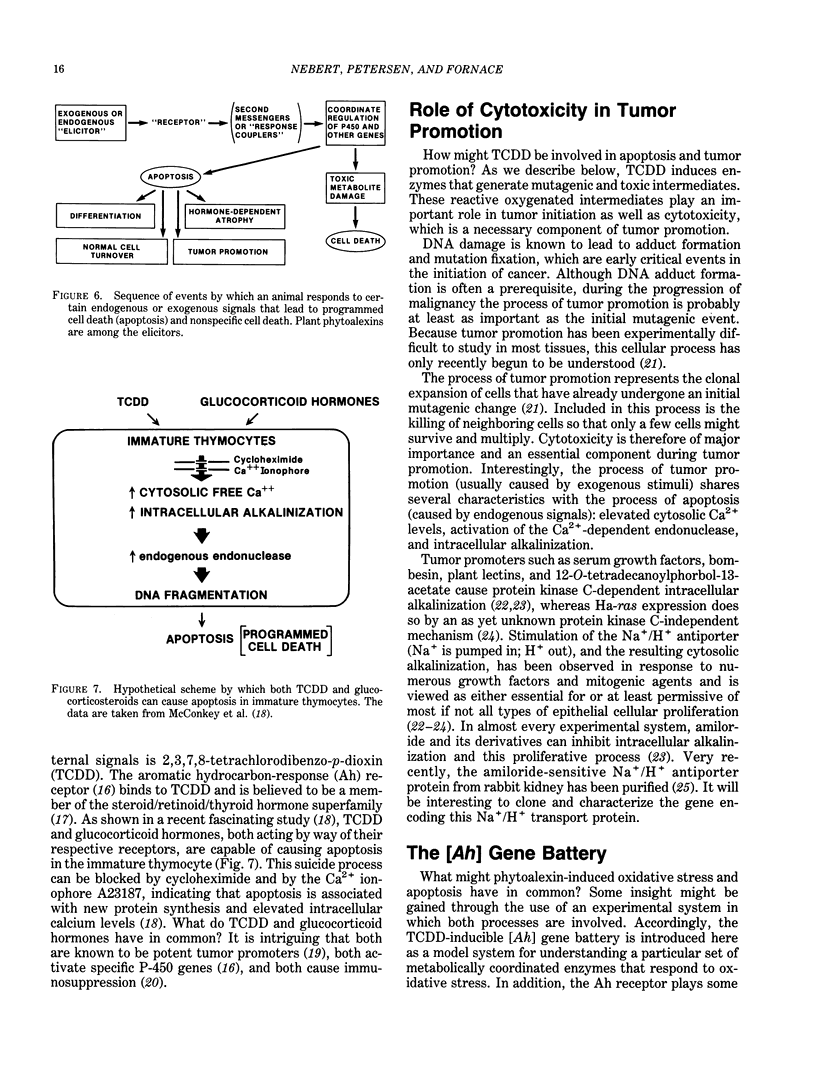
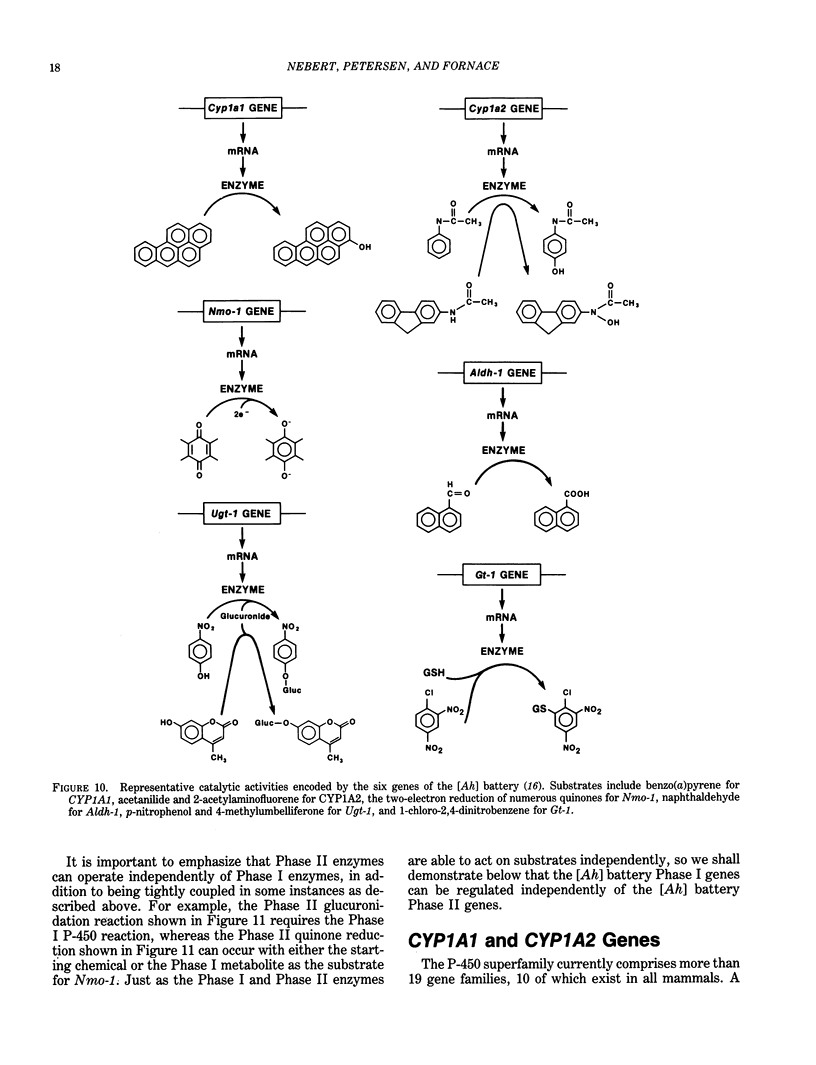
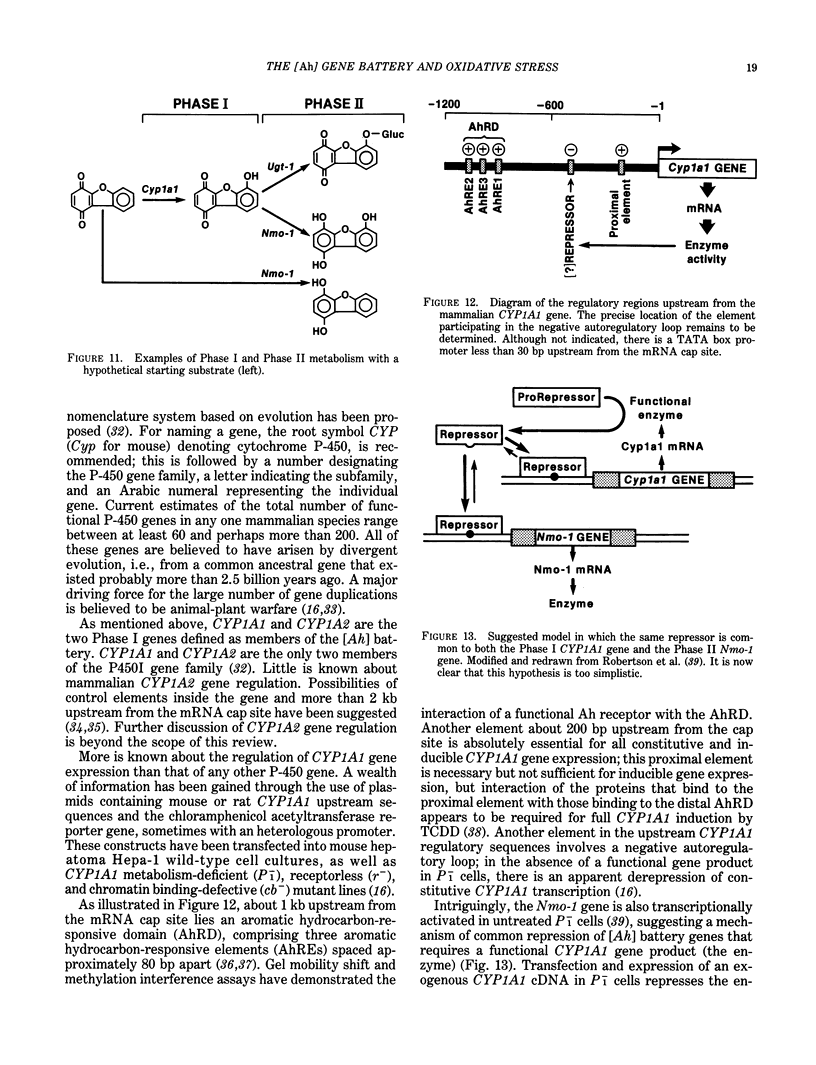
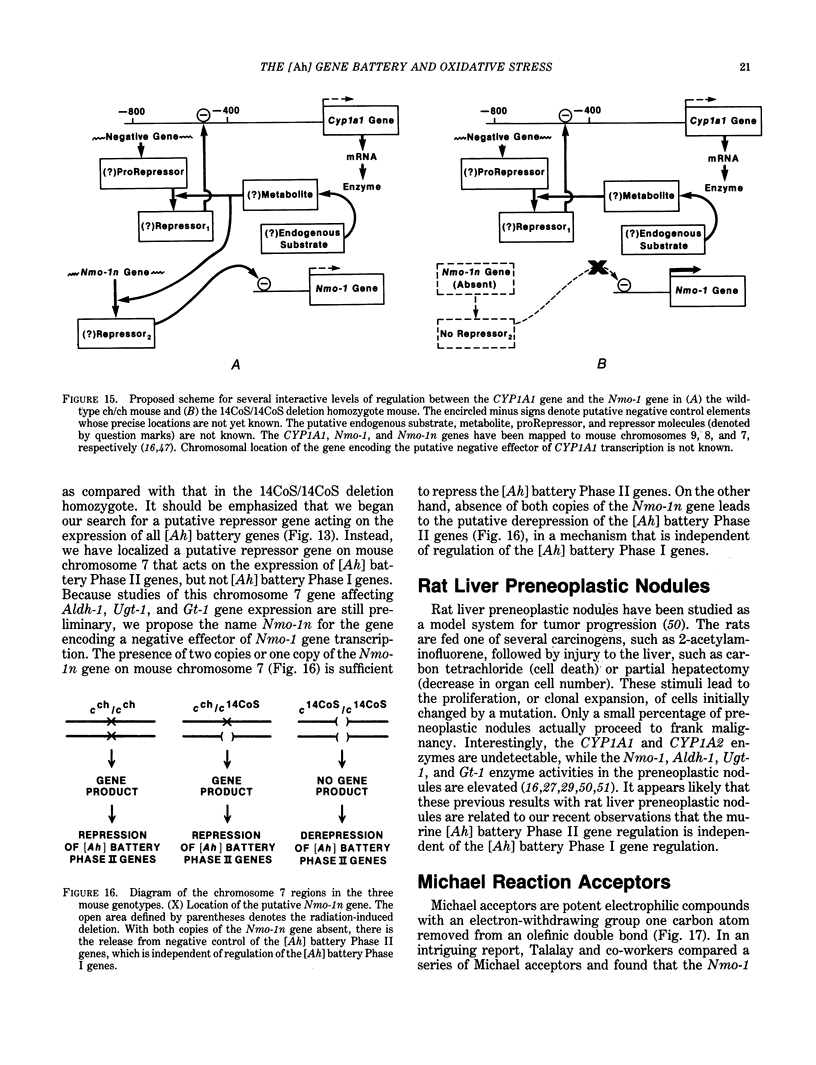
A major source of oxidative stress in animals is plant stress metabolites, also termed phytoalexins. The aromatic hydrocarbon-responsive [Ah] gene battery is considered here as a model system in which we can study metabolically coordinated enzymes that respond to phytoalexin-induced oxidative stress. In the mouse, the [Ah] battery comprises at least six genes: two Phase I genes, CYP1A1 and CYP1A2; and four Phase II genes, Nmo-1, Aldh-1, Ugt-1, and Gt-1. All six genes appear to be regulated positively by inducers such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other ligands of the Ah receptor. In the absence of foreign inducer, the control of Nmo-1 gene expression is independent of the control of CYP1A1 and CYP1A2 gene expression. The radiation deletion homozygote c14CoS/c14CoS mouse is lacking about 1.1 centiMorgans of chromosome 7. Although having no detectable CYP1A1 or CYP1A2 activation, the untreated c14CoS/c14CoS mouse exhibits markedly elevated transcripts of the Nmo-1 gene and three growth arrest- and DNA damage-inducible (gadd) genes. These data suggest that the missing region on chromosome 7 in the c14CoS/c14CoS mouse contains a gene(s), which we propose to call Nmo-1n, encoding a trans-acting factor(s) that is a negative effector of the Nmo-1 and gadd genes. The three other [Ah] battery Phase II genes behave similarly to Nmo-1 in the c14CoS/c14CoS mouse. This coordinated response to oxidative stress and DNA damage, by way of the release of a mammalian battery of genes from negative control, bears an interesting resemblance to the SOS response in bacteria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988 Mar 11;52(5):685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen. 1989;14 (Suppl 16):66–77. doi: 10.1002/em.2850140614. [DOI] [PubMed] [Google Scholar]
- Berkley S. F., Hightower A. W., Beier R. C., Fleming D. W., Brokopp C. D., Ivie G. W., Broome C. V. Dermatitis in grocery workers associated with high natural concentrations of furanocoumarins in celery. Ann Intern Med. 1986 Sep;105(3):351–355. doi: 10.7326/0003-4819-105-3-351. [DOI] [PubMed] [Google Scholar]
- Buchmann A., Kuhlmann W., Schwarz M., Kunz W., Wolf C. R., Moll E., Friedberg T., Oesch F. Regulation and expression of four cytochrome P-450 isoenzymes, NADPH-cytochrome P-450 reductase, the glutathione transferases B and C and microsomal epoxide hydrolase in preneoplastic and neoplastic lesions in rat liver. Carcinogenesis. 1985 Apr;6(4):513–521. doi: 10.1093/carcin/6.4.513. [DOI] [PubMed] [Google Scholar]
- Daniel V., Sharon R., Bensimon A. Regulatory elements controlling the basal and drug-inducible expression of glutathione S-transferase Ya subunit gene. DNA. 1989 Jul-Aug;8(6):399–408. doi: 10.1089/dna.1.1989.8.399. [DOI] [PubMed] [Google Scholar]
- DeFranco D., Morris S. M., Jr, Leonard C. M., Gluecksohn-Waelsch S. Metallothionein mRNA expression in mice homozygous for chromosomal deletions around the albino locus. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1161–1164. doi: 10.1073/pnas.85.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatmaitan Z., Lewis S., Turchin H., Arias I. M. Premature development of ligandin (GSH transferase B) in mice with an inherited defect in endoplasmic reticulum-Golgi structure and function. Biochem Biophys Res Commun. 1977 Mar 21;75(2):337–341. doi: 10.1016/0006-291x(77)91047-6. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Huot S. J., Cassel D., Igarashi P., Cragoe E. J., Jr, Slayman C. W., Aronson P. S. Identification and purification of a renal amiloride-binding protein with properties of the Na+-H+ exchanger. J Biol Chem. 1989 Jan 15;264(2):683–686. [PubMed] [Google Scholar]
- Ikeya K., Jaiswal A. K., Owens R. A., Jones J. E., Nebert D. W., Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989 Sep;3(9):1399–1408. doi: 10.1210/mend-3-9-1399. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Haniu M., Sogawa K., Fujii-Kuriyama Y., Watanabe S., Shively J. E., Anan K. F. Cloning and characterization of cDNA encoding 3-methylcholanthrene inducible rat mRNA for UDP-glucuronosyltransferase. J Biol Chem. 1986 Nov 25;261(33):15607–15614. [PubMed] [Google Scholar]
- Jones D. E., Jr, Brennan M. D., Hempel J., Lindahl R. Cloning and complete nucleotide sequence of a full-length cDNA encoding a catalytically functional tumor-associated aldehyde dehydrogenase. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1782–1786. doi: 10.1073/pnas.85.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. J., Boland M. P., Friedlos F., Coles B., Southan C., Roberts J. J. The nitroreductase enzyme in Walker cells that activates 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) to 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide is a form of NAD(P)H dehydrogenase (quinone) (EC 1.6.99.2). Biochem Pharmacol. 1988 Dec 15;37(24):4671–4677. doi: 10.1016/0006-2952(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Caskey C. T., Hood L. DNA diagnostics--molecular techniques and automation. Science. 1988 Oct 14;242(4876):229–237. doi: 10.1126/science.3051381. [DOI] [PubMed] [Google Scholar]
- Lavin M. F., Schroeder A. L. Damage-resistant DNA synthesis in eukaryotes. Mutat Res. 1988 May;193(3):193–206. doi: 10.1016/0167-8817(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Shaw P. A., Krauter K. S., Robinson C., Englard S., Hanson R. W., Gluecksohn-Waelsch S. Trans regulation of the phosphoenolpyruvate carboxykinase (GTP) gene, identified by deletions in chromosome 7 of the mouse. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5184–5188. doi: 10.1073/pnas.83.14.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster M. I., Germolec D. R., Clark G., Wiegand G., Rosenthal G. J. Selective effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and corticosteroid on in vitro lymphocyte maturation. J Immunol. 1988 Feb 1;140(3):928–935. [PubMed] [Google Scholar]
- Maly K., Uberall F., Loferer H., Doppler W., Oberhuber H., Groner B., Grunicke H. H. Ha-ras activates the Na+/H+ antiporter by a protein kinase C-independent mechanism. J Biol Chem. 1989 Jul 15;264(20):11839–11842. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Hargreaves A. D., Duvall E., Wyllie A. H. Hormone-induced cell death. 2. Surface changes in thymocytes undergoing apoptosis. Am J Pathol. 1984 Jun;115(3):426–436. [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jones J. E. Regulation of the mammalian cytochrome P1-450 (CYP1A1) gene. Int J Biochem. 1989;21(3):243–252. doi: 10.1016/0020-711x(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Petersen D. D., Gonzalez F. J., Rapic V., Kozak C. A., Lee J. Y., Jones J. E., Nebert D. W. Marked increases in hepatic NAD(P)H:oxidoreductase gene transcription and mRNA levels correlated with a mouse chromosome 7 deletion. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6699–6703. doi: 10.1073/pnas.86.17.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Quattrochi L. C., Tukey R. H. The human cytochrome Cyp1A2 gene contains regulatory elements responsive to 3-methylcholanthrene. Mol Pharmacol. 1989 Jul;36(1):66–71. [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S., Raymer G. D. Analysis of the albino-locus region of the mouse: IV. Characterization of 34 deficiencies. Genetics. 1982 Mar;100(3):427–453. doi: 10.1093/genetics/100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Müller G., Schütz G., Gluecksohn-Waelsch S. Deletions near the albino locus on chromosome 7 of the mouse affect the level of tyrosine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2866–2869. doi: 10.1073/pnas.82.9.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia L. A., Storz G., Ames B. N. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol. 1989 Dec 20;210(4):709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- Thaler M. M., Erickson R. P., Pelger A. Genetically determined abnormalities of microsomal enzymes in liver of mutant newborn mice. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1244–1250. doi: 10.1016/s0006-291x(76)80148-9. [DOI] [PubMed] [Google Scholar]
- Thor H., Mirabelli F., Salis A., Cohen G. M., Bellomo G., Orrenius S. Alterations in hepatocyte cytoskeleton caused by redox cycling and alkylating quinones. Arch Biochem Biophys. 1988 Nov 1;266(2):397–407. doi: 10.1016/0003-9861(88)90271-8. [DOI] [PubMed] [Google Scholar]
- Wagner G., Pott U., Bruckschen M., Sies H. Effects of 5-azacytidine and methyl-group deficiency on NAD(P)H: quinone oxidoreductase and glutathione S-transferase in liver. Biochem J. 1988 May 1;251(3):825–829. doi: 10.1042/bj2510825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr The regulation of gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Pharmacol Rev. 1987 Jun;39(2):147–161. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Poirier M. C. Chemical carcinogenesis: from animal models to molecular models in one decade. Adv Cancer Res. 1988;50:25–70. doi: 10.1016/s0065-230x(08)60434-0. [DOI] [PubMed] [Google Scholar]


