Abstract
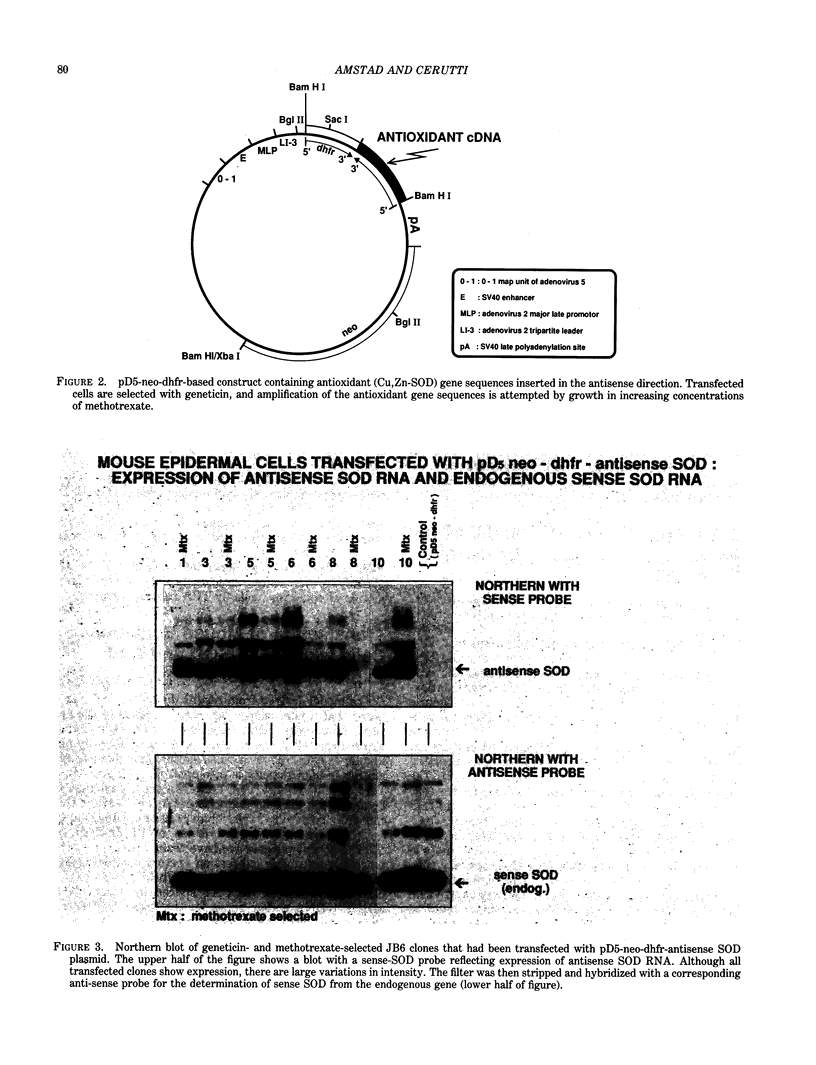
Oxidants are ubiquitous in our aerobic environment. While they are always toxic, they can also exert pathophysiological effects at low concentrations and play an etiological role in human disease. For example, oxidants can stimulate cell growth and act as tumor promoters. The cellular antioxidant defense system attenuates the effect of oxidants and consists of low molecular weight components and several enzymes. Most important are catalase (CAT), superoxide dismutases (SOD), and glutathione peroxidase. We are attempting to elucidate the role of CAT and Cu,Zn-SOD in oxidant tumor promotion of mouse epidermal cells JB6. We have found that the promotable clone 41 possesses 2- to 3-fold higher levels of activity, protein, and stationary mRNA of CAT and Cu,Zn-SOD than does the nonpromotable clone 30. We propose that the growth-stimulatory effect of oxidants is more pronounced in promotable clone 41 because it is better protected from oxidant toxicity. In order to corroborate this model, we have constructed JB6 cells with higher levels of Cu,Zn-SOD and CAT by transfection with expression vectors containing cDNA for these genes. On the other hand, cells with decreased amounts of Cu,Zn-SOD have been obtained by their stable transfection with a vector containing SOD-cDNA in the antisense orientation. These cell clones with modified antioxidant enzyme complements are being characterized. In particular, their promotability by oxidants and their sensitivity to killing and oxidative macromolecular damage are being measured. Certain tumor promoters that lack oxidizing properties may generate a cellular prooxidant state by a variety of mechanisms.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF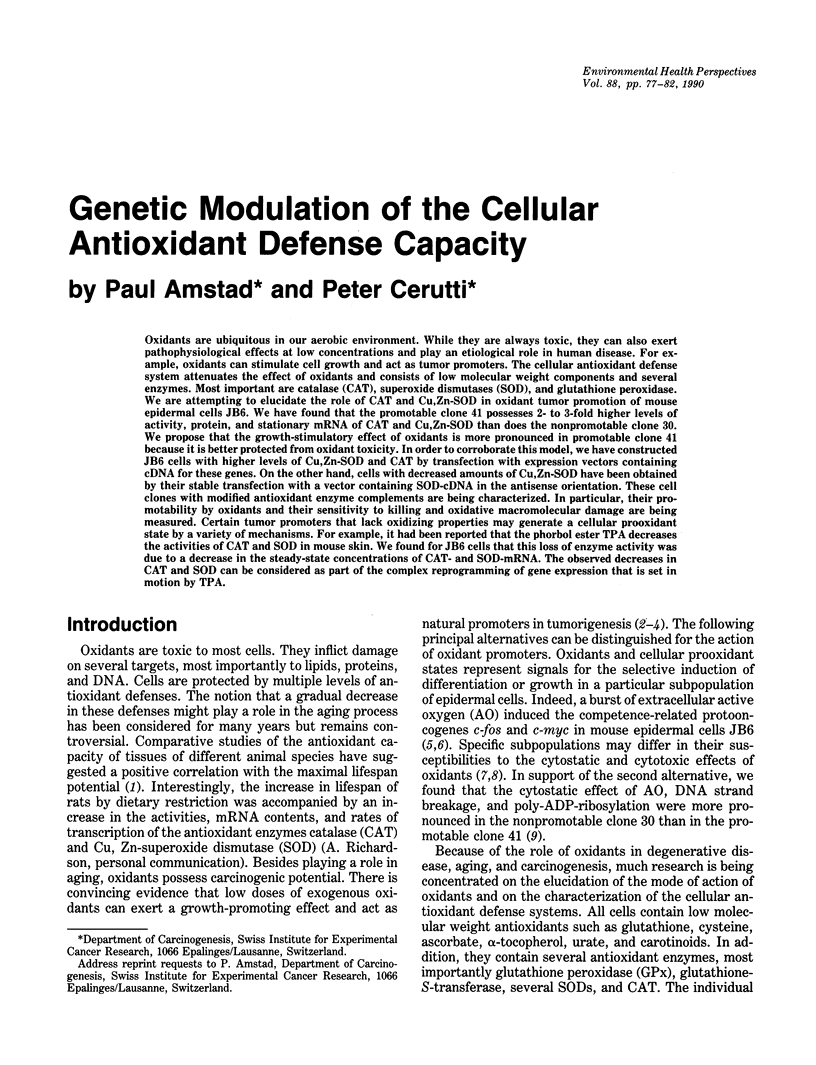




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Morrison D. P., Joyce T. L. Measurement of enzyme activities in mouse epidermis following phorbol ester treatment: a potential artifact. Carcinogenesis. 1986 Mar;7(3):495–497. doi: 10.1093/carcin/7.3.495. [DOI] [PubMed] [Google Scholar]
- Ceballos I., Delabar J. M., Nicole A., Lynch R. E., Hallewell R. A., Kamoun P., Sinet P. M. Expression of transfected human CuZn superoxide dismutase gene in mouse L cells and NS20Y neuroblastoma cells induces enhancement of glutathione peroxidase activity. Biochim Biophys Acta. 1988 Jan 25;949(1):58–64. doi: 10.1016/0167-4781(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Response modification creates promotability in multistage carcinogenesis. Carcinogenesis. 1988 Apr;9(4):519–526. doi: 10.1093/carcin/9.4.519. [DOI] [PubMed] [Google Scholar]
- Crawford D. R., Amstad P. A., Foo D. D., Cerutti P. A. Constitutive and phorbol-myristate-acetate regulated antioxidant defense of mouse epidermal JB6 cells. Mol Carcinog. 1989;2(3):136–143. doi: 10.1002/mc.2940020306. [DOI] [PubMed] [Google Scholar]
- Crawford D. R., Mirault M. E., Moret R., Zbinden I., Cerutti P. A. Molecular defect in human acatalasia fibroblasts. Biochem Biophys Res Commun. 1988 May 31;153(1):59–66. doi: 10.1016/s0006-291x(88)81189-6. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase--implication for gene dosage effects in Down syndrome. Cell. 1988 Jan 29;52(2):259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Fischer S. M., Baldwin J. K., Adams L. M. Effects of anti-promoters and strain of mouse on tumor promoter-induced oxidants in murine epidermal cells. Carcinogenesis. 1986 Jun;7(6):915–918. doi: 10.1093/carcin/7.6.915. [DOI] [PubMed] [Google Scholar]
- Gindhart T. D., Nakamura Y., Stevens L. A., Hegameyer G. A., West M. W., Smith B. M., Colburn N. H. Genes and signal transduction in tumor promotion: conclusions from studies with promoter resistant variants of JB-6 mouse epidermal cells. Carcinog Compr Surv. 1985;8:341–367. [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Zwelling L. A., Yuspa S. H. Association of DNA strand breaks with accelerated terminal differentiation in mouse epidermal cells exposed to tumor promoters. Cancer Res. 1985 Oct;45(10):4864–4870. [PubMed] [Google Scholar]
- Heckl K. Isolation of cDNAs encoding human manganese superoxide dismutase. Nucleic Acids Res. 1988 Jul 11;16(13):6224–6224. doi: 10.1093/nar/16.13.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Colburn N. H., Gindhart T. D. Role of reactive oxygen in tumor promotion: implication of superoxide anion in promotion of neoplastic transformation in JB-6 cells by TPA. Carcinogenesis. 1985 Feb;6(2):229–235. doi: 10.1093/carcin/6.2.229. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Gindhart T. D., Winterstein D., Tomita I., Seed J. L., Colburn N. H. Early superoxide dismutase-sensitive event promotes neoplastic transformation in mouse epidermal JB6 cells. Carcinogenesis. 1988 Feb;9(2):203–207. doi: 10.1093/carcin/9.2.203. [DOI] [PubMed] [Google Scholar]
- Perchellet J. P., Perchellet E. M., Orten D. K., Schneider B. A. Decreased ratio of reduced/oxidized glutathione in mouse epidermal cells treated with tumor promoters. Carcinogenesis. 1986 Mar;7(3):503–506. doi: 10.1093/carcin/7.3.503. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Solanki V., Rana R. S., Slaga T. J. Diminution of mouse epidermal superoxide dismutase and catalase activities by tumor promoters. Carcinogenesis. 1981;2(11):1141–1146. doi: 10.1093/carcin/2.11.1141. [DOI] [PubMed] [Google Scholar]
- Srinivas L., Colburn N. H. Preferential oxidation of cell surface sialic acid by periodate leads to promotion of transformation in JB6 cells. Carcinogenesis. 1984 Apr;5(4):515–519. doi: 10.1093/carcin/5.4.515. [DOI] [PubMed] [Google Scholar]




