Abstract
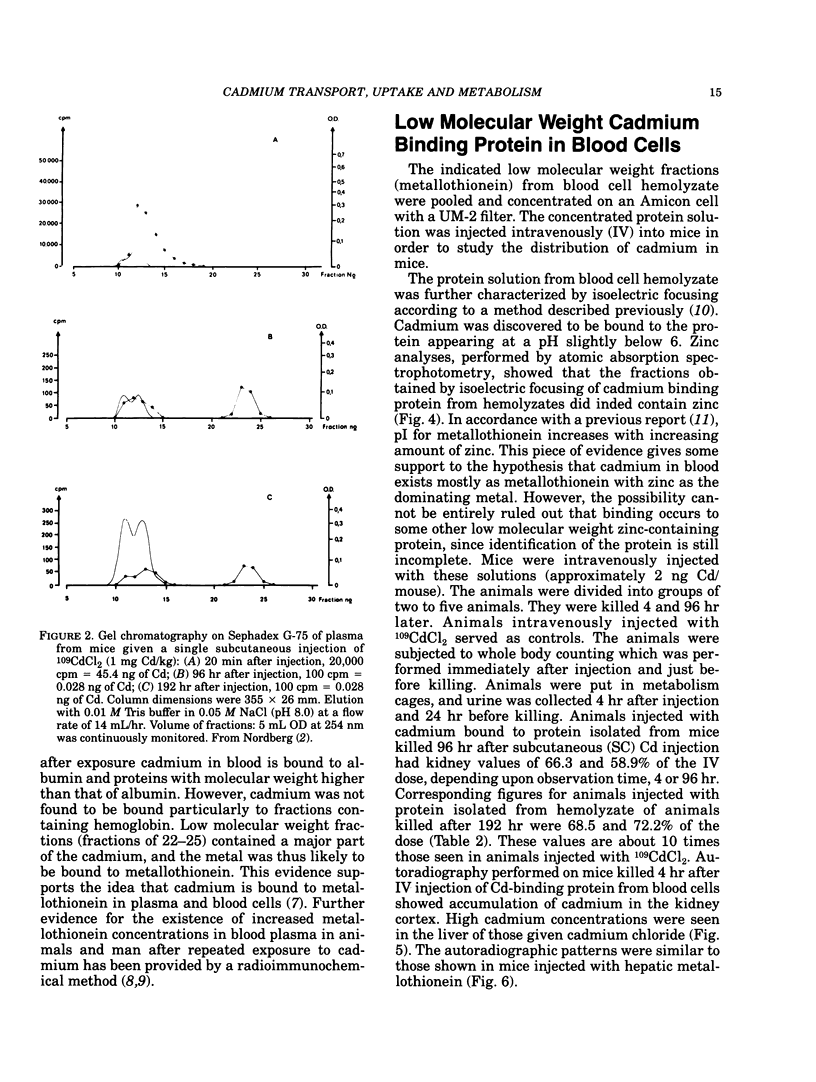
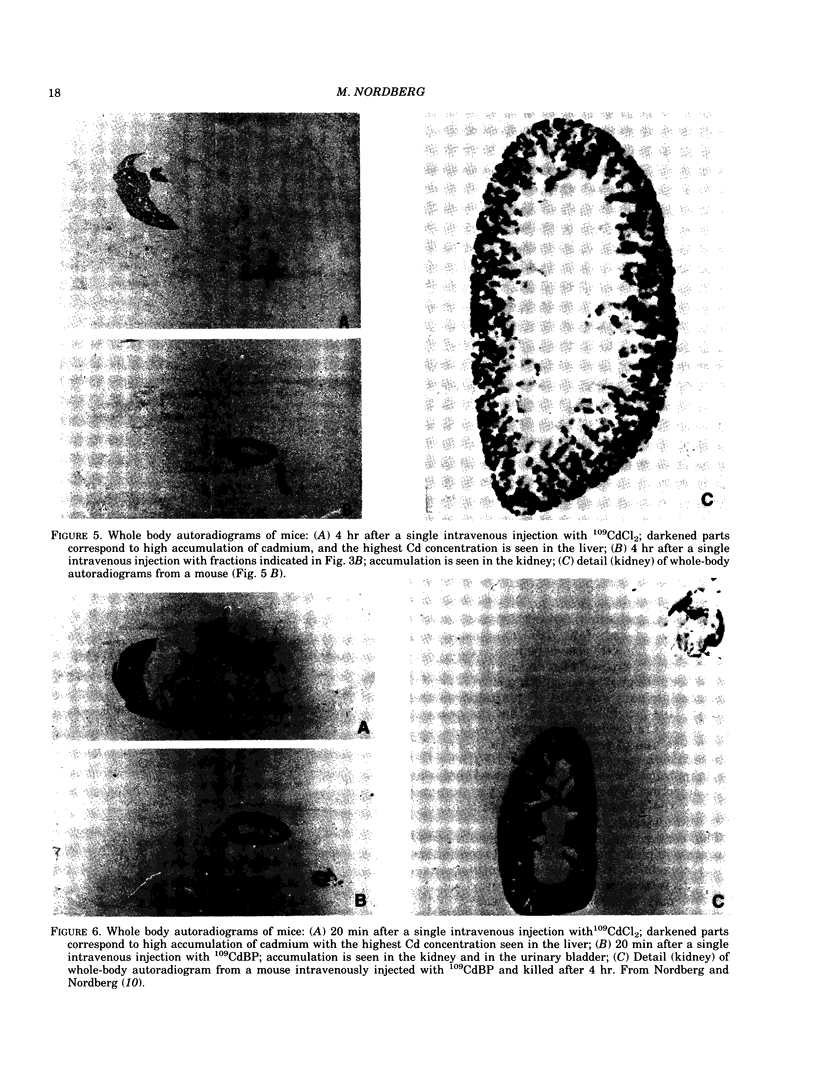
Cadmium taken up from lung and gastrointestinal tract is transported via blood to liver and kidney. On long-term exposure to cadmium, renal tubular dysfunction develops in humans and experimental animals. Data from animal experiments demonstrate that initially after exposure cadmium in blood is bound to albumin and proteins with higher molecular weight. Such cadmium is mainly taken up in liver. For a few days after exposure cadmium exists as metallothionein in plasma and blood cells. After both single and long-term administration of cadmium bound to metallothionein, cadmium is taken up by the kidney. The concentration of metallothionein-bound cadmium in plasma is quite low due to continuous renal clearance. Cadmium from metallothionein is taken up in renal tubules by pinocytosis and subsequently degraded in lysosomes, thereby releasing cadmium which stimulates de novo synthesis of metallothionein but also binds to reabsorbed metallothionein. Catabolizing and rebinding are continuous and prevent excretion of cadmium. Because of differences in transport, renal metabolic handling forms of cadmium are also different for different forms of cadmium administered and rate of administration. A single dose of metallothionein-bound cadmium given intravenously is almost immediately and completely taken up in the renal tubule. Under such conditions, resynthesis and rebinding processes are insufficient to sequester cadmium from sensitive tissue receptors, and renal damage occurs at total tissue concentrations much lower than when renal cadmium concentrations rise slowly. This explains the wide range (10-200 micrograms Cd/g wet weight) of cadmium in the renal cortex that associated with renal tubular dysfunction in experimental animals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogden J. D., Kemp F. W., Troiano R. A., Jortner B. S., Timpone C., Giuliani D. Effect of mercuric chloride and methylmercury chloride exposure on tissue concentrations of six essential minerals. Environ Res. 1980 Apr;21(2):350–359. doi: 10.1016/0013-9351(80)90037-7. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Vander Mallie R. J., Garvey J. S. A radioimmunoassay for human metallothionein. Toxicol Appl Pharmacol. 1980 Aug;55(1):94–102. doi: 10.1016/0041-008x(80)90224-0. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Goyer R. A., Delaquerriere-Richardson L. Cadmium-metallothionein-induced nephropathy. Toxicol Appl Pharmacol. 1976 Nov;38(2):399–408. doi: 10.1016/0041-008x(76)90146-0. [DOI] [PubMed] [Google Scholar]
- Elinder C. G., Lundgren G., Nordberg M., Palm B., Piscator M. Metallothionein in rabbit kidneys preserved for transplantation. Environ Health Perspect. 1984 Mar;54:275–280. doi: 10.1289/ehp.8454275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler B. A., Nordberg G. F. The renal toxicity of cadmium metallothionein: morphometric and X-ray microanalytical studies. Toxicol Appl Pharmacol. 1978 Dec;46(3):609–623. doi: 10.1016/0041-008x(78)90307-1. [DOI] [PubMed] [Google Scholar]
- Garty M., Wong K. L., Klaassen C. D. Redistribution of cadmium to blood of rats. Toxicol Appl Pharmacol. 1981 Jul;59(3):548–554. doi: 10.1016/0041-008x(81)90309-4. [DOI] [PubMed] [Google Scholar]
- Nordberg G. F., Garvey J. S., Chang C. C. Metallothionein in plasma and urine of cadmium workers. Environ Res. 1982 Jun;28(1):179–182. doi: 10.1016/0013-9351(82)90167-0. [DOI] [PubMed] [Google Scholar]
- Nordberg G. F., Goyer R., Nordberg M. Comparative toxicity of cadmium-metallothionein and cadmium chloride on mouse kidney. Arch Pathol. 1975 Apr;99(4):192–197. [PubMed] [Google Scholar]
- Nordberg G. F., Nordberg M., Piscator M., Vesterberg O. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972 Feb;126(3):491–498. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg M., Nordberg G. F. Distribution of metallothionein-bound cadmium and cadmium chloride in mice: preliminary studies. Environ Health Perspect. 1975 Dec;12:103–108. doi: 10.1289/ehp.7512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg M., Nordberg G. F., Piscator M. Isolation and characterization of a hepatic metallothionein from mice. Environ Physiol Biochem. 1975;5(6):396–403. [PubMed] [Google Scholar]
- Nordberg M. Studies on metallothionein and cadmium. Environ Res. 1978 Jun;15(3):381–404. doi: 10.1016/0013-9351(78)90120-2. [DOI] [PubMed] [Google Scholar]
- PISCATOR M. OM KADMIUM I NORMALA MAENNISKONJURAR SAMT REDOGOERELSE FOER ISOLERING AV METALLOTHIONEIN UR LEVER FRAN KADMIUMEXPONERADE KANINER. Nord Hyg Tidskr. 1964;45:76–82. [PubMed] [Google Scholar]
- Ridlington J. W., Winge D. R., Fowler B. A. Long-term turnover of cadmium metallothionein in liver and kidney following a single low dose of cadmium in rats. Biochim Biophys Acta. 1981 Mar 5;673(2):177–183. [PubMed] [Google Scholar]
- Squibb K. S., Ridlington J. W., Carmichael N. G., Fowler B. A. Early cellular effects of circulating cadmium-thionein on kidney proximal tubules. Environ Health Perspect. 1979 Feb;28:287–296. doi: 10.1289/ehp.7928287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Cadmium, copper, and zinc distribution in blood of rats after long-term cadmium administration. J Toxicol Environ Health. 1981 Feb;7(2):251–262. doi: 10.1080/15287398109529976. [DOI] [PubMed] [Google Scholar]