Abstract
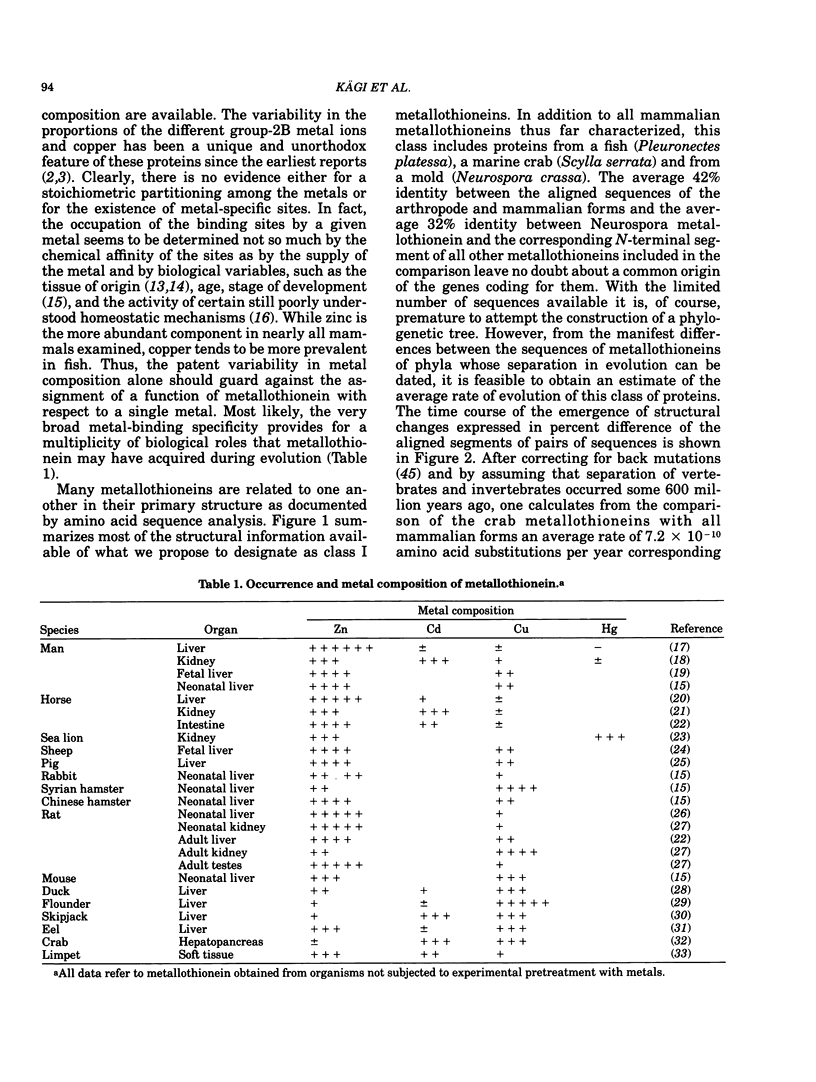
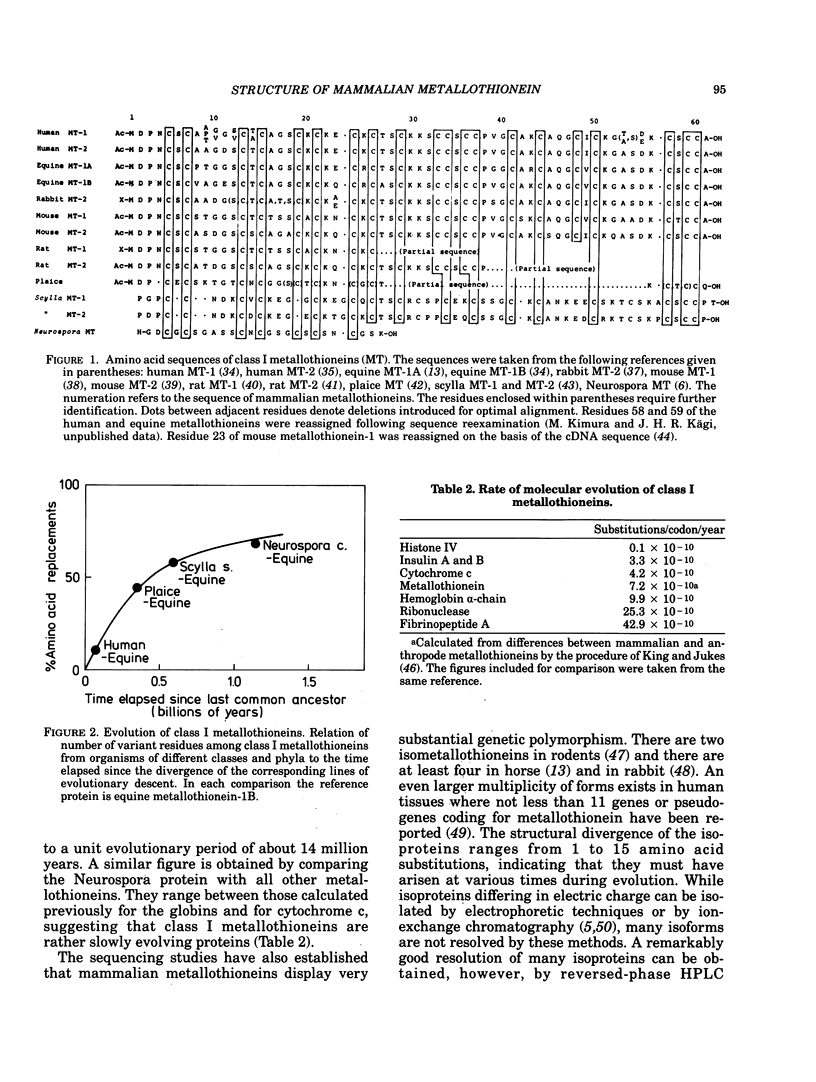
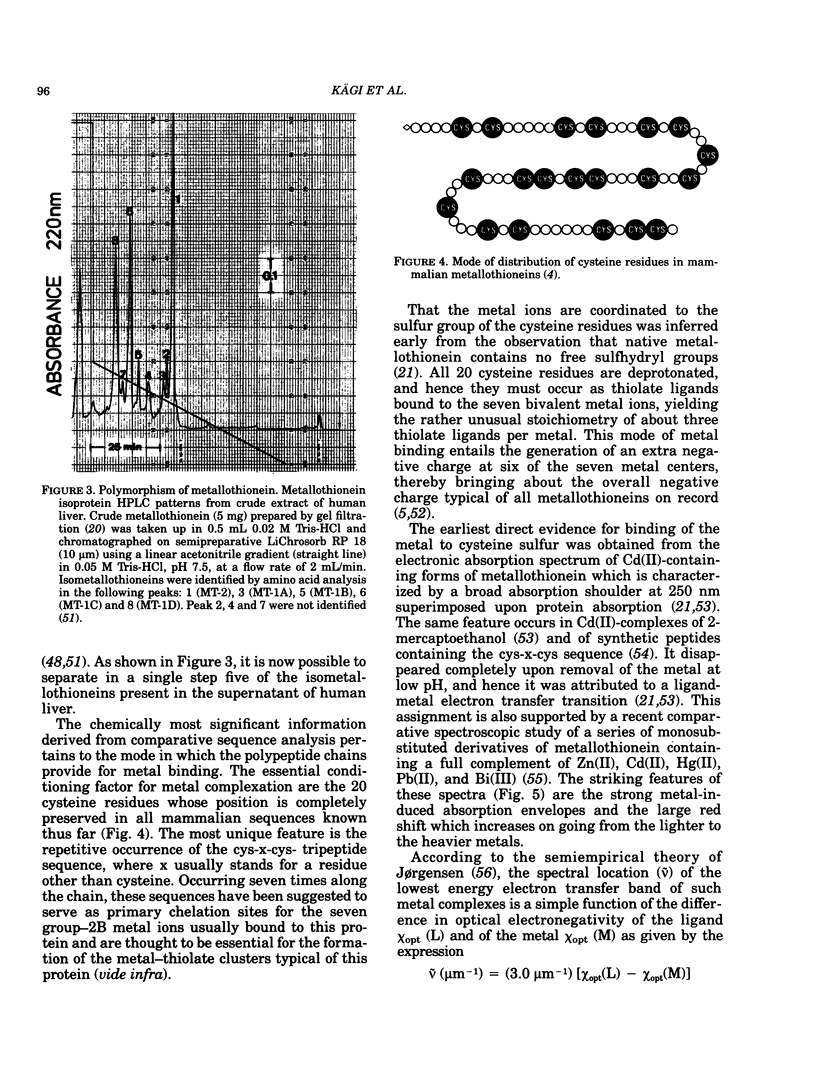
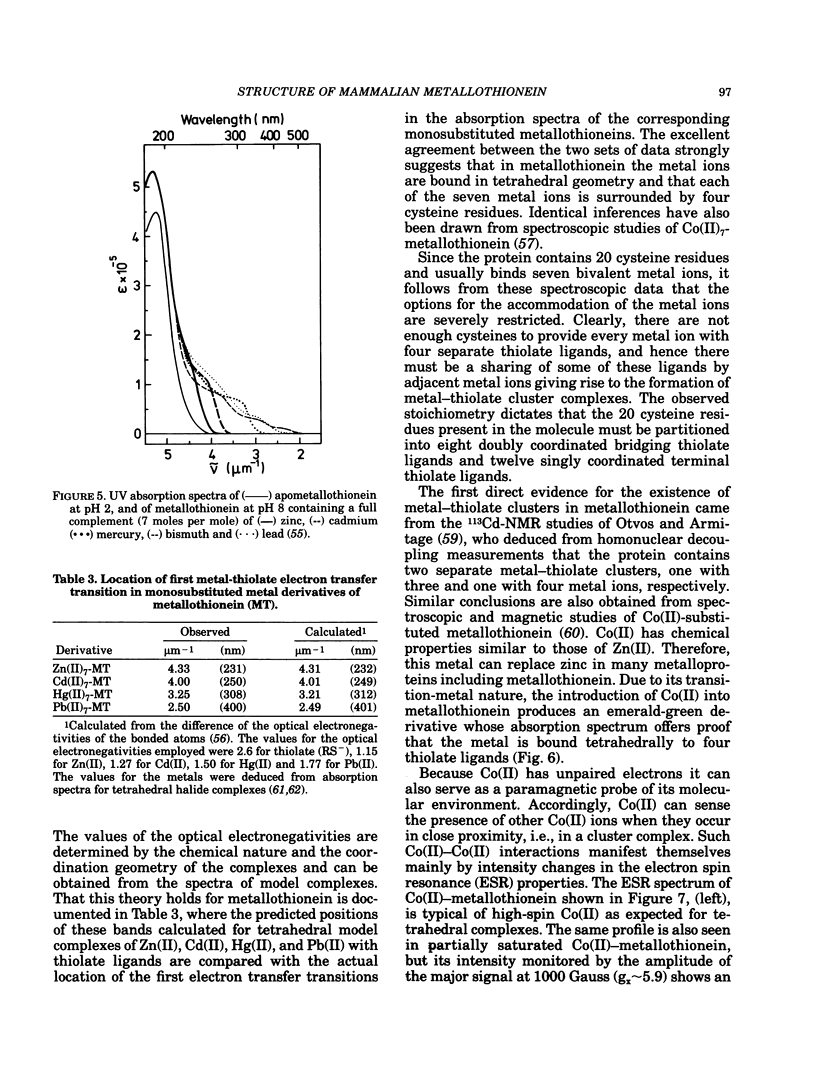
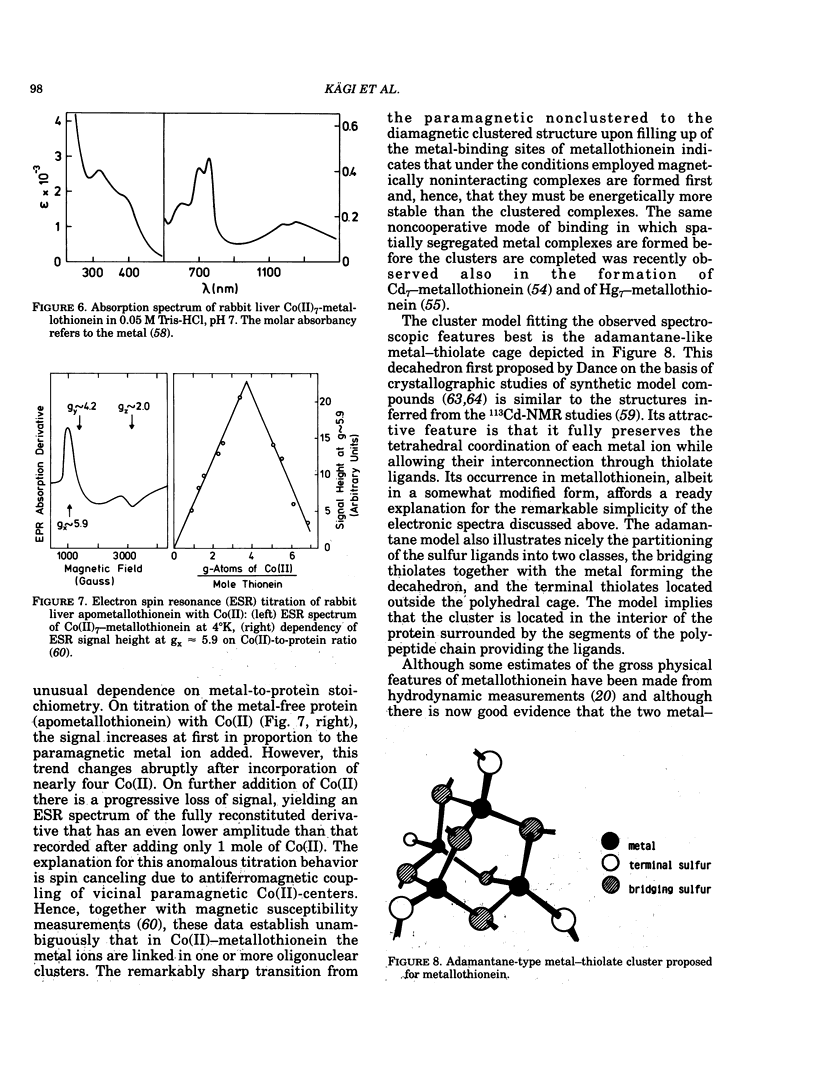
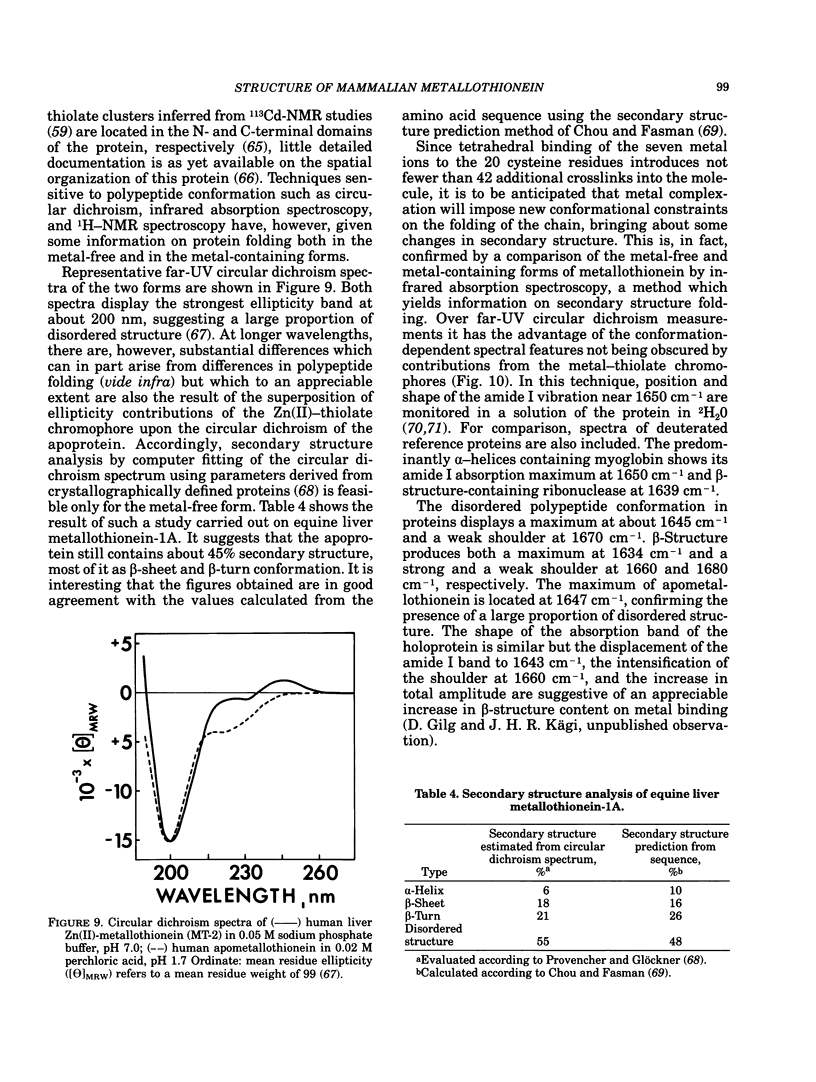
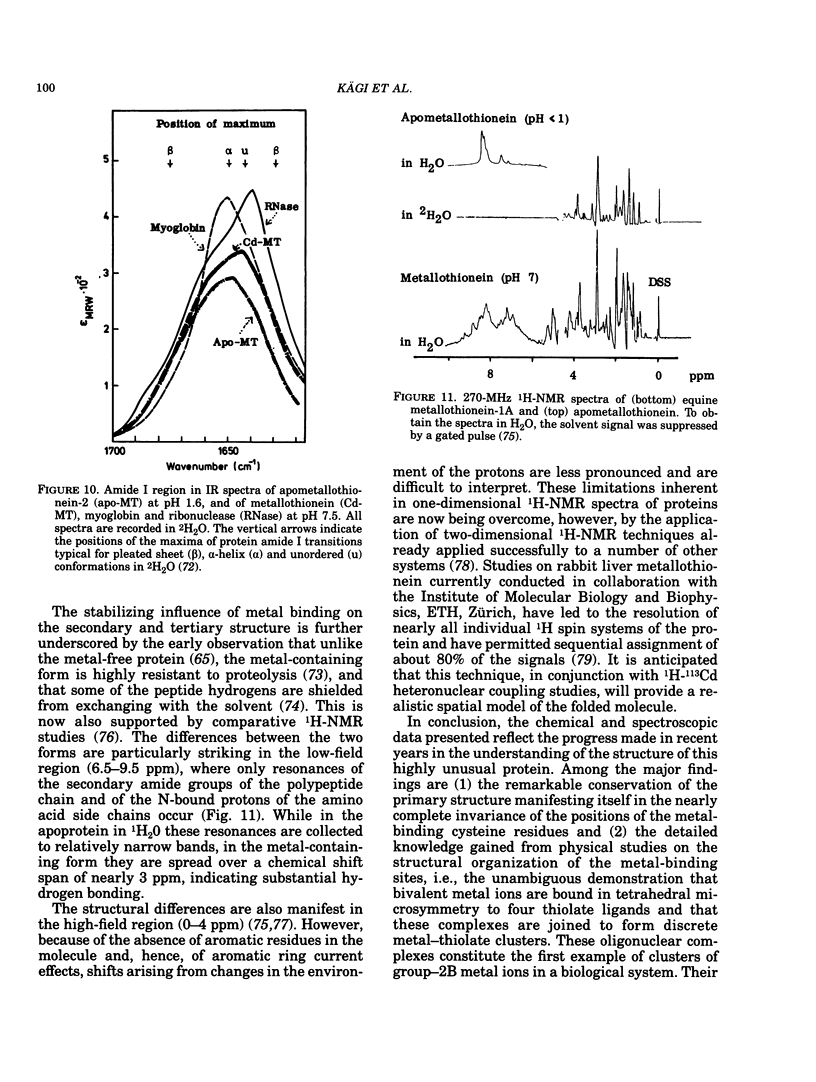
All mammalian metallothioneins characterized contain a single polypeptide chain of 61 amino acid residues, among them 20 cysteines providing the ligands for seven metal-binding sites. Native metallothioneins are usually heterogeneous in metal composition, with Zn, Cd, and Cu occurring in varying proportions. However, forms containing only a single metal species, i.e., Zn, Cd, Ni, Co, Hg, Pb, Bi, have now been prepared by in vitro reconstitution from the metal-free apoprotein. By spectroscopic analysis of such derivatives it was established that all cysteine residues participate in metal binding, that each metal ion is bound to four thiolate ligands, and that the symmetry of each complex is close to that of a tetrahedron. To satisfy the requirements of the overall Me7(Cys-)20 stoichiometry, the complexes must be combined to form metal-thiolate cluster structures. Experimental proof for the occurrence of such clusters comes from the demonstration of metal-metal interactions by spectroscopic and magnetic means. Thus, in Co(II)7-metallothionein, the Co(II)-specific ESR signals are effectively suppressed by antiferromagnetic coupling of juxtaposed paramagnetic metal ions. By monitoring changes in ESR signal size occurring on stepwise incorporation of Co(II) into the protein, it is possible to follow the building up of the clusters. This process is biphasic. Up to binding of four equivalents of Co(II), the ESR amplitude increases in proportion to the metal content, indicating generation of magnetically noninteracting high-spin complexes. However, upon addition of the remaining three equivalents of Co(II), these features are progressively suppressed, signaling the formation of clusters. The same mode of cluster formation has also been documented for Cd and Hg. The actual spatial organization of the clusters and the polypeptide chain remains to be established. An attractive possibility is the arrangement of the tetrahedral metal-thiolates in adamantane-like structures surrounded by properly folded segments of the chain providing the ligands. 1H-NMR data and infrared absorption measurements are consistent with a tightly folded structure rich in beta-type conformation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakka A., Webb M. Metabolism of zinc and copper in the neonate: changes in the concentrations and contents of thionein-bound Zn and Cu with age in the livers of the newborn of various mammalian species. Biochem Pharmacol. 1981 Apr 1;30(7):721–725. doi: 10.1016/0006-2952(81)90157-x. [DOI] [PubMed] [Google Scholar]
- Bartolf M., Brennan E., Price C. A. Partial Characterization of a Cadmium-binding Protein from the Roots of Cadmium-treated Tomato. Plant Physiol. 1980 Sep;66(3):438–441. doi: 10.1104/pp.66.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger Y., Goodman C. M., Forte C. P., Fesik S. W., Armitage I. M. Model for mammalian metallothionein structure. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1501–1505. doi: 10.1073/pnas.80.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady F. O., Webb M. Metabolism of zinc and copper in the neonate. (Zinc, copper)-thionein in the developing rat kidney and testis. J Biol Chem. 1981 Apr 25;256(8):3931–3935. [PubMed] [Google Scholar]
- Bremner I. The relationship between the zinc status of pigs and the occurrence of copper- and Zn-binding proteins in liver. Br J Nutr. 1976 Mar;35(2):245–252. doi: 10.1079/bjn19760028. [DOI] [PubMed] [Google Scholar]
- Bremner I., Williams R. B., Young B. W. Distribution of copper and zinc in the liver of the developing sheep foetus. Br J Nutr. 1977 Jul;38(1):87–92. doi: 10.1079/bjn19770064. [DOI] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Spectroscopic properties of zinc-metallothionein. Experientia Suppl. 1979;34:211–220. doi: 10.1007/978-3-0348-6493-0_14. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Eckert K., Grosse R., Malur J., Repke K. R. Calculation and use of protein-derived conformation-related spectra for the estimate of the secondary structure of proteins from their infrared spectra. Biopolymers. 1977 Nov;16(11):2549–2563. doi: 10.1002/bip.1977.360161116. [DOI] [PubMed] [Google Scholar]
- Howard A. G., Nickless G. Protein binding of cadmium, zinc and copper in environmentally insulted limpets Patella vulgata. J Chromatogr. 1975 Feb 12;104(2):457–459. doi: 10.1016/s0021-9673(00)91874-0. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Kimura M., Hata A., Tsunoo H., Yoshida A. Complete amino acid sequence of mouse liver metallothionein-II. J Biochem. 1981 Jun;89(6):1839–1845. doi: 10.1093/oxfordjournals.jbchem.a133385. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Yoshida A. Mouse liver metallothioneins. Complete amino acid sequence of metallothionein-I. J Biol Chem. 1977 Nov 25;252(22):8217–8221. [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Kimura M., Otaki N., Imano M. Rabbit liver metallothionein. Tentative amino acid sequence of metallothionein-B. Experientia Suppl. 1979;34:163–168. doi: 10.1007/978-3-0348-6493-0_7. [DOI] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- Kissling M. M., Berger C., Kägi J. H., Andersen R. D., Weser U. The amino-terminal sequence of a rat liver metallothionein (MT-2). Experientia Suppl. 1979;34:181–185. doi: 10.1007/978-3-0348-6493-0_10. [DOI] [PubMed] [Google Scholar]
- Kissling M. M., Kägi H. R. Primary structure of human hepatic metallothionein. FEBS Lett. 1977 Oct 15;82(2):247–250. doi: 10.1016/0014-5793(77)80594-2. [DOI] [PubMed] [Google Scholar]
- Klauser S., Kägi J. H., Wilson K. J. Characterization of isoprotein patterns in tissue extracts and isolated samples of metallothioneins by reverse-phase high-pressure liquid chromatography. Biochem J. 1983 Jan 1;209(1):71–80. doi: 10.1042/bj2090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Berger C., Kägi J. H. The amino acid sequence of equine metallothioneins. Experientia Suppl. 1979;34:153–161. doi: 10.1007/978-3-0348-6493-0_6. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Berger C., Vallee B. L., Kägi J. H. Amino-acid sequence of equine renal metallothionein-1B. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3413–3417. doi: 10.1073/pnas.73.10.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. Purification, molecular weight, amino acid composition, and metal content. J Biol Chem. 1974 Jun 10;249(11):3537–3542. [PubMed] [Google Scholar]
- Lerch K. Amino-acid sequence of copper-metallothionein from Neurospora crassa. Experientia Suppl. 1979;34:173–179. doi: 10.1007/978-3-0348-6493-0_9. [DOI] [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- Margoliash E., Fitch W. M. Evolutionary variability of cytochrome c primary structures. Ann N Y Acad Sci. 1968 Jun 14;151(1):359–381. doi: 10.1111/j.1749-6632.1968.tb11901.x. [DOI] [PubMed] [Google Scholar]
- Mason R., Bakka A., Samarawickrama G. P., Webb M. Metabolism of zinc and copper in the neonate: accumulation and function of (Zn, Cu)-metallothionein in the liver of the newborn rat. Br J Nutr. 1981 Mar;45(2):375–389. doi: 10.1079/bjn19810113. [DOI] [PubMed] [Google Scholar]
- Mbikay M., Maiti I. B., Thirion J. P. Cloning and sequencing of cDNA for mouse liver metallothionein-I. Biochem Biophys Res Commun. 1981 Dec 15;103(3):825–832. doi: 10.1016/0006-291x(81)90885-8. [DOI] [PubMed] [Google Scholar]
- Nagayama K. Two-dimensional NMR spectroscopy: an application to the study of flexibility of protein molecules. Adv Biophys. 1981;14:139–204. [PubMed] [Google Scholar]
- Ohtake H., Koga M. Purification and characterization of zinc-binding protein from the liver of the partially hepatectomized rat. Biochem J. 1979 Dec 1;183(3):683–690. doi: 10.1042/bj1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz R., Weser U. A naturally occurring Cu-thionein in Saccharomyces cerevisiae. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):767–776. doi: 10.1515/bchm2.1975.356.s1.767. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Pulido P., Kägi J. H., Vallee B. L. Isolation and some properties of human metallothionein. Biochemistry. 1966 May;5(5):1768–1777. doi: 10.1021/bi00869a046. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Richards V. Human fetal liver contains both zinc- and copper-rich forms of metallothionein. J Biol Chem. 1980 Jun 10;255(11):5380–5383. [PubMed] [Google Scholar]
- Rupp H., Voelter W., Weser U. 270 MHz proton magnetic resonance spectra of metallothionein. FEBS Lett. 1974 Mar 15;40(1):176–179. doi: 10.1016/0014-5793(74)80921-x. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T. Direct connection of high-speed liquid chromatograph (equipped with gel permeation column) to atomic absorption spectrophotometer for metalloprotein analyasis: metallothionein. Anal Biochem. 1980 Feb;102(1):31–34. doi: 10.1016/0003-2697(80)90312-7. [DOI] [PubMed] [Google Scholar]
- Vasák M., Galdes A., Hill H. A., Kägi J. H., Bremner I., Young B. W. Investigation of the structure of metallothioneins by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Feb 5;19(3):416–425. doi: 10.1021/bi00544a003. [DOI] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H., Hill H. A. Zinc(II), cadmium(II), and mercury(II) thiolate transitions in metallothionein. Biochemistry. 1981 May 12;20(10):2852–2856. doi: 10.1021/bi00513a022. [DOI] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H., Holmquist B., Vallee B. L. Spectral studies of cobalt (II)- and Nickel (II)-metallothionein. Biochemistry. 1981 Nov 10;20(23):6659–6664. doi: 10.1021/bi00526a021. [DOI] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H. Metal thiolate clusters in cobalt(II)-metallothionein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6709–6713. doi: 10.1073/pnas.78.11.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. Binding of cadmium ions by rat liver and kidney. Biochem Pharmacol. 1972 Oct 15;21(20):2751–2765. doi: 10.1016/0006-2952(72)90023-8. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]


