Abstract
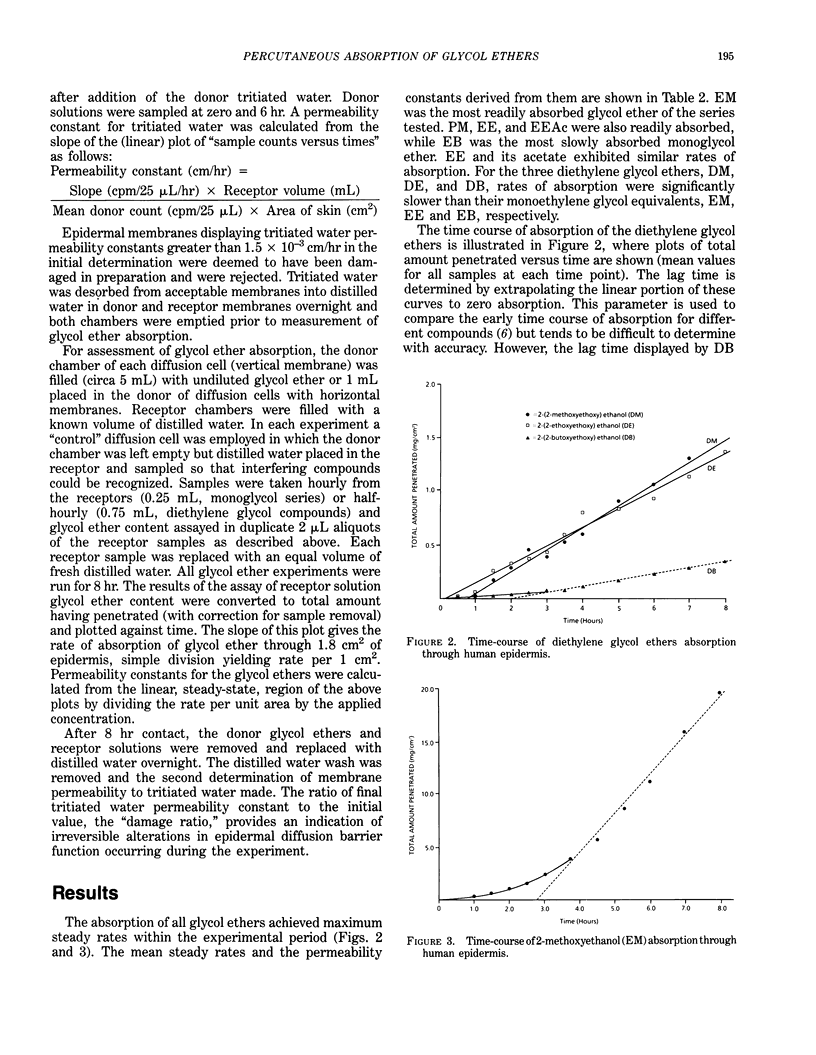
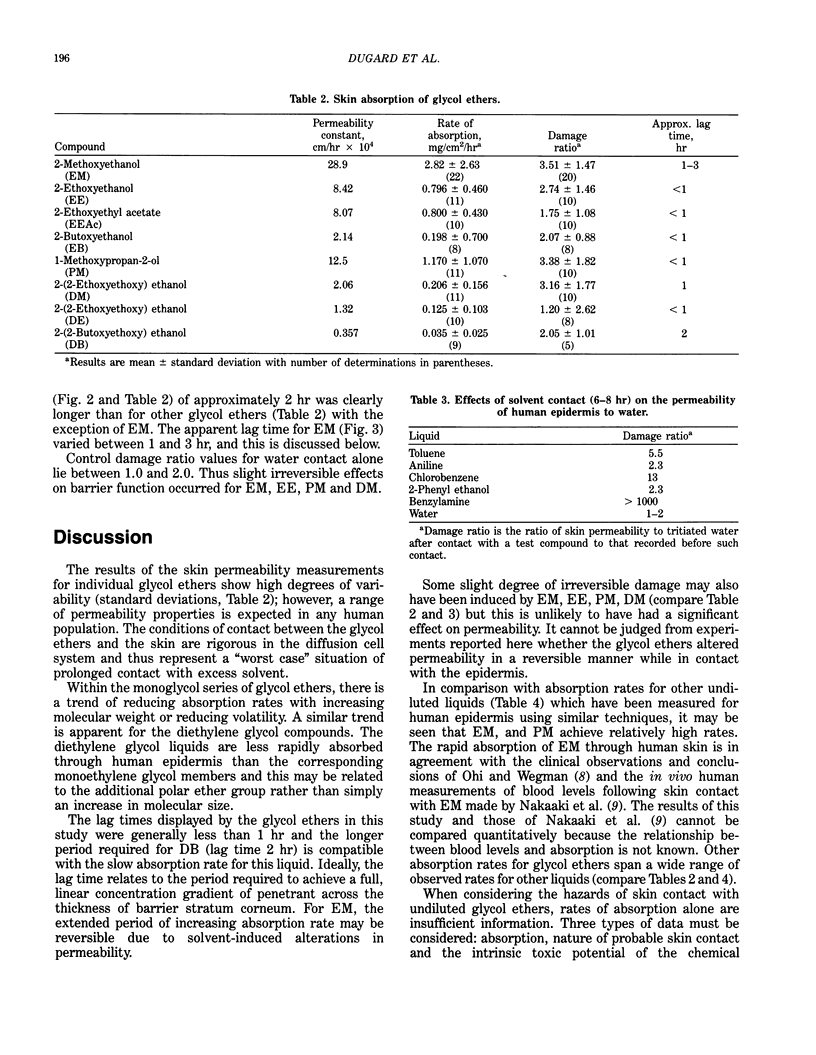
To assist evaluation of the hazards of skin contact with selected undiluted glycol ethers, their absorption across isolated human abdominal epidermis was measured in vitro. Epidermal membranes were set up in glass diffusion cells and, following an initial determination of permeability to tritiated water, excess undiluted glycol ether was applied to the outer surface for 8 hr. The appearance of glycol ether in an aqueous "receptor" phase bathing the underside of the epidermis was quantified by a gas chromatographic technique. A final determination of tritiated water permeability was compared with initial values to establish any irreversible alterations in epidermal barrier function induced by contact with the glycol ethers. 2-methoxyethanol (EM) was most readily absorbed (mean steady rate 2.82 mg/cm2/hr), and a relatively high absorption rate (1.17 mg/cm2/hr) was also apparent for 1-methoxypropan-2-ol (PM). There was a trend of reducing absorption rate with increasing molecular weight or reducing volatility for monoethylene glycol ethers (EM, 2.82 mg/cm2/hr; 2-ethoxyethanol, EE, 0.796 mg/cm2/hr; 2-butoxyethanol, EB, 0.198 mg/cm2/hr) and also within the diethylene glycol series: 2-(2-methoxyethoxy) ethanol (DM, 0.206 mg/cm2/hr); 2-(2-ethoxyethoxy) ethanol (DE, 0.125 mg/cm2/hr) and 2-(2-butoxyethoxy) ethanol (DB, 0.035 mg/cm2/hr). The rate of absorption of 2-ethoxyethyl acetate (EEAc) was similar to that of the parent alcohol, EE. Absorption rates of diethylene glycol ethers were slower than their corresponding monoethylene glycol equivalents. Combination of intrinsic toxicity and ability to pass across skin contribute to assessment of hazards of contact with undiluted glycol ethers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronaugh R. L., Stewart R. F., Congdon E. R., Giles A. L., Jr Methods for in vitro percutaneous absorption studies. I. Comparison with in vivo results. Toxicol Appl Pharmacol. 1982 Mar 15;62(3):474–480. doi: 10.1016/0041-008x(82)90148-x. [DOI] [PubMed] [Google Scholar]
- Bronaugh R. L., Stewart R. F., Congdon E. R. Methods for in vitro percutaneous absorption studies. II. Animal models for human skin. Toxicol Appl Pharmacol. 1982 Mar 15;62(3):481–488. doi: 10.1016/0041-008x(82)90149-1. [DOI] [PubMed] [Google Scholar]
- Ohi G., Wegman D. H. Transcutaneous ethylene glycol monomethyl ether poisoning in the work setting. J Occup Med. 1978 Oct;20(10):675–676. [PubMed] [Google Scholar]
- Scheuplein R. J., Blank I. H. Permeability of the skin. Physiol Rev. 1971 Oct;51(4):702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]


