Abstract
The nasal passages of laboratory animals and man are complex, and lesions induced in the delicate nasal lining by inhaled air pollutants vary considerably in location and nature. The distribution of nasal lesions is generally a consequence of regional deposition of the inhaled material, local tissue susceptibility, or a combination of these factors. Nasal uptake and regional deposition are are influenced by numerous factors including the physical and chemical properties of the inhaled material, such as water solubility and reactivity; airborne concentration and length of exposure; the presence of other air contaminants such as particulate matter; nasal metabolism, and blood and mucus flow. For certain highly water-soluble or reactive gases, nasal airflow patterns play a major role in determining lesion distribution. Studies of nasal airflow in rats and monkeys, using casting and molding techniques combined with a water-dye model, indicate that nasal airflow patterns are responsible for characteristic differences in the distribution of nasal lesions induced by formaldehyde in these species. Local tissue susceptibility is also a complex issue that may be a consequence of many factors, including physiologic and metabolic characteristics of the diverse cell populations that comprise each of the major epithelial types lining the airways. Identification of the principal factors that influence the distribution and nature of nasal lesions is important when attempting the difficult process of determining potential human risks using data derived from laboratory animals. Toxicologic pathologists can contribute to this process by carefully identifying the site and nature of nasal lesions induced by inhaled materials.
Full text
PDF
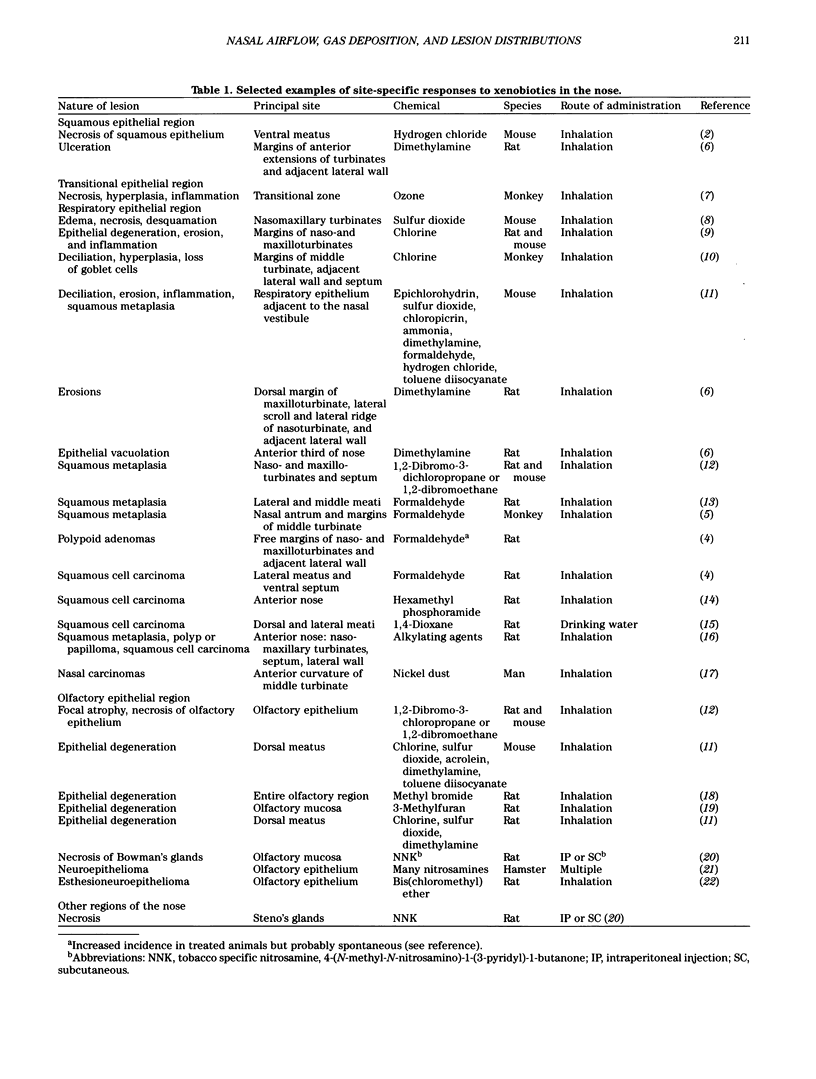
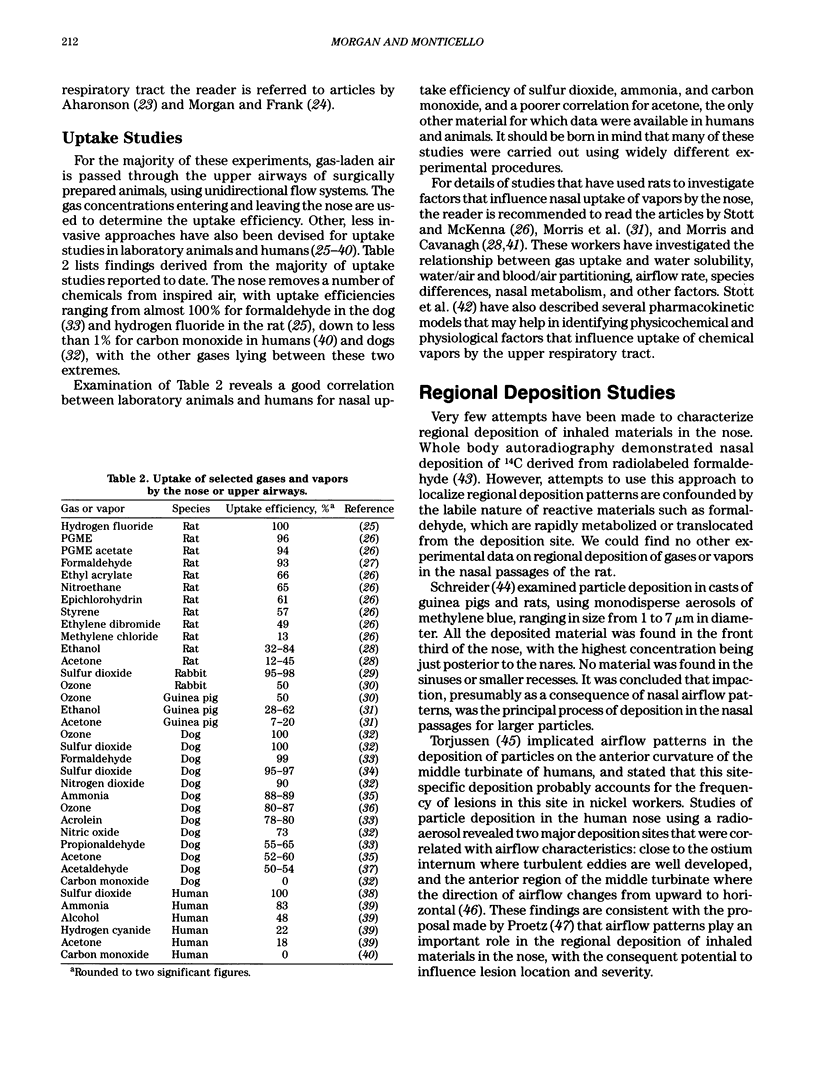

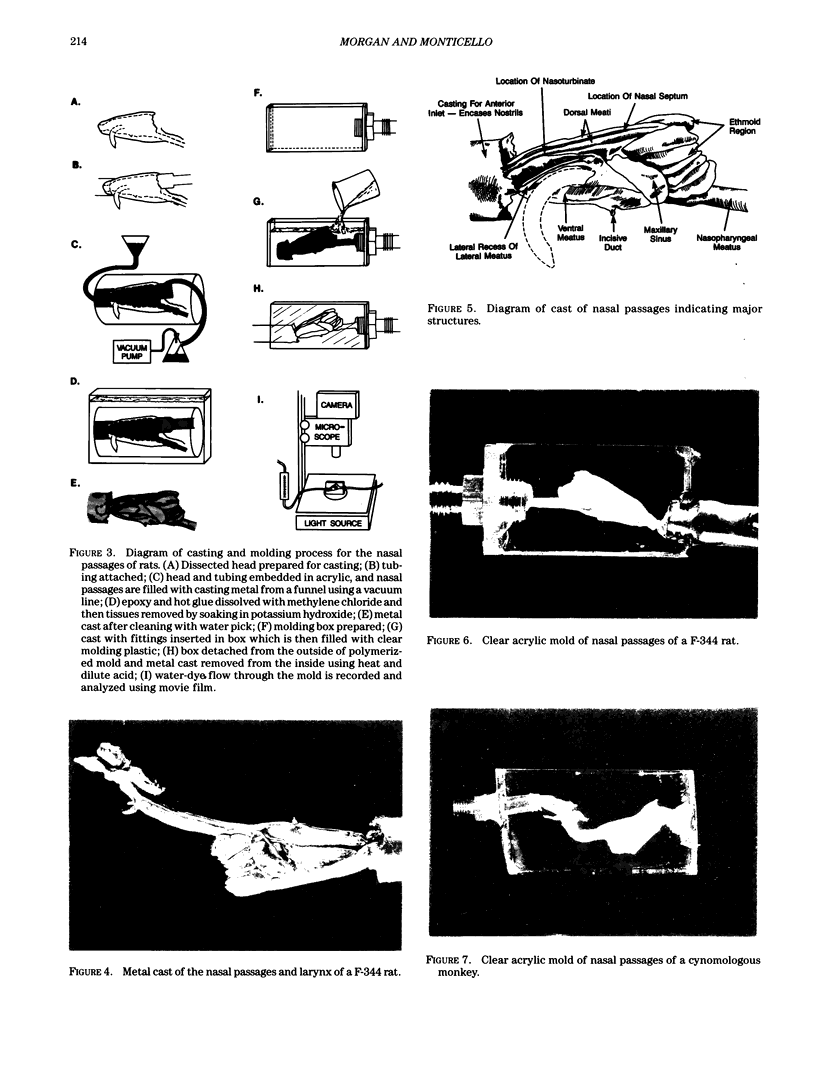




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J., Burke J. P., Guengerich F. P., Jakoby W. B., Voigt J. M. Sites for xenobiotic activation and detoxication within the respiratory tract: implications for chemically induced toxicity. Toxicol Appl Pharmacol. 1988 May;93(3):493–505. doi: 10.1016/0041-008x(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Belinsky S. A., Walker V. E., Maronpot R. R., Swenberg J. A., Anderson M. W. Molecular dosimetry of DNA adduct formation and cell toxicity in rat nasal mucosa following exposure to the tobacco specific nitrosamine 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone and their relationship to induction of neoplasia. Cancer Res. 1987 Nov 15;47(22):6058–6065. [PubMed] [Google Scholar]
- Bond J. A., Harkema J. R., Russell V. I. Regional distribution of xenobiotic metabolizing enzymes in respiratory airways of dogs. Drug Metab Dispos. 1988 Jan-Feb;16(1):116–124. [PubMed] [Google Scholar]
- Brain J. D. The uptake of inhaled gases by the nose. Ann Otol Rhinol Laryngol. 1970 Jun;79(3):529–539. doi: 10.1177/000348947007900315. [DOI] [PubMed] [Google Scholar]
- Bridger M. W., van Nostrand A. W. The nose and paranasal sinuses--applied surgical anatomy. A histologic study of whole organ sections in three planes. J Otolaryngol Suppl. 1978 Dec;6:1–33. [PubMed] [Google Scholar]
- Buckley L. A., Jiang X. Z., James R. A., Morgan K. T., Barrow C. S. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol. 1984 Jul;74(3):417–429. doi: 10.1016/0041-008x(84)90295-3. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Gross E. A., Swenberg J. A., Barrow C. S. Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 1983 Apr;68(2):161–176. doi: 10.1016/0041-008x(83)90001-7. [DOI] [PubMed] [Google Scholar]
- Collins M. P. A practical guide to the construction of a "cire perdue" model of the human nose. Rhinology. 1985 Mar;23(1):71–78. [PubMed] [Google Scholar]
- DALHAMN T., STRANDBERG L. Acute effect of sulphur dioxide on the rate of ciliary beat in the trachea of rabbit, in vivo and in vitro, with studies on the absorptional capacity of the nasal cavity. Int J Air Water Pollut. 1961 Sep;4:154–167. [PubMed] [Google Scholar]
- Dahl A. R., Hadley W. M., Hahn F. F., Benson J. M., McClellan R. O. Cytochrome P-450-dependent monooxygenases in olfactory epithelium of dogs: possible role in tumorigenicity. Science. 1982 Apr 2;216(4541):57–59. doi: 10.1126/science.7063870. [DOI] [PubMed] [Google Scholar]
- Egle J. L., Jr Retention of inhaled acetaldehyde in the dog. Arch Environ Health. 1972 May;24(5):354–357. doi: 10.1080/00039896.1972.10666103. [DOI] [PubMed] [Google Scholar]
- Egle J. L., Jr Retention of inhaled acetone and ammonia in the dog. Am Ind Hyg Assoc J. 1973 Dec;34(12):533–539. doi: 10.1080/0002889738506894. [DOI] [PubMed] [Google Scholar]
- Egle J. L., Jr Retention of inhaled formaldehyde, propionaldehyde, and acrolein in the dog. Arch Environ Health. 1972 Aug;25(2):119–124. doi: 10.1080/00039896.1972.10666147. [DOI] [PubMed] [Google Scholar]
- Giddens W. E., Jr, Fairchild G. A. Effects of sulfur dioxide on the nasal mucosa of mice. Arch Environ Health. 1972 Sep;25(3):166–173. doi: 10.1080/00039896.1972.10666156. [DOI] [PubMed] [Google Scholar]
- Gross E. A., Patterson D. L., Morgan K. T. Effects of acute and chronic dimethylamine exposure on the nasal mucociliary apparatus of F-344 rats. Toxicol Appl Pharmacol. 1987 Sep 30;90(3):359–376. doi: 10.1016/0041-008X(87)90129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt A. R., Holmes M. A., Cumming G. Can carbon monoxide be absorbed from the upper respiratory tract in man? Eur J Respir Dis. 1981 Dec;62(6):383–390. [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of the macaque nasal epithelium to ambient levels of ozone. A morphologic and morphometric study of the transitional and respiratory epithelium. Am J Pathol. 1987 Jul;128(1):29–44. [PMC free article] [PubMed] [Google Scholar]
- Haschek W. M., Morse C. C., Boyd M. R., Hakkinen P. J., Witschi H. P. Pathology of acute inhalation exposure to 3-methylfuran in the rat and hamster. Exp Mol Pathol. 1983 Dec;39(3):342–354. doi: 10.1016/0014-4800(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Hurtt M. E., Thomas D. A., Working P. K., Monticello T. M., Morgan K. T. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: pathology, cell kinetics, and olfactory function. Toxicol Appl Pharmacol. 1988 Jun 30;94(2):311–328. doi: 10.1016/0041-008x(88)90273-6. [DOI] [PubMed] [Google Scholar]
- Jiang X. Z., Buckley L. A., Morgan K. T. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol Appl Pharmacol. 1983 Nov;71(2):225–236. doi: 10.1016/0041-008x(83)90339-3. [DOI] [PubMed] [Google Scholar]
- Kerns W. D., Pavkov K. L., Donofrio D. J., Gralla E. J., Swenberg J. A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983 Sep;43(9):4382–4392. [PubMed] [Google Scholar]
- Klonne D. R., Ulrich C. E., Riley M. G., Hamm T. E., Jr, Morgan K. T., Barrow C. S. One-year inhalation toxicity study of chlorine in rhesus monkeys (Macaca mulatta). Fundam Appl Toxicol. 1987 Oct;9(3):557–572. doi: 10.1016/0272-0590(87)90037-6. [DOI] [PubMed] [Google Scholar]
- LANDAHL H. D., HERRMANN R. G. Retention of vapors and gases in the human nose and lung. Arch Ind Hyg Occup Med. 1950 Jan;1(1):36–45. [PubMed] [Google Scholar]
- Lee K. P., Trochimowicz H. J. Metaplastic changes of nasal respiratory epithelium in rats exposed to hexamethylphosphoramide (HMPA) by inhalation. Am J Pathol. 1982 Jan;106(1):8–19. [PMC free article] [PubMed] [Google Scholar]
- Leong B. K., Kociba R. J., Jersey G. C. A lifetime study of rats and mice exposed to vapors of bis(chloromethyl)ether. Toxicol Appl Pharmacol. 1981 Apr;58(2):269–281. doi: 10.1016/0041-008x(81)90432-4. [DOI] [PubMed] [Google Scholar]
- McCLENAHAN J. L., VOGEL F. S. The use of fusible metal as a radiopaque contrast medium and in the preparation of anatomical castings. Am J Roentgenol Radium Ther Nucl Med. 1952 Sep;68(3):406–412. [PubMed] [Google Scholar]
- Miller F. J., McNeal C. A., Kirtz J. M., Gardner D. E., Coffin D. L., Menzel D. B. Nasopharyngeal removal of ozone in rabbits and guinea pigs. Toxicology. 1979 Nov;14(3):273–281. doi: 10.1016/0300-483x(79)90009-x. [DOI] [PubMed] [Google Scholar]
- Monticello T. M., Morgan K. T., Everitt J. I., Popp J. A. Effects of formaldehyde gas on the respiratory tract of rhesus monkeys. Pathology and cell proliferation. Am J Pathol. 1989 Mar;134(3):515–527. [PMC free article] [PubMed] [Google Scholar]
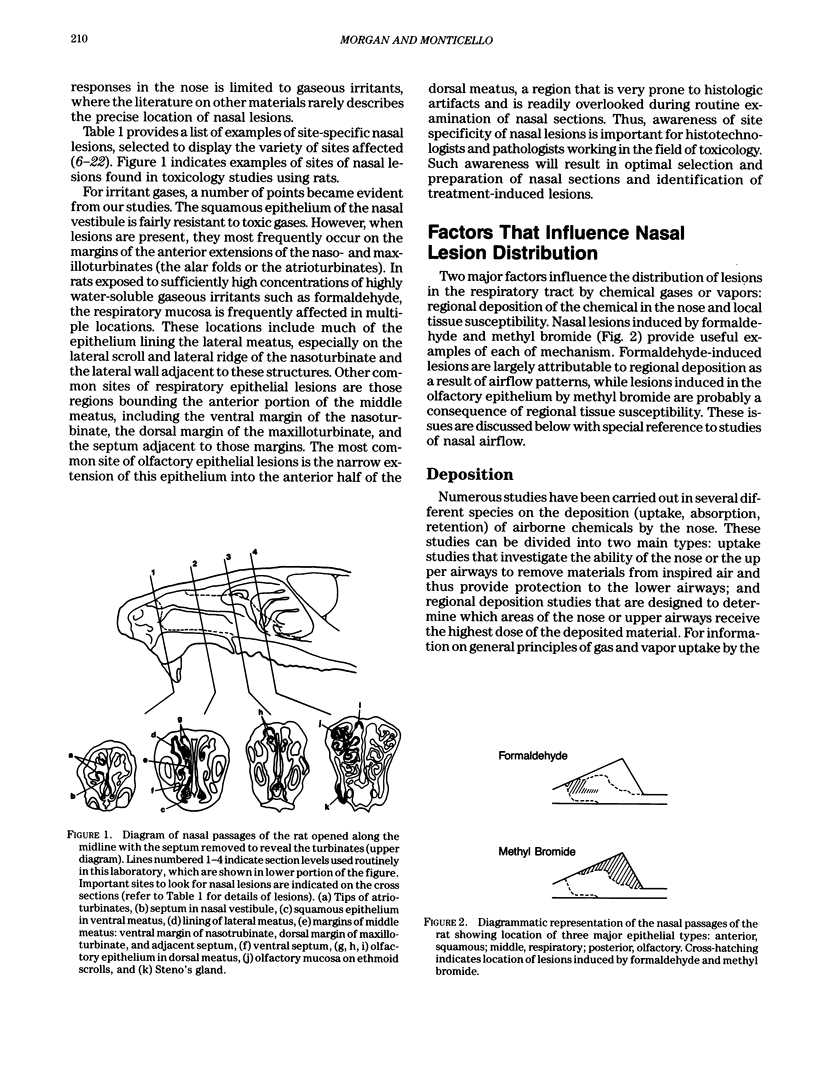
- Morgan K. T., Gross E. A., Patterson D. L. Distribution, progression, and recovery of acute formaldehyde-induced inhibition of nasal mucociliary function in F-344 rats. Toxicol Appl Pharmacol. 1986 Dec;86(3):448–456. doi: 10.1016/0041-008x(86)90372-8. [DOI] [PubMed] [Google Scholar]
- Morgan K. T., Patterson D. L., Gross E. A. Responses of the nasal mucociliary apparatus of F-344 rats to formaldehyde gas. Toxicol Appl Pharmacol. 1986 Jan;82(1):1–13. doi: 10.1016/0041-008x(86)90431-x. [DOI] [PubMed] [Google Scholar]
- Morris J. B., Cavanagh D. G. Deposition of ethanol and acetone vapors in the upper respiratory tract of the rat. Fundam Appl Toxicol. 1986 Jan;6(1):78–88. doi: 10.1016/0272-0590(86)90266-6. [DOI] [PubMed] [Google Scholar]
- Morris J. B., Cavanagh D. G. Metabolism and deposition of propanol and acetone vapors in the upper respiratory tract of the hamster. Fundam Appl Toxicol. 1987 Jul;9(1):34–40. doi: 10.1016/0272-0590(87)90151-5. [DOI] [PubMed] [Google Scholar]
- Morris J. B., Clay R. J., Cavanagh D. G. Species differences in upper respiratory tract deposition of acetone and ethanol vapors. Fundam Appl Toxicol. 1986 Nov;7(4):671–680. doi: 10.1016/0272-0590(86)90117-x. [DOI] [PubMed] [Google Scholar]
- Morris J. B., Smith F. A. Regional deposition and absorption of inhaled hydrogen fluoride in the rat. Toxicol Appl Pharmacol. 1982 Jan;62(1):81–89. doi: 10.1016/0041-008x(82)90104-1. [DOI] [PubMed] [Google Scholar]
- PROETZ A. W. Air currents in the upper respiratory tract and their clinical importance. Ann Otol Rhinol Laryngol. 1951 Jun;60(2):439–467. doi: 10.1177/000348945106000216. [DOI] [PubMed] [Google Scholar]
- Patra A. L., Gooya A., Morgan K. T. Airflow characteristics in a baboon nasal passage cast. J Appl Physiol (1985) 1986 Nov;61(5):1959–1966. doi: 10.1152/jappl.1986.61.5.1959. [DOI] [PubMed] [Google Scholar]
- Randall H. W., Bogdanffy M. S., Morgan K. T. Enzyme histochemistry of the rat nasal mucosa embedded in cold glycol methacrylate. Am J Anat. 1987 May;179(1):10–17. doi: 10.1002/aja.1001790103. [DOI] [PubMed] [Google Scholar]
- Reznik G., Stinson S. F., Ward J. M. Respiratory pathology in rats and mice after inhalation of 1,2-dibromo-3-chloropropane or 1,2 dibromoethane for 13 weeks. Arch Toxicol. 1980 Dec;46(3-4):233–240. doi: 10.1007/BF00310439. [DOI] [PubMed] [Google Scholar]
- Sellakumar A. R., Snyder C. A., Albert R. E. Inhalation carcinogenesis of various alkylating agents. J Natl Cancer Inst. 1987 Aug;79(2):285–289. [PubMed] [Google Scholar]
- Speizer F. E., Frank N. R. The uptake and release of SO2 by the human nose. Arch Environ Health. 1966 Jun;12(6):725–728. doi: 10.1080/00039896.1966.10664471. [DOI] [PubMed] [Google Scholar]
- Stott W. T., McKenna M. J. The comparative absorption and excretion of chemical vapors by the upper, lower, and intact respiratory tract of rats. Fundam Appl Toxicol. 1984 Aug;4(4):594–602. doi: 10.1016/0272-0590(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Torjussen W., Solberg L. A., Høgetveit A. C. Histopathological changes of the nasal mucosa in active and retired nickel workers. Br J Cancer. 1979 Oct;40(4):568–580. doi: 10.1038/bjc.1979.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan T. R., Jr, Jennelle L. F., Lewis T. R. Long-term exposure to low levels of air pollutants. Effects on pulmonary function in the beagle. Arch Environ Health. 1969 Jul;19(1):45–50. doi: 10.1080/00039896.1969.10666803. [DOI] [PubMed] [Google Scholar]
- Yokoyama E., Frank R. Respiratory uptake of ozone in dogs. Arch Environ Health. 1972 Aug;25(2):132–138. doi: 10.1080/00039896.1972.10666149. [DOI] [PubMed] [Google Scholar]
- Young J. T. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981 Jul-Aug;1(4):309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]