Abstract
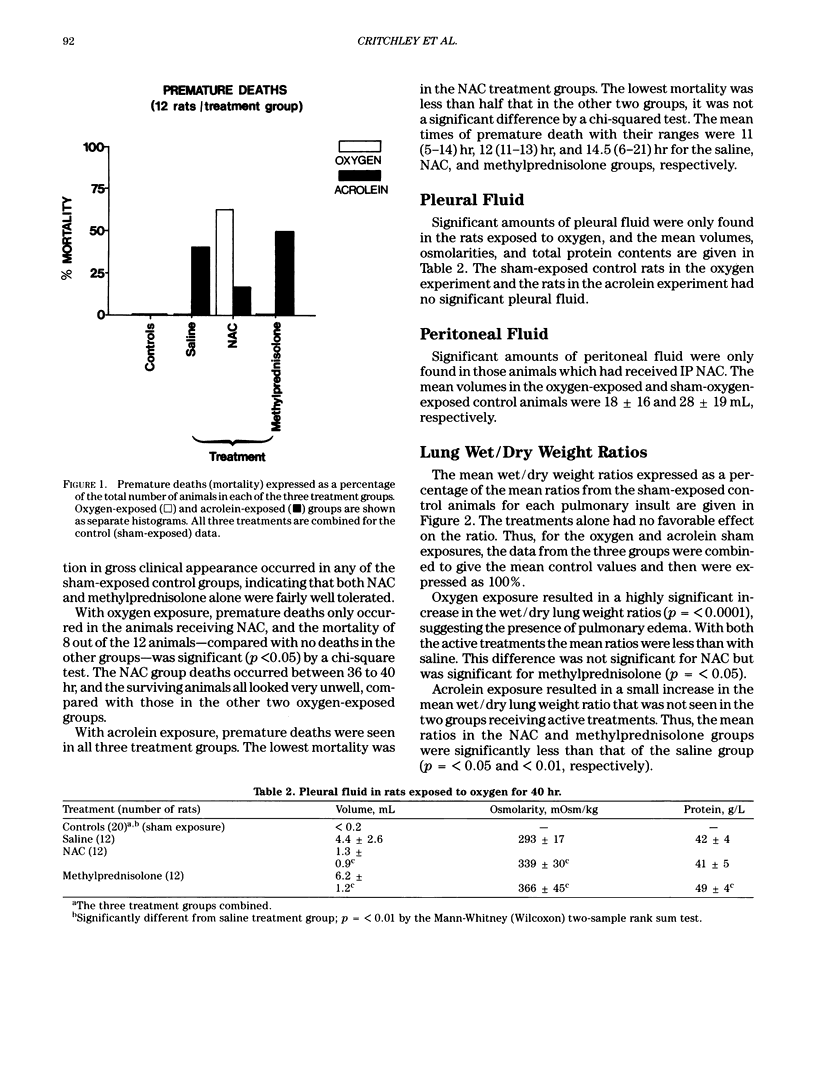
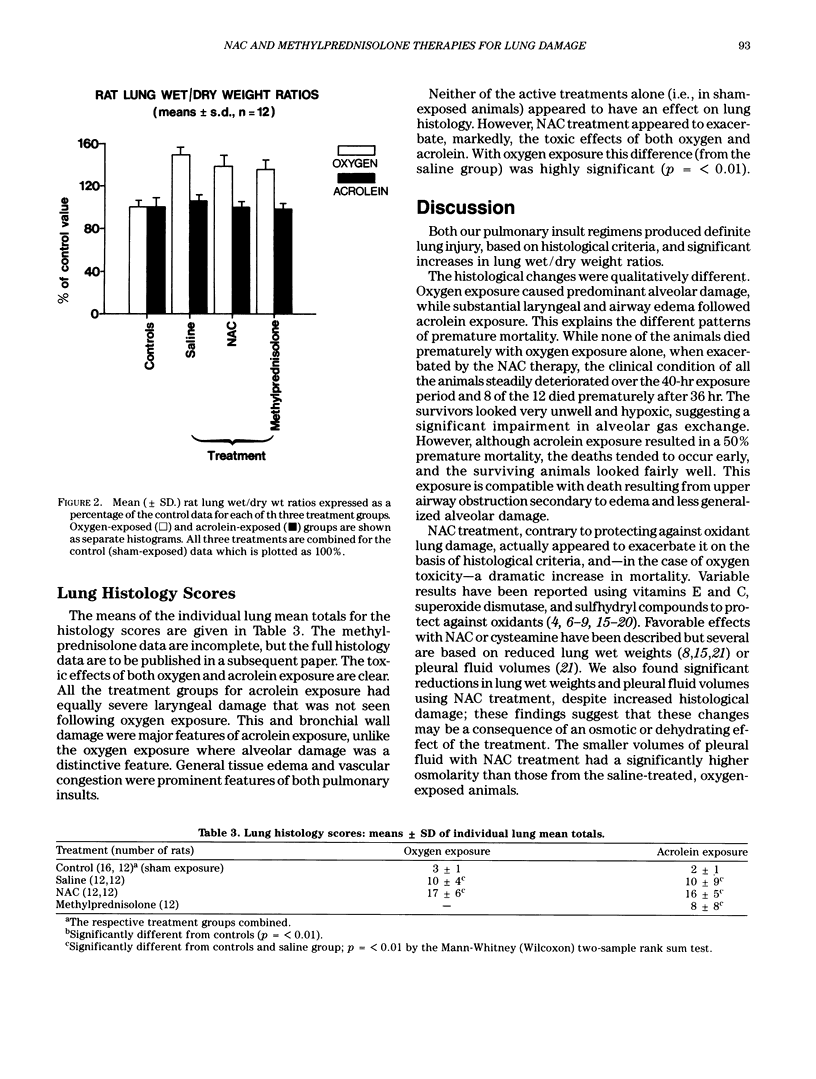
Reactive oxidizing species are implicated in the etiology of a range of inhalational pulmonary injuries. Consequently, various free radical scavengers have been tested as potential prophylactic agents. The sulfydryl compound, N-acetylcysteine (NAC) is the only such compound clinically available for use in realistic dosages, and it is well established as an effective antidote for the hepatic and renal toxicity of paracetamol. Another approach in pulmonary injury prophylaxis is methylprednisolone therapy. We evaluated NAC and methylprednisolone in two rat models of inhalational injury: 40-hr exposure to greater than 97% oxygen at 1.1 bar and 15-min exposure to acrolein vapor (210 ppm). For oxygen toxicity, NAC (80 mg) or methylprednisolone (10 mg) were given IP every 2 or 6 hr, respectively. For acrolein, single doses of NAC (1 g/kg) and methylprednisolone (30 mg/kg) were given intravenously 15 min before exposure. In sham-exposed control animals, neither treatment favorably effected mortality, lung wet/dry weight ratios, or pulmonary histology. The increases in lung wet/dry weight ratios, seen with both oxygen and acrolein toxicity were reduced with both treatments. However, with oxygen, NAC therapy was associated with considerably increased mortality and histological changes. Furthermore, IP NAC administration resulted in large volumes of ascitic fluid. With acrolein, IV, NAC had no significant effect on mortality or pulmonary histological damage. Methylprednisolone had no beneficial effects on either the mortality or histological damage observed in either toxicity model. We caution against the ad hoc use of NAC in the management of inhalational pulmonary injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeley J. M., Crow J., Jones J. G., Minty B., Lynch R. D., Pryce D. P. Mortality and lung histopathology after inhalation lung injury. The effect of corticosteroids. Am Rev Respir Dis. 1986 Feb;133(2):191–196. doi: 10.1164/arrd.1986.133.2.191. [DOI] [PubMed] [Google Scholar]
- Boor P. J., Sanduja R., Nelson T. J., Ansari G. A. In vivo metabolism of the cardiovascular toxin, allylamine. Biochem Pharmacol. 1987 Dec 15;36(24):4347–4353. doi: 10.1016/0006-2952(87)90683-6. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P. Lung protection by thiol-containing antioxidants. Bull Eur Physiopathol Respir. 1987 Jul-Aug;23(4):275–277. [PubMed] [Google Scholar]
- Currie W. D., Gelein R. M., Jr, Sanders A. P. Comparison of protective agents against hyperbaric oxygen in large animals. Aerosp Med. 1973 Sep;44(9):996–998. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GERSCHMAN R., GILBERT D. L., CACCAMISE D. Effect of various substances on survival times of mice exposed to different high oxygen tensions. Am J Physiol. 1958 Mar;192(3):563–571. doi: 10.1152/ajplegacy.1958.192.3.563. [DOI] [PubMed] [Google Scholar]
- GERSCHMAN R., GILBERT D. L., NYE S. W., DWYER P., FENN W. O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954 May 7;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortsø E., Qvist J., Bud M. I., Thomsen J. L., Andersen J. B., Wiberg-Jørgensen F., Jensen N. K., Jones R., Reid L. M., Zapol W. M. ARDS after accidental inhalation of zinc chloride smoke. Intensive Care Med. 1988;14(1):17–24. doi: 10.1007/BF00254116. [DOI] [PubMed] [Google Scholar]
- Jamieson D. D., Kerr D. R., Unsworth I. Interaction of N-acetylcysteine and bleomycin on hyperbaric oxygen-induced lung damage in mice. Lung. 1987;165(4):239–247. doi: 10.1007/BF02714441. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Jensen T., Kharazmi A., Schiøtz P. O., Nielsen H., Stenvang Pedersen S., Stafanger G., Koch C., Høiby N. Effect of oral N-acetylcysteine administration on human blood neutrophil and monocyte function. APMIS. 1988 Jan;96(1):62–67. doi: 10.1111/j.1699-0463.1988.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Hawkins H. C., Weikel J. H., Jr The toxicity of N-acetylcysteine in laboratory animals. Semin Oncol. 1983 Mar;10(1 Suppl 1):17–24. [PubMed] [Google Scholar]
- Patterson C. E., Butler J. A., Byrne F. D., Rhodes M. L. Oxidant lung injury: intervention with sulfhydryl reagents. Lung. 1985;163(1):23–32. doi: 10.1007/BF02713803. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Critchley J. A. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87–101. doi: 10.1146/annurev.pa.23.040183.000511. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Illingworth R. N., Critchley J. A., Stewart M. J., Adam R. D., Proudfoot A. T. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979 Nov 3;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Schatte C., Swansinger A. Effect of dietary "antioxidant" supplementation on the susceptibility to oxygen toxicity in mice. Aviat Space Environ Med. 1976 Feb;47(2):147–150. [PubMed] [Google Scholar]


