Abstract
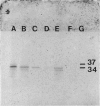
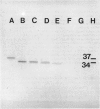
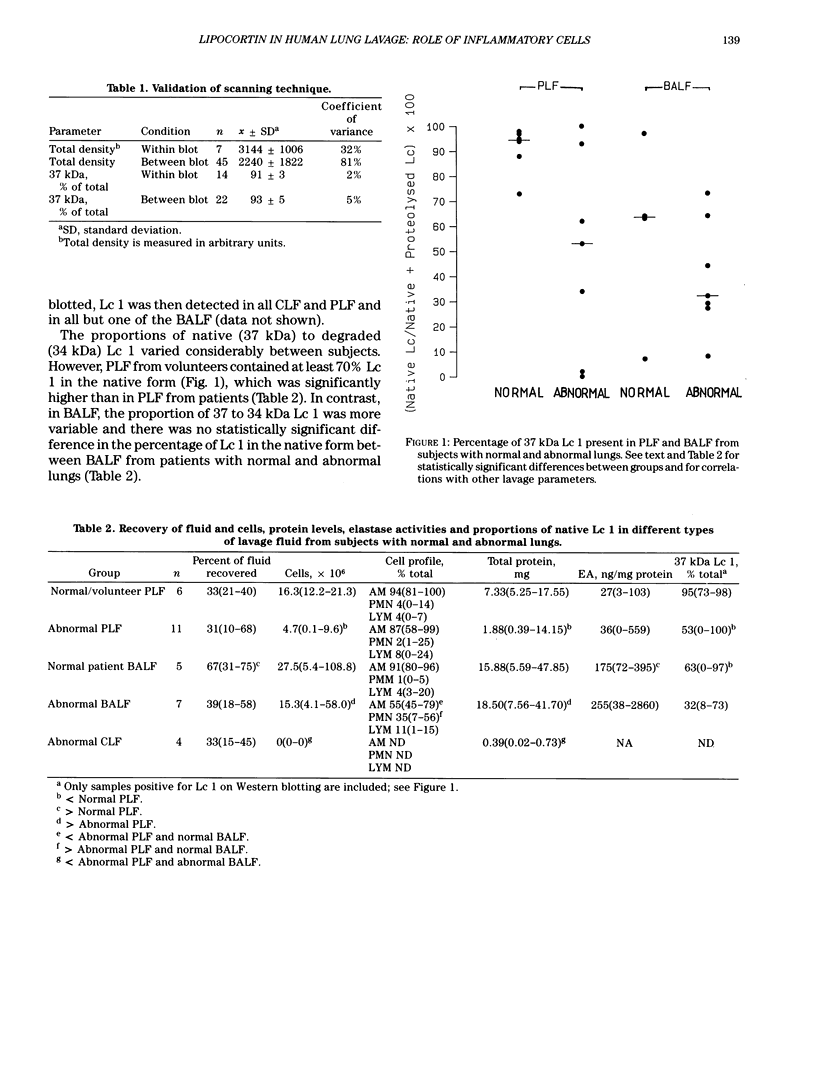
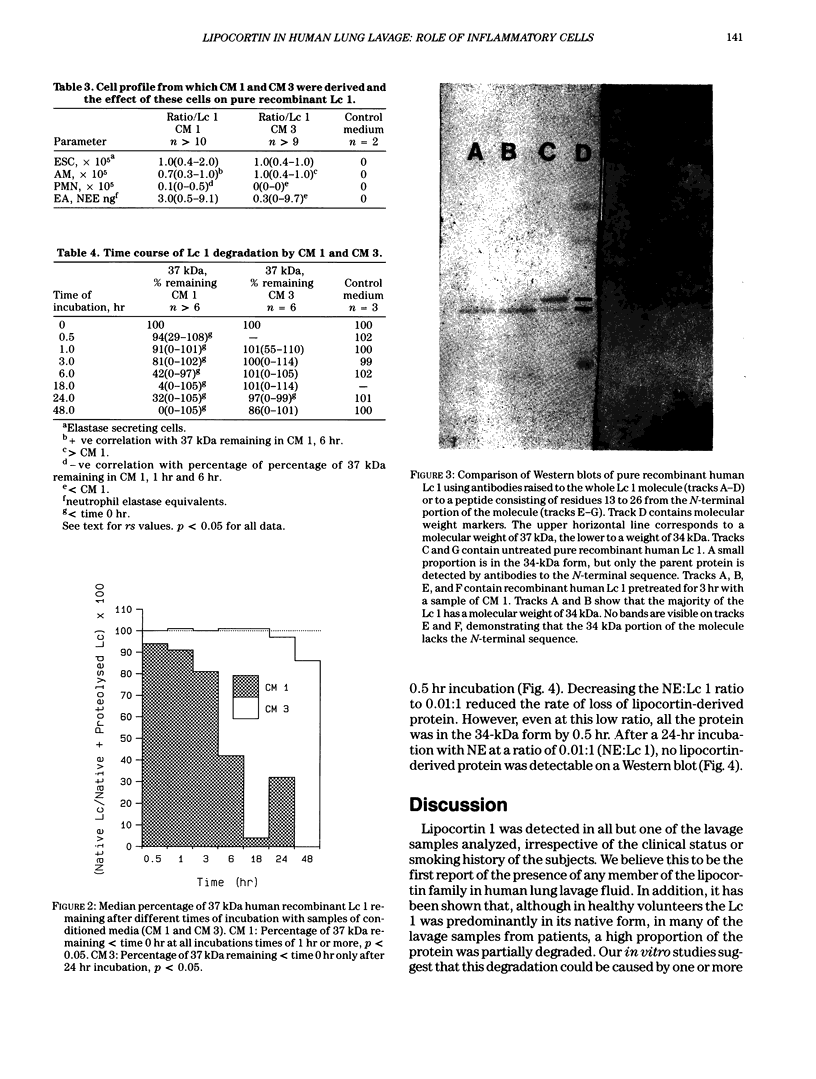
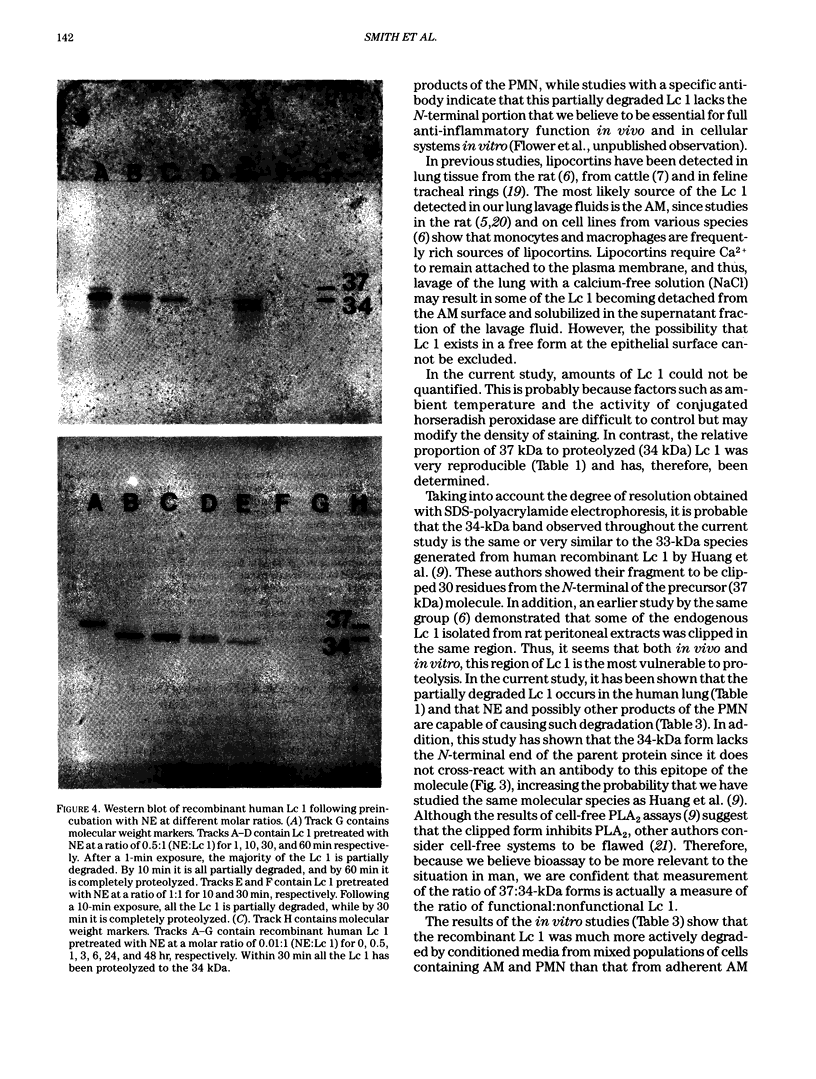
Lipocortins are structurally related, glucocorticoid-inducible proteins that inhibit phospholipase A2 (PLA2), thereby reducing the liberation of arachidonic acid from phospholipids and so limiting the synthesis of eicosanoid inflammatory mediators. This study is the first demonstration of one lipocortin, lipocortin 1 (Lc 1; 37 kDa), in human lung lavage supernatants. In lavage fluid from healthy volunteers, a higher percentage (greater than 70%) of the detected Lc 1 was in its native form, compared to that from patients with abnormal lungs. In patients' lavage fluids, Lc 1 was more likely to be partially degraded (34 kDa). In abnormal bronchoalveolar lavage fluid (BALF), the more polymorphonuclear neutrophils (PMN)/lavage, the lower the proportion of Lc 1 in the native (37 kDa) form (n = 7 pairs, rs = -0.8214, p less than 0.05). Furthermore, when BALF cells were cultured and the harvested conditioned media incubated with pure human recombinant Lc 1, degradation of the 37 kDa form increased with the percentage of PMN (n = 10 pairs, s = -0.7200 after 1 hr; n = 6 pairs, rs = -0.9241 after 6 hr). These results suggest that factors released from the PMN are responsible for Lc 1 degradation in man. When recombinant human Lc 1 was incubated with human neutrophil elastase, the enzyme degraded Lc 1 in a dose-dependent way, suggesting that neutrophil elastase may be one such factor. Since PMNs are ubiquitous at sites of inflammation, it is possible that Lc 1 degradation is a permissive mechanism, which ensures that sufficient inflammation occurs to destroy the provocative stimulus. However, it is equally possible that, in some circumstances, the mechanism may be pathological and that the inactivation of Lc 1 leads to chronic, uncontrolled inflammation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J. Specificity and inhibition of glucocorticoid-induced macrocortin secretion from rat peritoneal macrophages. Br J Pharmacol. 1983 Jun;79(2):587–594. doi: 10.1111/j.1476-5381.1983.tb11033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Errasfa M., Rothhut B., Fradin A., Billardon C., Junien J. L., Bure J., Russo-Marie F. The presence of lipocortin in human embryonic skin fibroblasts and its regulation by anti-inflammatory steroids. Biochim Biophys Acta. 1985 Nov 20;847(2):247–254. doi: 10.1016/0167-4889(85)90027-8. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Background and discovery of lipocortins. Agents Actions. 1986 Jan;17(3-4):255–262. doi: 10.1007/BF01982616. [DOI] [PubMed] [Google Scholar]
- Gurpide E., Markiewicz L., Schatz F., Hirata F. Lipocortin output by human endometrium in vitro. J Clin Endocrinol Metab. 1986 Jul;63(1):162–166. doi: 10.1210/jcem-63-1-162. [DOI] [PubMed] [Google Scholar]
- Hirata F., Notsu Y., Matsuda K., Vasanthakumar G., Schiffmann E., Wong T. W., Goldberg A. R. Inhibition of leukocyte chemotaxis by Glu-Glu-Glu-Glu-Tyr-Pro-Met-Glu and Leu-Ile-Glu-Asp-Asn-Glu-Tyr-Thr-Ala-Arg-Gln-Gly. Biochem Biophys Res Commun. 1984 Jan 30;118(2):682–690. doi: 10.1016/0006-291x(84)91357-3. [DOI] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Huang K. S., McGray P., Mattaliano R. J., Burne C., Chow E. P., Sinclair L. K., Pepinsky R. B. Purification and characterization of proteolytic fragments of lipocortin I that inhibit phospholipase A2. J Biol Chem. 1987 Jun 5;262(16):7639–7645. [PubMed] [Google Scholar]
- Khanna N. C., Tokuda M., Waisman D. M. Purification of three forms of lipocortin from bovine lung. Cell Calcium. 1987 Jun;8(3):217–228. doi: 10.1016/0143-4160(87)90020-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundgren J. D., Hirata F., Marom Z., Logun C., Steel L., Kaliner M., Shelhamer J. Dexamethasone inhibits respiratory glycoconjugate secretion from feline airways in vitro by the induction of lipocortin (lipomodulin) synthesis. Am Rev Respir Dis. 1988 Feb;137(2):353–357. doi: 10.1164/ajrccm/137.2.353. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Niewoehner D. E. Cigarette smoking, lung inflammation, and the development of emphysema. J Lab Clin Med. 1988 Jan;111(1):15–27. [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Browning J. L., Mattaliano R. J., Smart J. E., Chow E. P., Falbel T., Ribolini A., Garwin J. L., Wallner B. P. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J Biol Chem. 1986 Mar 25;261(9):4239–4246. [PubMed] [Google Scholar]
- Sablonniere B., Scharfman A., Lafitte J. J., Laine A., Aerts C., Hayem A. Enzymatic activities of bronchoalveolar lavages in coal workers pneumoconiosis. Lung. 1983;161(4):219–228. doi: 10.1007/BF02713867. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Davidson D. A., Lichtenstein L. M., Adkinson N. F., Jr Selective inhibition of arachidonic acid metabolite release from human lung tissue by antiinflammatory steroids. J Immunol. 1986 Apr 15;136(8):3006–3011. [PubMed] [Google Scholar]
- Smith S. F., Guz A., Cooke N. T., Burton G. H., Tetley T. D. Extracellular elastolytic activity in human lung lavage: a comparative study between smokers and non-smokers. Clin Sci (Lond) 1985 Jul;69(1):17–27. doi: 10.1042/cs0690017. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Tetley T. D., Smith S. F., Burton G. H., Winning A. J., Cooke N. T., Guz A. Effects of cigarette smoking and drugs on respiratory tract proteases and antiproteases. Eur J Respir Dis Suppl. 1987;153:93–102. [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]