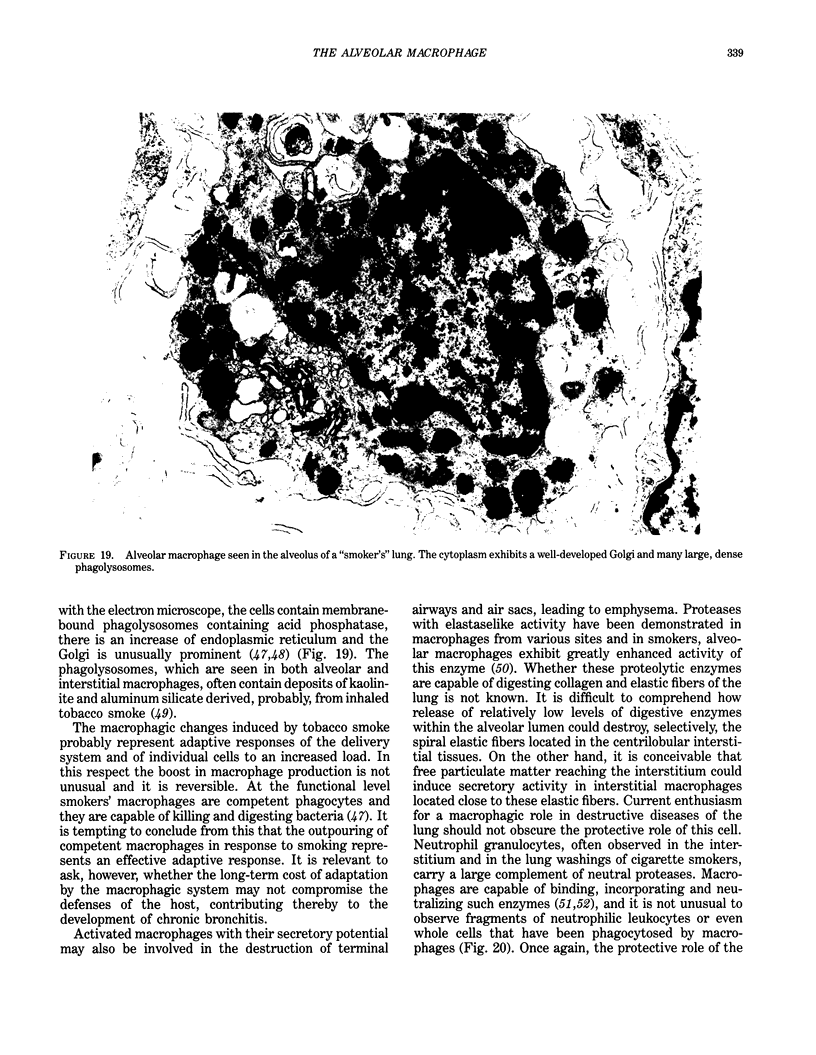
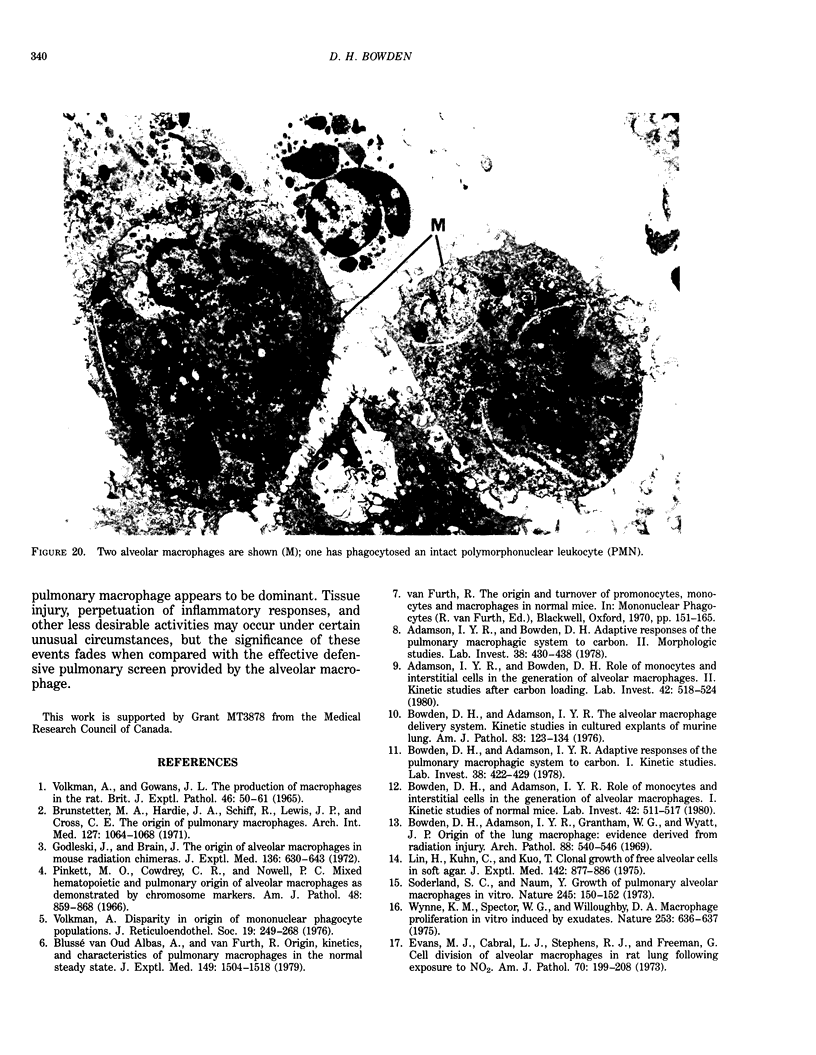
Abstract
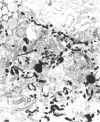
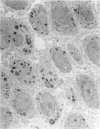
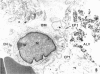
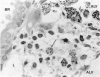
The pulmonary macrophagic system is critical to the defense of the lung, keeping the alveoli clean and sterile and responding on demand with an adaptive outpouring of new cells into the air sacs. Under basal conditions alveolar macrophages, in common with other mononuclear phagocytes, are derived from the bone marrow. A population of macrophage precursors within the pulmonary interstitium provides a reserve pool capable of proliferation and delivery of phagocytes in response to unusually heavy loads of inhaled particles. This reserve system also produces macrophages when monocytic precursors in the bone marrow are depleted by diseases such as leukemia. The alveolar macrophage is destined to ingest particulate matter and to be eliminated along the mucociliary pathway; clearance by lymphatics is of minor importance and macrophages probably do not recross the alveolar epithelium to reach the pulmonary interstitial compartment. Although the protective role of the macrophage is dominant, this cell may participate, directly or indirectly, in the genesis of two major groups of chronic pulmonary disease, interstitial fibrosis and emphysema. Such inappropriate responses involve interactions with fibroblastic cells and tissue injury initiated by proteases secreted by the macrophage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Adaptive responses of the pulmonary macrophagic system to carbon. II. Morphologic studies. Lab Invest. 1978 Apr;38(4):430–438. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975 Jun;32(6):736–745. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981 Aug;2(3):165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Bernstein D. M., Drew R. T., Kuschner M. Experimental approaches for exposure to sized glass fibers. Environ Health Perspect. 1980 Feb;34:47–57. doi: 10.1289/ehp.803447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin J., Rosenberg R., Janoff A. An inhibitor in human lung macrophages active against human neutrophil elastase. Am Rev Respir Dis. 1972 Sep;106(3):477–479. doi: 10.1164/arrd.1972.106.3.477. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Adaptive responses of the pulmonary macrophagic system to carbon. I. Kinetic studies. Lab Invest. 1978 Apr;38(4):422–429. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Alveolar macrophage response to carbon in monocyte-depleted mice. Am Rev Respir Dis. 1982 Oct;126(4):708–711. doi: 10.1164/arrd.1982.126.4.708. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y., Grantham W. G., Wyatt J. P. Origin of the lung macrophage. Evidence derived from radiation injury. Arch Pathol. 1969 Nov;88(5):540–546. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Role of monocytes and interstitial cells in the generation of alveolar macrophages I. Kinetic studies of normal mice. Lab Invest. 1980 May;42(5):511–517. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The alveolar macrophage delivery system. Kinetic studies in cultured explants of murine lung. Am J Pathol. 1976 Apr;83(1):123–134. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Craighead J. E. Cytoplasmic inclusions in pulmonary macrophages of cigarette smokers. Lab Invest. 1975 Feb;32(2):125–132. [PubMed] [Google Scholar]
- Brunstetter M. A., Hardie J. A., Schiff R., Lewis J. P., Cross C. E. The origin of pulmonary alveolar macrophages. Studies of stem cells using the Es-2 marker of mice. Arch Intern Med. 1971 Jun;127(6):1064–1068. [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Curle D. C., Adamson I. Y. Retarded development of noenatal rat lung by maternal malnutrition. J Histochem Cytochem. 1978 May;26(5):401–408. doi: 10.1177/26.5.659840. [DOI] [PubMed] [Google Scholar]
- Dauber J. H., Daniele R. P. Chemotactic activity of guinea pig alveolar macrophages. Am Rev Respir Dis. 1978 Apr;117(4):673–684. doi: 10.1164/arrd.1978.117.4.673. [DOI] [PubMed] [Google Scholar]
- Dauber J. H., Daniele R. P. Secretion of chemotaxins by guinea pig lung macrophages. I. The spectrum of inflammatory cell responses. Exp Lung Res. 1980 Mar;1(1):23–32. doi: 10.3109/01902148009057510. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Cell division of alveolar macrophages in rat lung following exposure to NO2. Am J Pathol. 1973 Feb;70(2):199–208. [PMC free article] [PubMed] [Google Scholar]
- Godleski J. J., Brain J. D. The origin of alveolar macrophages in mouse radiation chimeras. J Exp Med. 1972 Sep 1;136(3):630–643. doi: 10.1084/jem.136.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. The pulmonary macrophage in acute leukemia. N Engl J Med. 1974 Apr 18;290(16):875–878. doi: 10.1056/NEJM197404182901603. [DOI] [PubMed] [Google Scholar]
- HEISE E. R., MYRVIK Q. N., LEAKE E. S. EFFECT OF BACILLUS CALMETTE-GU'ERIN ON THE LEVELS OF ACID PHOSPHATASE, LYSOZYME AND CATHEPSIN IN RABBIT ALVEOLAR MACROPHAGES. J Immunol. 1965 Jul;95:125–130. [PubMed] [Google Scholar]
- Harris J. O., Olsen G. N., Castle J. R., Maloney A. S. Comparison of proteolytic enzyme activity in pulmonary alveolar macrophages and blood leukocytes in smokers and nonsmokers. Am Rev Respir Dis. 1975 May;111(5):579–586. doi: 10.1164/arrd.1975.111.5.579. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Kilburn K. H. A hypothesis for pulmonary clearance and its implications. Am Rev Respir Dis. 1968 Sep;98(3):449–463. doi: 10.1164/arrd.1968.98.3.449. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Kuhn C., Kuo T. Clonal growth of hamster free alveolar cells in soft agar. J Exp Med. 1975 Oct 1;142(4):877–886. doi: 10.1084/jem.142.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas N. G., O'Neill C. H., Stanton M. F. Fibroblast anchorage in carcinogenesis by fibres. Lancet. 1973 Apr 14;1(7807):807–809. doi: 10.1016/s0140-6736(73)90604-1. [DOI] [PubMed] [Google Scholar]
- Nerurkar L. S., Zeligs B. J., Bellanti J. A. Maturation of the rabbit alveolar macrophage during animal development. II. Biochemical and enzymatic studies. Pediatr Res. 1977 Dec;11(12):1202–1207. doi: 10.1203/00006450-197712000-00007. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Participation of mononuclear phagocytes in chronic inflammatory diseases. J Reticuloendothel Soc. 1974 May;15(5):413–438. [PubMed] [Google Scholar]
- Pinkett M. O., Cowdrey C. R., Nowell P. C. Mixed hematopoietic and pulmonary origin of 'alveolar macrophages' as demonstrated by chromosome markers. Am J Pathol. 1966 May;48(5):859–867. [PMC free article] [PubMed] [Google Scholar]
- Pratt S. A., Smith M. H., Ladman A. J., Finley T. N. The ultrastructure of alveolar macrophages from human cigarette smokers and nonsmokers. Lab Invest. 1971 May;24(5):331–338. [PubMed] [Google Scholar]
- Privalova L. I., Katsnelson B. A., Osipenko A. B., Yushkov B. N., Babushkina L. G. Response of a phagocyte cell system to products of macrophage breakdown as a probable mechanism of alveolar phagocytosis adaptation to deposition of particles of different cytotoxicity. Environ Health Perspect. 1980 Apr;35:205–218. doi: 10.1289/ehp.8035205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderland S. C., Naum Y. Letter: Growth of pulmonary alveolar macrophages in vitro. Nature. 1973 Sep 21;245(5421):150–152. doi: 10.1038/245150a0. [DOI] [PubMed] [Google Scholar]
- Spector W. G. Pulmonary fibrosis due to chemicals and particles. Ann N Y Acad Sci. 1974;221:309–311. doi: 10.1111/j.1749-6632.1974.tb28230.x. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 1972 Mar;48(3):797–821. [PubMed] [Google Scholar]
- Suzuki Y., Churg J. Structure and development of the asbestos body. Am J Pathol. 1969 Apr;55(1):79–107. [PMC free article] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE PRODUCTION OF MACROPHAGES IN THE RAT. Br J Exp Pathol. 1965 Feb;46:50–61. [PMC free article] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne K. M., Spector W. G., Willoughby D. A. Macrophage proliferation in vitro induced by exudates. Nature. 1975 Feb 20;253(5493):636–637. doi: 10.1038/253636a0. [DOI] [PubMed] [Google Scholar]
- Zeligs B. J., Nerurkar L. S., Bellanti J. A., Zeligs J. D. Maturation of the rabbit alveolar macrophage during animal development. I. Perinatal influx into alveoli and ultrastructural differentiation. Pediatr Res. 1977 Mar;11(3 Pt 1):197–208. doi: 10.1203/00006450-197703000-00011. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]