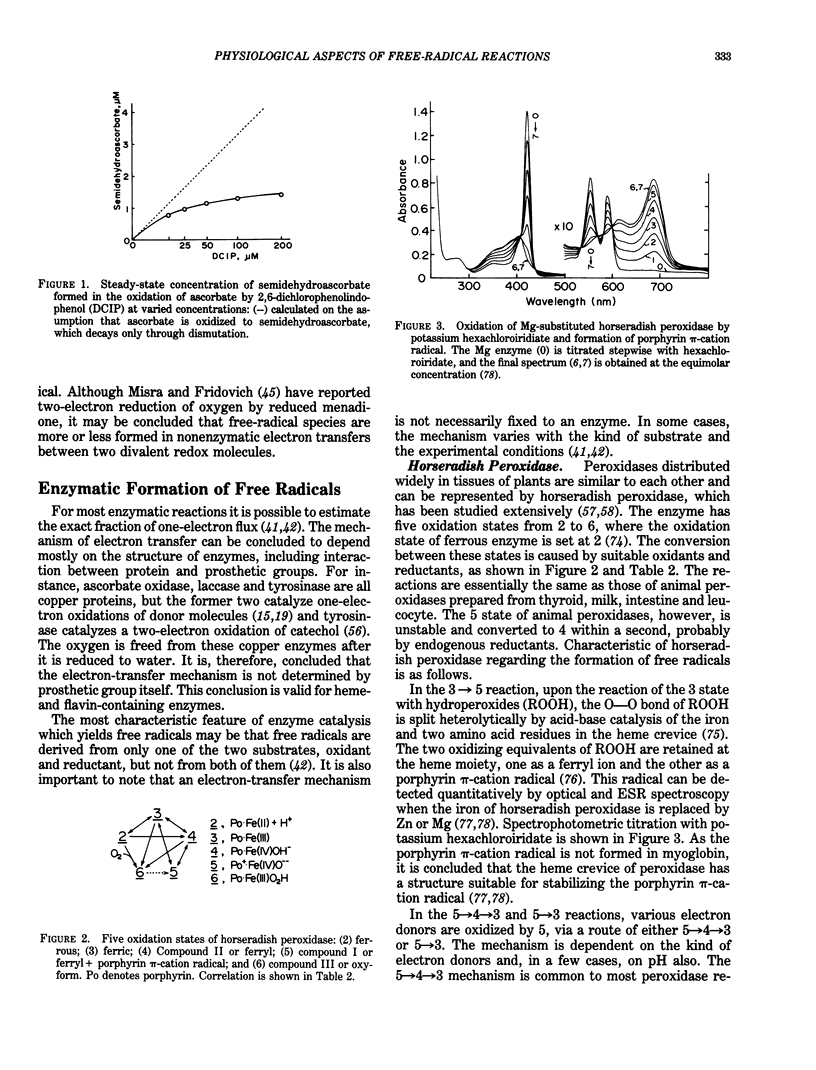
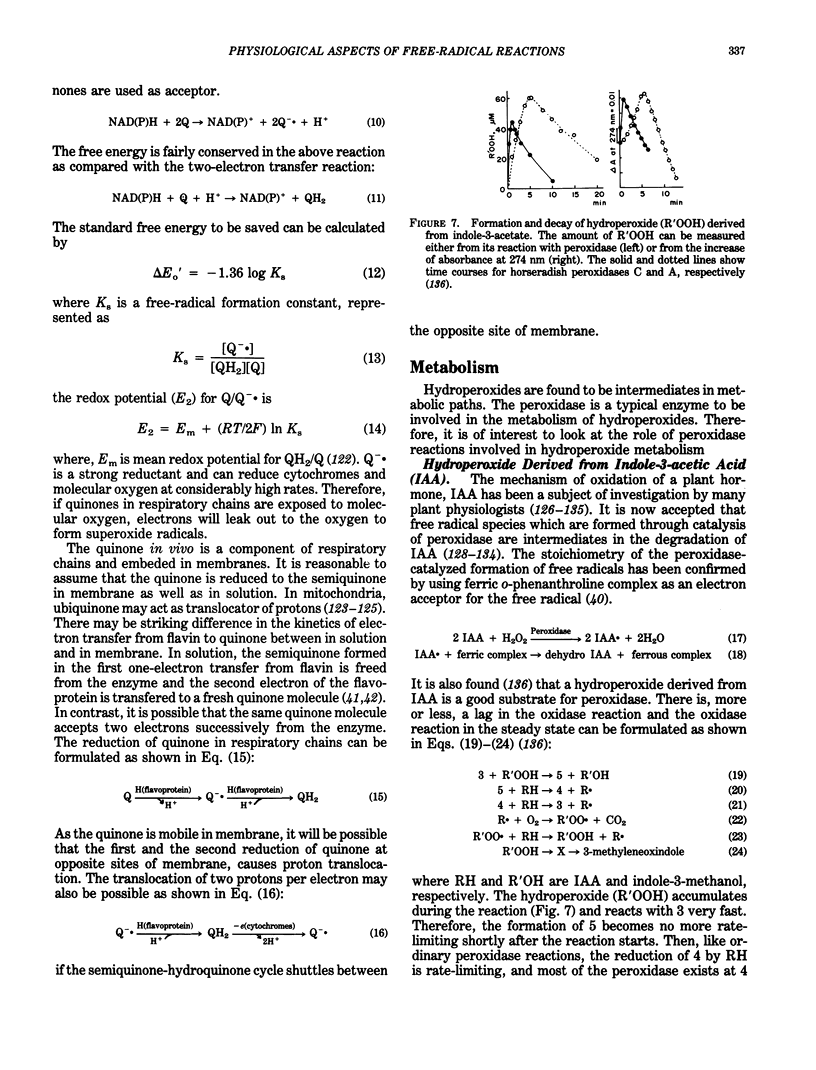
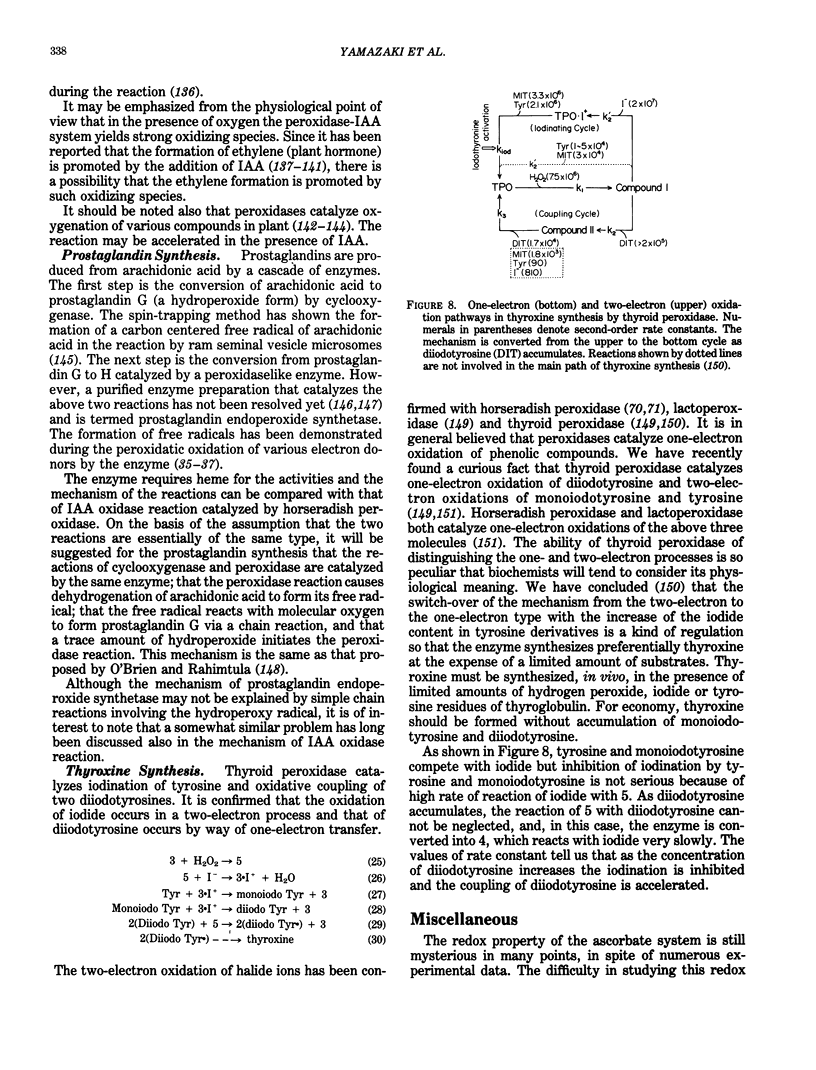
Abstract
Enzymes which catalyze the formation of free radicals in vitro will catalyze similar reactions in vivo. We believe that the formation of some kinds of free radicals has definite physiological meanings in metabolism. In this sense, the enzymes forming such free radicals are concluded to be in evolutionally advanced states. Elaborated structure and function of enzymes such as horseradish peroxidase and microsomal flavoproteins support the idea. Deleterious and side reactions caused by free radicals are assumed to be minimized in vivo by localizing the reactions, but this assumption should be verified by future studies.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Yang S. F. Auxin-induced ethylene production as related to auxin metabolism in leaf discs of tobacco and sugar beet. Plant Physiol. 1983 Nov;73(3):598–604. doi: 10.1104/pp.73.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araiso T., Miyoshi K., Yamazaki I. Mechanisms of electron transfer from sulfite to horseradish peroxidase-hydroperoxide compounds. Biochemistry. 1976 Jul 13;15(14):3059–3063. doi: 10.1021/bi00659a019. [DOI] [PubMed] [Google Scholar]
- Ashley P. L., Griffin B. W. Involvement of radical species in the oxidation of aminopyrine and 4-aminoantipyrine by cumene hydroperoxide in rat liver microsomes. Mol Pharmacol. 1981 Jan;19(1):146–152. [PubMed] [Google Scholar]
- Augusto O., Beilan H. S., Ortiz de Montellano P. R. The catalytic mechanism of cytochrome P-450. Spin-trapping evidence for one-electron substrate oxidation. J Biol Chem. 1982 Oct 10;257(19):11288–11295. [PubMed] [Google Scholar]
- Ballou D., Palmer G., Massey V. Direct demonstration of superoxide anion production during the oxidation of reduced flavin and of its catalytic decomposition by erythrocuprein. Biochem Biophys Res Commun. 1969 Sep 10;36(6):898–904. doi: 10.1016/0006-291x(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bernhardt F. H., Kuthan H. Dioxygen activation by putidamonooxin. The oxygen species formed and released under uncoupling conditions. Eur J Biochem. 1981 Dec;120(3):547–555. doi: 10.1111/j.1432-1033.1981.tb05735.x. [DOI] [PubMed] [Google Scholar]
- Björkstén F. The horseradish peroxidase-catalyzed oxidation of iodide. Outline of the mechanism. Biochim Biophys Acta. 1970 Sep 16;212(3):396–406. doi: 10.1016/0005-2744(70)90245-7. [DOI] [PubMed] [Google Scholar]
- Blake R. C., 2nd, Coon M. J. On the mechanism of action of cytochrome P-450. Evaluation of homolytic and heterolytic mechanisms of oxygen-oxygen bond cleavage during substrate hydroxylation by peroxides. J Biol Chem. 1981 Dec 10;256(23):12127–12133. [PubMed] [Google Scholar]
- Blankenhorn G. Nicotinamide-dependent one-electron and two-electron (flavin) oxidoreduction: thermodynamics, kinetics, and mechanism. Eur J Biochem. 1976 Aug 1;67(1):67–80. doi: 10.1111/j.1432-1033.1976.tb10634.x. [DOI] [PubMed] [Google Scholar]
- Bors W., Michel C., Saran M., Lengfelder E. Kinetic investigations of the autoxidation of adrenalin. Z Naturforsch C. 1978 Nov-Dec;33(11-12):891–896. doi: 10.1515/znc-1978-11-1215. [DOI] [PubMed] [Google Scholar]
- Bors W., Michel C., Saran M., Lengfelder E. The involvement of oxygen radicals during the autoxidation of adrenalin. Biochim Biophys Acta. 1978 Apr 19;540(1):162–172. doi: 10.1016/0304-4165(78)90445-2. [DOI] [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Michel C., Fuchs C., Frenzel C. Detection of oxygen radicals in biological reactions. Photochem Photobiol. 1978 Oct-Nov;28(4-5):629–638. doi: 10.1111/j.1751-1097.1978.tb06982.x. [DOI] [PubMed] [Google Scholar]
- Boyd J. A., Harvan D. J., Eling T. E. The oxidation of 2-aminofluorene by prostaglandin endoperoxide synthetase. Comparison with other peroxidases. J Biol Chem. 1983 Jul 10;258(13):8246–8254. [PubMed] [Google Scholar]
- Caldwell E. S., Steelink C. Phenoxy radical intermediates in the enzymatic degradation of lignin model compounds. Biochim Biophys Acta. 1969 Jul 30;184(2):420–431. doi: 10.1016/0304-4165(69)90046-4. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Cohen G. Effect of hydroxyl radical scavengers on microsomal oxidation of alcohols and on associated microsomal reactions. Biochemistry. 1978 Jul 25;17(15):3058–3064. doi: 10.1021/bi00608a018. [DOI] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Microsomal metabolism of hydroxyl radical scavenging agents: relationship to the microsomal oxidation of alcohols. Arch Biochem Biophys. 1980 Feb;199(2):438–447. doi: 10.1016/0003-9861(80)90300-8. [DOI] [PubMed] [Google Scholar]
- De Vries S., Albracht S. P., Berden J. A., Slater E. C. The pathway of electrons through OH2:cytochrome c oxidoreductase studied by pre-steady -state kinetics. Biochim Biophys Acta. 1982 Jul 22;681(1):41–53. doi: 10.1016/0005-2728(82)90276-6. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Dean G., Carter C., Allen P. L. Tissue, subcellular, and submitochondrial distributions of semidehydroascorbate reductase: possible role of semidehydroascorbate reductase in cofactor regeneration. J Neurochem. 1982 Aug;39(2):563–568. doi: 10.1111/j.1471-4159.1982.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Dolphin D., Forman A., Borg D. C., Fajer J., Felton R. H. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971 Mar;68(3):614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré S., Federici G., Santoro L., Rossi Fanelli M. R., Cavallini D. The involvement of superoxide anions in the autoxidation of various cofactors of cysteamine-oxygenase. Mol Cell Biochem. 1975 Dec 31;9(3):149–154. doi: 10.1007/BF01751310. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Baptista E. M., Kennicott K. L., VandenHeuvel W. J., Walker R. W., Fagerness P. E., Kuehl F. A., Jr Oxidation reactions by prostaglandin cyclooxygenase-hydroperoxidase. J Biol Chem. 1981 Jul 25;256(14):7352–7361. [PubMed] [Google Scholar]
- Feierman D. E., Cederbaum A. I. The effect of EDTA and iron on the oxidation of hydroxyl radical scavenging agents and ethanol by rat liver microsomes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):765–770. doi: 10.1016/0006-291x(83)90590-9. [DOI] [PubMed] [Google Scholar]
- Fox L. R., Purves W. K., Nakada H. I. The role of horseradish peroxidase in indole-3-acetic acid oxidation. Biochemistry. 1965 Dec;4(12):2754–2763. doi: 10.1021/bi00888a028. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- GEORGE P. The third intermediate compound of horseradish peroxidase and hydrogen peroxide. J Biol Chem. 1953 Mar;201(1):427–434. [PubMed] [Google Scholar]
- Gelinas D. A. Proposed Model for the Peroxidase-Catalyzed Oxidation of Indole-3-acetic Acid in the Presence of the Inhibitor Ferulic Acid. Plant Physiol. 1973 May;51(5):967–972. doi: 10.1104/pp.51.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. W., Marth C., Yasukochi Y., Masters B. S. Radical mechanism of aminopyrine oxidation by cumene hydroperoxide catalyzed by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1980 Dec;205(2):543–553. doi: 10.1016/0003-9861(80)90137-x. [DOI] [PubMed] [Google Scholar]
- Griffin B. W., Ting P. L. Mechanism of N-demethylation of aminopyrine by hydrogen peroxide catalyzed by horseradish peroxidase, metmyoglobin, and protohemin. Biochemistry. 1978 May 30;17(11):2206–2211. doi: 10.1021/bi00604a029. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Ehrenberg A., Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem. 1982 May 25;257(10):5711–5715. [PubMed] [Google Scholar]
- Gutman M. Electron flux through the mitochondrial ubiquinone. Biochim Biophys Acta. 1980 Dec 22;594(1):53–84. doi: 10.1016/0304-4173(80)90013-0. [DOI] [PubMed] [Google Scholar]
- HINMAN R. L., LANG J. PEROXIDASE-CATALYZED OXIDATION OF INDOLE-3-ACETIC ACID. Biochemistry. 1965 Jan;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Lindenau D., Tamura M. A pulse-radiolysis study on active oxygen. Adv Exp Med Biol. 1977 Jul 4;94:353–359. doi: 10.1007/978-1-4684-8890-6_46. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Yamazaki I. The oxidation-reduction potentials of compound I/compound II and compound II/ferric couples of horseradish peroxidases A2 and C. J Biol Chem. 1979 Sep 25;254(18):9101–9106. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. J Biol Chem. 1971 Dec 25;246(24):7825–7826. [PubMed] [Google Scholar]
- Hlavica P., Golly I., Mietaschk J. Comparative studies on the cumene hydroperoxide- and NADPH-supported N-oxidation of 4-chloroaniline by cytochrome P-450. Biochem J. 1983 Jun 15;212(3):539–547. doi: 10.1042/bj2120539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg P. F., Hager L. P. The P-450 nature of the carbon monoxide complex of ferrous chloroperoxidase. J Biol Chem. 1973 Apr 10;248(7):2630–2633. [PubMed] [Google Scholar]
- Hrycay E. G., Gustafsson J. A., Ingelman-Sundberg M., Ernster L. The involvement of cytochrome P-450 in hepatic microsomal steroid hydroxylation reactions supported by sodium periodate, sodium chlorite, and organic hydroperoxides. Eur J Biochem. 1976 Jan 2;61(1):43–52. doi: 10.1111/j.1432-1033.1976.tb09995.x. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971 Nov;147(1):14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Ekström G. Aniline is hydroxylated by the cytochrome P-450-dependent hydroxyl radical-mediated oxygenation mechanism. Biochem Biophys Res Commun. 1982 May 31;106(2):625–631. doi: 10.1016/0006-291x(82)91156-1. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes P-450. J Biol Chem. 1984 May 25;259(10):6447–6458. [PubMed] [Google Scholar]
- Iyanagi T., Makino N., Mason H. S. Redox properties of the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 and reduced nicotinamide adenine dinucleotide-cytochrome b5 reductases. Biochemistry. 1974 Apr 9;13(8):1701–1710. doi: 10.1021/bi00705a023. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Mason H. S. Some properties of hepatic reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochemistry. 1973 Jun 5;12(12):2297–2308. doi: 10.1021/bi00736a018. [DOI] [PubMed] [Google Scholar]
- Iyanagi T. Redox properties of microsomal reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase and cytochrome b5. Biochemistry. 1977 Jun 14;16(12):2725–2730. doi: 10.1021/bi00631a021. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. 3. One-electron reduction of quinones by microsomal flavin enzymes. Biochim Biophys Acta. 1969 Apr 8;172(3):370–381. doi: 10.1016/0005-2728(69)90133-9. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Johansson I., Ingelman-Sundberg M. Hydroxyl radical-mediated, cytochrome P-450-dependent metabolic activation of benzene in microsomes and reconstituted enzyme systems from rabbit liver. J Biol Chem. 1983 Jun 25;258(12):7311–7316. [PubMed] [Google Scholar]
- Josephy P. D., Eling T., Mason R. P. The horseradish peroxidase-catalyzed oxidation of 3,5,3',5'-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J Biol Chem. 1982 Apr 10;257(7):3669–3675. [PubMed] [Google Scholar]
- Josephy P. D., Mason R. P., Eling T. Cooxidation of the clinical reagent 3,5,3'5'-tetramethylbenzidine by prostaglandin synthase. Cancer Res. 1982 Jul;42(7):2567–2570. [PubMed] [Google Scholar]
- Kalyanaraman B., Felix C. C., Sealy R. C. Electron spin resonance-spin stabilization of semiquinones produced during oxidation of epinephrine and its analogues. J Biol Chem. 1984 Jan 10;259(1):354–358. [PubMed] [Google Scholar]
- Kalyanaraman B., Felix C. C., Sealy R. C. Peroxidatic oxidation of catecholamines. A kinetic electron spin resonance investigation using the spin stabilization approach. J Biol Chem. 1984 Jun 25;259(12):7584–7589. [PubMed] [Google Scholar]
- Kaneko Y., Tamura M., Yamazaki I. Formation of porphyrin pi cation radical in zinc-substituted horseradish peroxidase. Biochemistry. 1980 Dec 9;19(25):5795–5799. doi: 10.1021/bi00566a020. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Gibson J. F., Pick F. M., Bray R. C. Electron-spin-resonance evidence for enzymic reduction of oxygen to a free radical, the superoxide ion. Biochem J. 1969 Jan;111(1):53–58. doi: 10.1042/bj1110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Hayashi K. One-electron reduction in oxyform of hemoproteins. J Biol Chem. 1981 Dec 10;256(23):12350–12354. [PubMed] [Google Scholar]
- Kokkinakis D. M., Brooks J. L. Hydrogen Peroxide-mediated Oxidation of Indole-3-acetic Acid by Tomato Peroxidase and Molecular Oxygen. Plant Physiol. 1979 Aug;64(2):220–223. doi: 10.1104/pp.64.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem. 1982 Sep 1;126(3):583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- Kuwahara Y., Tamura M., Yamazaki I. The reactivity of Mg-substituted horseradish peroxidases. J Biol Chem. 1982 Oct 10;257(19):11517–11522. [PubMed] [Google Scholar]
- Lasker J. M., Sivarajah K., Mason R. P., Kalyanaraman B., Abou-Donia M. B., Eling T. E. A free radical mechanism of prostaglandin synthase-dependent aminopyrine demethylation. J Biol Chem. 1981 Aug 10;256(15):7764–7767. [PubMed] [Google Scholar]
- Lichtenberger F., Nastainczyk W., Ullrich V. Cytochrome P450 as an oxene transferase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):939–946. doi: 10.1016/0006-291x(76)90682-3. [DOI] [PubMed] [Google Scholar]
- MARRE E., ARRIGONI O. Ascorbic acid and photosynthesis. I. Monodehydroascorbic acid reductase of chloroplasts. Biochim Biophys Acta. 1958 Dec;30(3):453–457. doi: 10.1016/0006-3002(58)90089-1. [DOI] [PubMed] [Google Scholar]
- MASON H. S., SPENCER E., YAMAZAKI I. Identification by electron spin resonance spectroscopy of the primary product of tyrosinase-catalyzed catechol oxidation. Biochem Biophys Res Commun. 1961 Mar 10;4:236–238. doi: 10.1016/0006-291x(61)90278-9. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Marklund S., Ohlsson P. I., Opara A., Paul K. G. The substrate profiles of the acidic and slightly basic horseradish peroxidases. Biochim Biophys Acta. 1974 Jun 18;350(2):304–313. doi: 10.1016/0005-2744(74)90504-x. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Siedlik P. H., Fung L. W. Oxidation of phenidone and BW755C by prostaglandin endoperoxide synthetase. J Biol Chem. 1982 Jun 25;257(12):6957–6964. [PubMed] [Google Scholar]
- Mason R. P., Kalyanaraman B., Tainer B. E., Eling T. E. A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. Spin trapping and oxygen uptake studies. J Biol Chem. 1980 Jun 10;255(11):5019–5022. [PubMed] [Google Scholar]
- McCarthy M. B., White R. E. Competing modes of peroxyacid flux through cytochrome P-450. J Biol Chem. 1983 Oct 10;258(19):11610–11616. [PubMed] [Google Scholar]
- McCarthy M. B., White R. E. Functional differences between peroxidase compound I and the cytochrome P-450 reactive oxygen intermediate. J Biol Chem. 1983 Aug 10;258(15):9153–9158. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Mottley C., Trice T. B., Mason R. P. Direct detection of the sulfur trioxide radical anion during the horseradish peroxidase-hydrogen peroxide oxidation of sulfite (aqueous sulfur dioxide). Mol Pharmacol. 1982 Nov;22(3):732–737. [PubMed] [Google Scholar]
- Nakajima R., Yamazaki I. The mechanism of indole-3-acetic acid oxidation by horseradish peroxidases. J Biol Chem. 1979 Feb 10;254(3):872–878. [PubMed] [Google Scholar]
- Nakamura M., Kurebayashi H., Yamazaki I. One-electron and two-electron reductions of acceptors by xanthine oxidase and xanthine dehydrogenase. J Biochem. 1978 Jan;83(1):9–17. doi: 10.1093/oxfordjournals.jbchem.a131916. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I., Nakagawa H., Ohtaki S. Steady state kinetics and regulation of thyroid peroxidase-catalyzed iodination. J Biol Chem. 1983 Mar 25;258(6):3837–3842. [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I. One-electron transfer reactions in biochemical systems. VI. Changes in electron transfer mechanism of lipoamide dehydrogenase by modification of sulfhydryl groups. Biochim Biophys Acta. 1972 May 25;267(2):249–257. doi: 10.1016/0005-2728(72)90113-2. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I. One-electron transfer reactions in biochemical systems. VII. Two types of electron outlets in milk xanthine oxidase. Biochim Biophys Acta. 1973 Dec 19;327(2):247–256. doi: 10.1016/0005-2744(73)90407-5. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I. Preparation of bovine milk xanthine oxidase as a dehydrogenase form. J Biochem. 1982 Oct;92(4):1279–1286. doi: 10.1093/oxfordjournals.jbchem.a134046. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yamazaki I. One-electron transfer reactions in biochemical systems. IV. A mixed mechanism in the reaction of milk xanthine oxidase with electron acceptors. Biochim Biophys Acta. 1969 Sep 16;189(1):29–37. doi: 10.1016/0005-2728(69)90221-7. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Appaji N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972 Jan 31;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Gibson Q. H. The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J Biol Chem. 1970 May 10;245(9):2409–2413. [PubMed] [Google Scholar]
- O'Brien P. J., Rahimtula A. The possible involvement of a peroxidase in prostaglandin biosynthesis. Biochem Biophys Res Commun. 1976 Jun 7;70(3):832–838. doi: 10.1016/0006-291x(76)90667-7. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloneperoxidase of the leukocyte of normal blood. 3. The reaction of ferric myeloperoxidase with superoxide anion. Biochim Biophys Acta. 1972 Oct 12;284(2):355–359. doi: 10.1016/0005-2744(72)90130-1. [DOI] [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Ohnishi T., Yamazaki H., Iyanagi T., Nakamura T., Yamazaki I. One-electron-transfer reactions in biochemical systems. II. The reaction of free radicals formed in the enzymic oxidation. Biochim Biophys Acta. 1969 Apr 8;172(3):357–369. doi: 10.1016/0005-2728(69)90132-7. [DOI] [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Kimura S., Yamazaki I. Analyses of catalytic intermediates of hog thyroid peroxidase during its iodinating reaction. J Biol Chem. 1981 Jan 25;256(2):805–810. [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Nakamura M., Yamazaki I. One- and two-electron oxidations of tyrosine, monoiodotyrosine, and diiodotyrosine catalyzed by hog thyroid peroxidase. J Biol Chem. 1982 Nov 25;257(22):13398–13403. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Beilan H. S., Wheeler C. Destruction of cytochrome P-450 by vinyl fluoride, fluroxene, and acetylene. Evidence for a radical intermediate in olefin oxidation. Biochemistry. 1982 Mar 16;21(6):1331–1339. doi: 10.1021/bi00535a035. [DOI] [PubMed] [Google Scholar]
- PIETTE L. H., BULOW G., YAMAZAKI I. ELECTRON-PARAMAGNETIC-RESONANCE STUDIES OF THE CHLORPROMAZINE FREE RADICAL FORMED DURING ENZYMIC OXIDATION BY PEROXIDASE-HYDROGEN PEROXIDE. Biochim Biophys Acta. 1964 Jul 29;88:120–129. doi: 10.1016/0926-6577(64)90160-3. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Mason R. P. Characterization of the structure and reactions of free radicals from serotonin and related indoles. J Biol Chem. 1981 Mar 10;256(5):2427–2432. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J., Hrycay E. G., Peterson J. A., Estabrook R. W. Possible higher valence states of cytochrome P-450 during oxidative reactions. Biochem Biophys Res Commun. 1974 Sep 23;60(2):695–702. doi: 10.1016/0006-291x(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Rathmell W. G., Bendall D. S. The peroxidase-catalysed oxidation of a chalcone and its possible physiological significance. Biochem J. 1972 Mar;127(1):125–132. doi: 10.1042/bj1270125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneberg R., Damerau W., Jung C., Ebert B., Scheller F. Study of H2O2-supported N-demethylations catalyzed by cytochrome P-450 and horseradish peroxidase. Biochem Biophys Res Commun. 1983 May 31;113(1):332–339. doi: 10.1016/0006-291x(83)90470-9. [DOI] [PubMed] [Google Scholar]
- Renneberg R., Scheller F., Ruckpaul K., Pirrwitz J., Mohr P. NADPH and H2O2-dependent reactions of cytochrome P-450LM compared with peroxidase catalysis. FEBS Lett. 1978 Dec 15;96(2):349–353. doi: 10.1016/0014-5793(78)80434-7. [DOI] [PubMed] [Google Scholar]
- Ricard J., Job D. Reaction mechanisms of indole-3-acetate degradation by peroxidases. A stopped-flow and low-temperature spectroscopic study. Eur J Biochem. 1974 May 15;44(2):359–374. doi: 10.1111/j.1432-1033.1974.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Roman R., Dumbord H. B. pH dependence of the oxidation of iodide by compound I of horseradish peroxidase. Biochemistry. 1972 May 23;11(11):2076–2082. doi: 10.1021/bi00761a013. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W., STAUDINGER H. REDUCED NICOTINAMIDE-ADENINE DINUCLEOTIDE-DEPENDENT REDUCTION OF SEMIDEHYDROASCORBIC ACID. Biochim Biophys Acta. 1965 Jan;96:157–159. doi: 10.1016/0005-2787(65)90620-9. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Iyanagi T., Yamazaki I. Relation between redox potentials and rate constants in reactions coupled with the system oxygen-superoxide. Biochemistry. 1975 Aug 26;14(17):3761–3764. doi: 10.1021/bi00688a007. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Yamazaki I. One-electron transfer reactions in biochemical systems. 8. Kinetic study of superoxide dismutase. Biochim Biophys Acta. 1973 Dec 19;327(2):257–265. doi: 10.1016/0005-2744(73)90408-7. [DOI] [PubMed] [Google Scholar]
- Scarpa M., Stevanato R., Viglino P., Rigo A. Superoxide ion as active intermediate in the autoxidation of ascorbate by molecular oxygen. Effect of superoxide dismutase. J Biol Chem. 1983 Jun 10;258(11):6695–6697. [PubMed] [Google Scholar]
- Schonbaum G. R., Lo S. Interaction of peroxidases with aromatic peracids and alkyl peroxides. Product analysis. J Biol Chem. 1972 May 25;247(10):3353–3360. [PubMed] [Google Scholar]
- Schulze H. U., Schott H. H., Staudinger H. Isolierung und Charakterisierung einer NADH: Semidehydroascorbinsäure-Oxidoreduktase aus Neurospora crassa. Hoppe Seylers Z Physiol Chem. 1972 Dec;353(12):1931–1942. [PubMed] [Google Scholar]
- Shiga T., Imaizumi K. Electron spin resonance study on peroxidase- and oxidase-reactions of horse radish peroxidase and methemoglobin. Arch Biochem Biophys. 1975 Apr;167(2):469–479. doi: 10.1016/0003-9861(75)90489-0. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Kobayashi K., Hayashi K. The reaction of superoxide radical with catalase. Mechanism of the inhibition of catalase by superoxide radical. J Biol Chem. 1984 Apr 10;259(7):4414–4418. [PubMed] [Google Scholar]
- Sinha B. K. Irreversible binding of quinacrine to nucleic acids during horseradish peroxidase- and prostaglandin synthetase-catalyzed oxidation. Biochem Pharmacol. 1983 Sep 1;32(17):2604–2607. doi: 10.1016/0006-2952(83)90028-x. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Gräslund A., Eckstein F. A substrate radical intermediate in the reaction between ribonucleotide reductase from Escherichia coli and 2'-azido-2'-deoxynucleoside diphosphates. J Biol Chem. 1983 Jul 10;258(13):8060–8067. [PubMed] [Google Scholar]
- Skotland T., Ljones T. Direct spectrophotometric detection of ascorbate free radical formed by dopamine beta-monooxygenase and by ascorbate oxidase. Biochim Biophys Acta. 1980 Jun 5;630(1):30–35. doi: 10.1016/0304-4165(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Smith A. M., Morrison W. L., Milham P. J. Oxidation of indole-3-acetic acid by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry. 1982 Aug 31;21(18):4414–4419. doi: 10.1021/bi00261a034. [DOI] [PubMed] [Google Scholar]
- Tamura M., Yamazaki I. Reactions of the oxyform of horseradish peroxidase. J Biochem. 1972 Feb;71(2):311–319. doi: 10.1093/oxfordjournals.jbchem.a129768. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Sono M., Hirata F., Hayaishi O., Tamura M., Hayashi K., Iizuka T., Ishimura Y. Indoleamine 2,3-dioxygenase. Kinetic studies on the binding of superoxide anion and molecular oxygen to enzyme. J Biol Chem. 1979 May 10;254(9):3288–3294. [PubMed] [Google Scholar]
- White R. E., Sligar S. G., Coon M. J. Evidence for a homolytic mechanism of peroxide oxygen--oxygen bond cleavage during substrate hydroxylation by cytochrome P-450. J Biol Chem. 1980 Dec 10;255(23):11108–11111. [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. I. Oxidation of hydroxyl radical scavenging agents. J Biol Chem. 1983 Feb 10;258(3):1508–1513. [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. II. Role in microsomal oxidation of ethanol. J Biol Chem. 1983 Feb 10;258(3):1514–1519. [PubMed] [Google Scholar]
- Wittenberg J. B., Noble R. W., Wittenberg B. A., Antonini E., Brunori M., Wyman J. Studies on the equilibria and kinetics of the reactions of peroxidase with ligands. II. The reaction of ferroperoxidase with oxygen. J Biol Chem. 1967 Feb 25;242(4):626–634. [PubMed] [Google Scholar]
- YAMAZAKI I., MASON H. S., PIETTE L. Identification, by electron paramagnetic resonance spectroscopy, of free radicals generated from substrates by peroxidase. J Biol Chem. 1960 Aug;235:2444–2449. [PubMed] [Google Scholar]
- YAMAZAKI I., PIETTE L. H. Mechanism of free radical formation and disappearance during the ascorbic acid oxidase and peroxidase reactions. Biochim Biophys Acta. 1961 Jun 10;50:62–69. doi: 10.1016/0006-3002(61)91060-5. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I., PIETTE L. H. THE MECHANISM OF AEROBIC OXIDASE REACTION CATALYZED BY PEROXIDASE. Biochim Biophys Acta. 1963 Sep 3;77:47–64. doi: 10.1016/0006-3002(63)90468-2. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I., SOUZU H. The mechanism of indoleacetic acid oxidase reaction catalyzed by turnip peroxidase. Arch Biochem Biophys. 1960 Feb;86:294–301. doi: 10.1016/0003-9861(60)90421-5. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I. The reduction of cytochrome c by enzyme-generated ascorbic free radical. J Biol Chem. 1962 Jan;237:224–229. [PubMed] [Google Scholar]
- Yamada H., Yamazaki I. Proton balance in conversions between five oxidation-reduction states of horseradish peroxidase. Arch Biochem Biophys. 1974 Dec;165(2):728–738. doi: 10.1016/0003-9861(74)90301-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki I. One-electron and two-electron transfer mechanisms in enzymic oxidation-reduction reactions. Adv Biophys. 1971;2:33–76. [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. VII. Electron paramagnetic resonance absorptions of the enzyme and complex ES in dissolved and crystalline forms. J Biol Chem. 1966 Jul 10;241(13):3240–3243. [PubMed] [Google Scholar]
- de Beer R., Duine J. A., Frank J., Westerling J. The role of pyrrolo-quinoline semiquinone forms in the mechanism of action of methanol dehydrogenase. Eur J Biochem. 1983 Jan 17;130(1):105–109. doi: 10.1111/j.1432-1033.1983.tb07123.x. [DOI] [PubMed] [Google Scholar]
- von Foerster G., Weis W., Staudinger H. Kinetik der Entstehung von Semidehydroascorbinsäure. Hoppe Seylers Z Physiol Chem. 1966;344(4):217–222. [PubMed] [Google Scholar]


