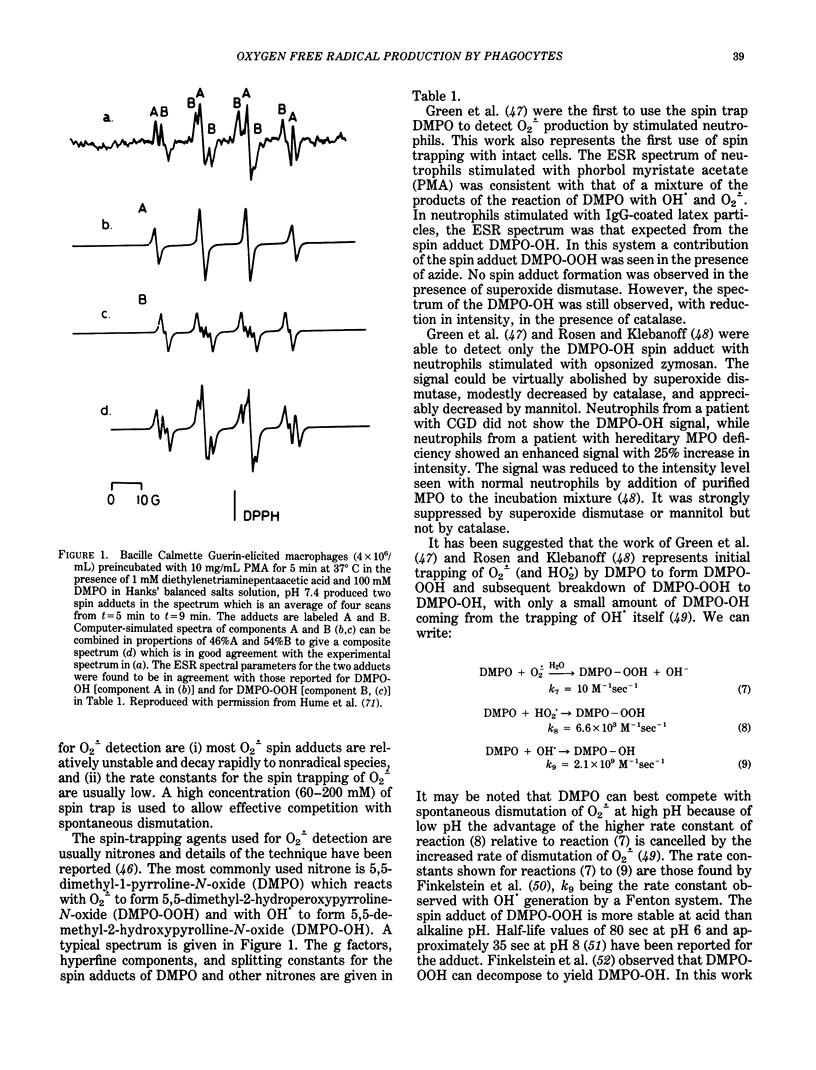
Abstract
Neutrophils and macrophages undergo a respiratory burst and an increase in the activity of the hexose monophosphate pathway in response to particulate or soluble agents. The increase in oxygen consumption was found to be associated with the production of oxygen-centered radicals. The ESR technique of spin trapping showed that besides a superoxide spin adduct, a hydroxyl spin adduct is also produced. ESR is considered to be the least ambiguous technique for the detection of free radicals. The spin-trapping agents used for oxygen-centered radical detection are usually nitrones. The most commonly used nitrone is 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), which reacts with O2-. to form 5,5-dimethyl-2-hydroperoxypyrroline-N-oxide (DMPO-OOH) and with OH. to form 5,5-dimethyl-2-hydroxypyrroline-N-oxide (DMPO-OH). Although spin-adduct formation is considered to be the most direct technique for the detection of free radicals, some disadvantages are encountered. There has been considerable interest in the isolation of the O2-. generating activity from phagocytic cells. The enzyme can be extracted with deoxycholate and gel filtration indicates that it is a high molecular weight complex. Maximum activity was between pH 7.0 and pH 7.5. The Km value was 15.8 microM for NADPH and 434 micron for NADH, indicating that NADPH is the preferred substrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Production of superoxide and hydrogen peroxide by an NADH-oxidase in guinea pig polymorphonuclear leukocytes. Modulation by nucleotides and divalent cations. J Biol Chem. 1979 Nov 25;254(22):11530–11537. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Thornalley P. J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982 Mar 15;715(1):116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bellavite P., Davoli A., Thornalley P. J., Rossi F. The generation of hydroxyl radicals following superoxide production by neutrophil NADPH oxidase. FEBS Lett. 1982 Dec 27;150(2):300–302. doi: 10.1016/0014-5793(82)80755-2. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bellavite P., Serra M. C., Thornalley P. J., Rossi F. An EPR study of the production of superoxide radicals by neutrophil NADPH oxidase. FEBS Lett. 1982 Aug 23;145(2):323–326. doi: 10.1016/0014-5793(82)80192-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bellavite P., Serra M. C., Davoli A., Bannister J. V., Rossi F. The NADPH oxidase of guinea pig polymorphonuclear leucocytes. Properties of the deoxycholate extracted enzyme. Mol Cell Biochem. 1983;52(1):17–25. doi: 10.1007/BF00230585. [DOI] [PubMed] [Google Scholar]
- Bielski B. H., Arudi R. L., Sutherland M. W. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J Biol Chem. 1983 Apr 25;258(8):4759–4761. [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E. NAD(P)H-dependent superoxide production by phagocytic vesicles from guinea pig and human granulocytes. J Biol Chem. 1980 Jul 25;255(14):6584–6588. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M. R., Baehner R. L., Nathan D. G. Amino acid oxidase of leukocytes in relation to H 2 O 2 -mediated bacterial killing. J Clin Invest. 1971 Sep;50(9):1985–1991. doi: 10.1172/JCI106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J., Paxton J. Spin trapping of superoxide. Mol Pharmacol. 1979 Sep;16(2):676–685. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L. Evidence for superoxide-dependent reduction of Fe3+ and its role in enzyme-generated hydroxyl radical formation. Chem Biol Interact. 1976 Sep;15(1):77–89. doi: 10.1016/0009-2797(76)90130-7. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Gabig T. G., Kipnes R. S., Babior B. M. Solubilization of the O2(-)-forming activity responsible for the respiratory burst in human neutrophils. J Biol Chem. 1978 Oct 10;253(19):6663–6665. [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Green T. R., Wirtz M. K., Wu D. E. Delineation of the catalytic components of the NADPH-dependent O2- generating oxidoreductase of human neutrophils. Biochem Biophys Res Commun. 1983 Feb 10;110(3):873–879. doi: 10.1016/0006-291x(83)91042-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Gordon S., Thornalley P. J., Bannister J. V. The production of oxygen-centered radicals by bacillus-Calmette-Guerin-activated macrophages. An electron paramagnetic resonance study of the response to phorbol myristate acetate. Biochim Biophys Acta. 1983 Oct 25;763(3):245–250. doi: 10.1016/0167-4889(83)90131-3. [DOI] [PubMed] [Google Scholar]
- Jacques Y. V., Bainton D. F. Changes in pH within the phagocytic vacuoles of human neutrophils and monocytes. Lab Invest. 1978 Sep;39(3):179–185. [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. P., Ghai G., Petrone W. F., McCord J. M. Calmodulin-dependent stimulation of the NADPH oxidase of human neutrophils. Biochim Biophys Acta. 1982 Jan 12;714(1):152–156. doi: 10.1016/0304-4165(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Motohashi N., Mori I. Superoxide-dependent formation of hydroxyl radical catalyzed by transferrin. FEBS Lett. 1983 Jun 27;157(1):197–199. doi: 10.1016/0014-5793(83)81144-2. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Basford R. E., Cramer R., Dri P., Rossi F. Studies on the NADPH oxidizing activity in polymorphonuclear leucocytes. The mode of association with the granule membrane the relationship to myeloperoxidase and the interference of hemoglobin with NADPH oxidase determination. Biochim Biophys Acta. 1974 Sep 5;362(2):221–232. doi: 10.1016/0304-4165(74)90215-3. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. OXIDATION OF REDUCED TRIPHOSPHOPYRIDINE NUCLEOTIDE BY GUINEA PIG POLYMORPHONUCLEAR LEUCOCYTES. Nature. 1964 Apr 4;202:85–86. doi: 10.1038/202085a0. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Subcellular distribution of superoxide dismutases in human neutrophils. Influence of myeloperoxidase on the measurement of superoxide dismutase activity. Biochem J. 1977 Aug 15;166(2):145–153. doi: 10.1042/bj1660145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Wyss S. R., Aebi H. E. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980 Jun;65(6):1515–1522. doi: 10.1172/JCI109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978 Nov 30;276(5687):515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T., Webster D., Allison A. C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978 Aug 26;2(8087):446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- Serra M. C., Bellavite P., Davoli A., Bannister J. V., Rossi F. Isolation from neutrophil membranes of a complex containing active NADPH oxidase and cytochrome b-245. Biochim Biophys Acta. 1984 Jul 17;788(1):138–146. doi: 10.1016/0167-4838(84)90306-6. [DOI] [PubMed] [Google Scholar]
- Stjernholm R. L., Manak R. C. Carbohydrate metabolism in leukocytes. XIV. Regulation of pentose cycle activity and glycogen metabolism during phagocytosis. J Reticuloendothel Soc. 1970 Dec;8(6):550–560. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen J. W., Raap A., Koppenol W. H., Nauta H. A tunnelling model to explain the reduction of ferricytochrome c by H and OH radicals. Biochim Biophys Acta. 1978 Jul 6;503(1):1–9. doi: 10.1016/0005-2728(78)90157-3. [DOI] [PubMed] [Google Scholar]


