Abstract
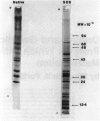
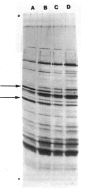
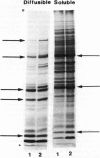
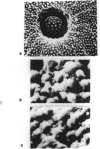
The major function of pollen is to deliver the sperm nuclei to the embryo sac. It does this by germinating and producing a pollen tube and thus provides a relatively simple developmental system for study. Mutants for many pollen functions are accessible, as it is a haploid cell. Mature pollen was fractionated into diffusible proteins, soluble proteins, and proteins insolubly associated with membrane or wall; these protein fractions have been quantified and cataloged by native and SDS polyacrylamide gel electrophoresis. Diffusible proteins are localized in the pollen grain wall whereas soluble proteins are cytoplasmic. The roles of haploid and diploid genomes in specifying these proteins is discussed. Pollen from maximally divergent maize lines was examined for quantitative and qualitative variation in the diffusible proteins. A surprising conservation was found for these proteins indicating some functional role which is, at present, unknown. Initial experiments on the incorporation of 35S-methionine into germinating pollen indicate that major representatives of the diffusible proteins are made within the pollen grain itself. They are presumably included in the pollen wall during development and diffuse out through the pore region. Studies with pollen mRNA and experiments on incorporation of 35S-methionine into developing anthers are underway and will identify the origin of these proteins. A knowledge of the basic developmental biology of maize pollen is a prerequisite to its judicious use as a monitor of environmental mutagens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Clarke A., Gleeson P., Harrison S., Knox R. B. Pollen-stigma interactions: Identification and characterization of surface components with recognition potential. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3358–3362. doi: 10.1073/pnas.76.7.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Pollen wall development. The succession of events in the growth of intricately patterned pollen walls is described and discussed. Science. 1968 Jul 19;161(3838):230–237. doi: 10.1126/science.161.3838.230. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Recognition and response in the pollen-stigma interaction. Symp Soc Exp Biol. 1978;32:121–138. [PubMed] [Google Scholar]
- Howlett B. J., Knox R. B., Heslop-Harrison J. Pollen-wall proteins: release of the allergen Antigen E from intine and exine sites in pollen grains of ragweed and Cosmos. J Cell Sci. 1973 Sep;13(2):603–619. doi: 10.1242/jcs.13.2.603. [DOI] [PubMed] [Google Scholar]
- Knox R. B., Heslop-Harrison J. Pollen-wall proteins: electron-microscopic localization of acid phosphatase in the intine of Crocus vernus. J Cell Sci. 1971 May;8(3):727–733. doi: 10.1242/jcs.8.3.727. [DOI] [PubMed] [Google Scholar]
- Knox R. B., Heslop-Harrison J. Pollen-wall proteins: localization and enzymic activity. J Cell Sci. 1970 Jan;6(1):1–27. doi: 10.1242/jcs.6.1.1a. [DOI] [PubMed] [Google Scholar]
- Knox R. B. Pollen wall proteins: pollen-stigma interactions in ragweed and Cosmos (Compositae). J Cell Sci. 1973 Mar;12(2):421–443. doi: 10.1242/jcs.12.2.421. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Weeden N. F., Gottlieb L. D. Isolation of cytoplasmic enzymes from pollen. Plant Physiol. 1980 Sep;66(3):400–403. doi: 10.1104/pp.66.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]