Abstract
Model systems to study the effects of chemicals of environmental concern on bacterial and parasitic diseases as well as the immunosurveillance and destruction of transplantable tumor cells were described and evaluated. Studies were conducted in female B6C3F1 mice following adult or pre/postnatal exposure to several prototype chemicals. The prototype chemicals employed included the synthetic estrogen diethylstilbestrol (DES), the polycyclic aromatic hydrocarbon benzo(a)pyrene (B[a]P), and the carcinogenesis promoting agent 12-0-tetradecanoyl-phorbol-13-0-acetate (TPA).
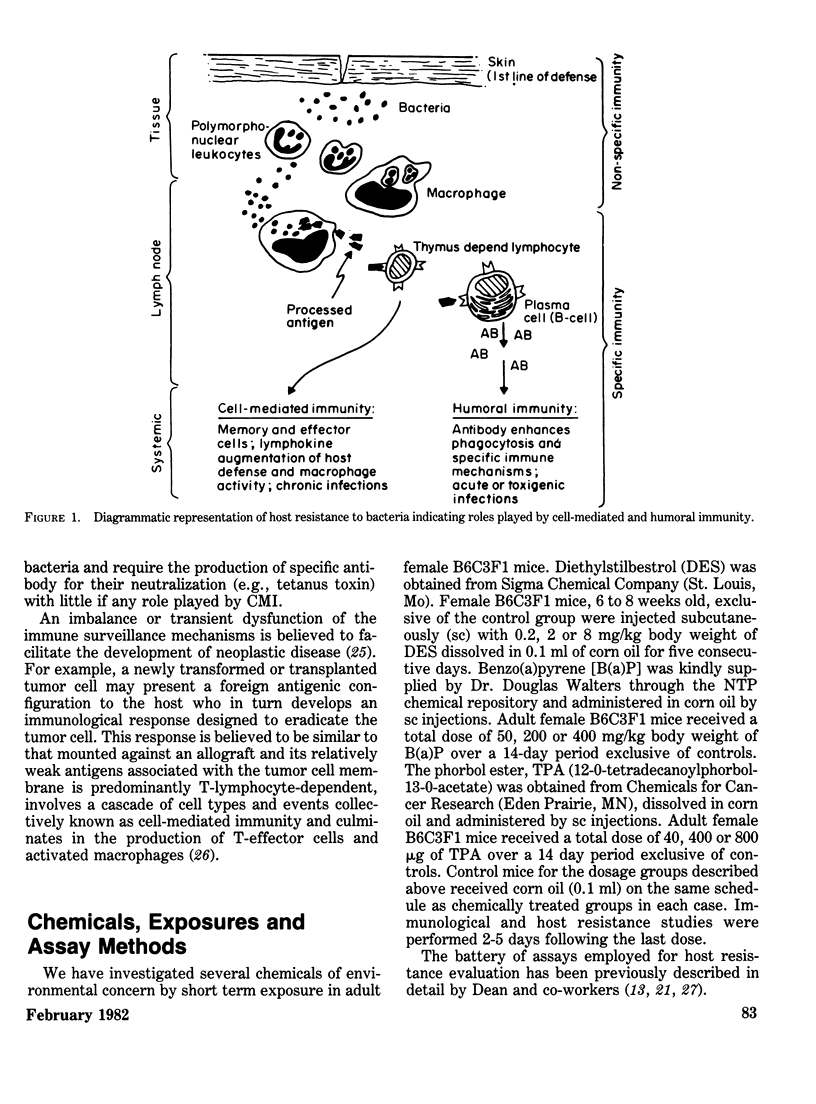
The host resistance models employed depend primarily on functional thymus-dependent immunity, although humoral immunity is suggested to have a role in the parasite model as well. These models include: subcutaneous challenge with a dose of PYB6 tumor cell causing a 10-20% incidence (TD10–20) of tumor; intravenous challenge with B16 melanoma cells; challenge with a dose of Listeria monocytogenes causing a 10-20% incidence of mortality (LD10–20); challenge with a dose of E. coli lipopolysaccharide endotoxin causing a 10-20% incidence of lethality (LD10–20); and challenge with larvae of Trichinella spiralis for parasite expulsion kinetic studies.
Increased mortality was observed following Listeria monocytogenes challenge in DES-exposed mice. B(a)P and TPA exposure did not alter host resistance to this organism. The increased mortality observed following DES was associated with a significant increase in the number of viable Listeria in the spleens and livers at 4 days, a time when T-cell immunity is thought to be expressed, but bacterial counts were similar to control mice at day 1, a time when MΦ are thought to exert their greatest effect. These data suggest that the increased Listeria susceptibility found following DES exposure may result from a T-cell defect, although the intracellular killing capacity of DES-treated Mϕ's has not been well examined.
Tumor susceptibility studies following challenge with 5 × 103 viable syngeneic PYB6 tumor cells revealed that nontreated adult B6C3F1 mice resisted tumor formation, with only a 10-20% incidence of tumor formation. In contrast, mice exposed to DES or TPA as adults had a tumor frequency of from 70-100% following TPA and up to 90% following DES exposure. In all cases the tumors were progressive and resulted in death. B(a)P did not alter the frequency of tumor incidence from controls in this model.
Preliminary data, using the B16 melanoma intravenous challenge model and 125IUdR to quantitate tumor mass revealed this model was sensitive to non-specifically activated macrophage kill. DES treated mice with activated macrophages did not demonstrate increased tumor mass, while mice exposed to TPA or the potent immunosuppressive agent cyclophosphamide had a significantly increased tumor mass in their lungs.
Expulsion of Trichinella spiralis adults from the gut also apparently required functional T-cells and possibly some element of humoral immunity. Mice exposed to DES and B(a)P exhibited increased numbers of adult worms in the gut at day 14.
Sensitivity to gram-negative endotoxin (LPS) was apparently increased following exposure to DES or B(a)P. These data suggest that the detoxification of LPS is related to an intact Mϕ population. The data presented here demonstrate the sensitivity of the host resistance assay panel proposed for detecting immune alteration. Alteration of T-cell function appeared to correlate with increased susceptibility to bacterial and tumor cell challenge.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUDE A. I., CAREY F. J., ZALESKY M. Studies with radioactive endotoxin. II. Correlation of physiologic effects with distribution of radioactivity in rabbits injected with radioactive sodium chromate. J Clin Invest. 1955 Jun;34(6):858–866. doi: 10.1172/JCI103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J. K. Immunosuppression and carcinogenesis: contrasting effects with 7,12-dimethylbenz[a]anthracene, benz[a]pyrene, and 3-methylcholanthrene. J Natl Cancer Inst. 1970 Jan;44(1):1–10. [PubMed] [Google Scholar]
- Bern H. A., Jones L. A., Mori T., Young P. N. Exposure of neonatal mice to steroids: longterm effects on the mammary gland and other reproductive structures. J Steroid Biochem. 1975 May;6(5):673–676. doi: 10.1016/0022-4731(75)90051-5. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., Luster M. I., Dean J. H., Wilson R. E. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J Reticuloendothel Soc. 1980 Dec;28(6):547–560. [PubMed] [Google Scholar]
- Burnet F. M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Luster M. I., Boorman G. A., Luebke R. W., Lauer L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in tumor susceptibility and host resistance parameters. J Reticuloendothel Soc. 1980 Dec;28(6):571–583. [PubMed] [Google Scholar]
- Dean J. H., Padarathsingh M. L., Jerrells T. R., Keys L., Northing J. W. Assessment of immunobiological effects induced by chemicals, drugs or food additives. II. Studies with cyclophosphamide. Drug Chem Toxicol. 1979;2(1-2):133–153. doi: 10.3109/01480547908993186. [DOI] [PubMed] [Google Scholar]
- Fairchild G. A., Roan J., McCarroll J. Atmospheric pollutants and the pathogenesis of viral respiratory infection. Sulfur dioxide and influenza infection in mice. Arch Environ Health. 1972 Sep;25(3):174–182. doi: 10.1080/00039896.1972.10666157. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Friend M., Trainer D. O. Polychlorinated biphenyl: interaction with duck hepatitis virus. Science. 1970 Dec 18;170(3964):1314–1316. doi: 10.1126/science.170.3964.1314. [DOI] [PubMed] [Google Scholar]
- Gainer J. H., Pry T. W. Effects of arsenicals on viral infections in mice. Am J Vet Res. 1972 Nov;33(11):2299–2307. [PubMed] [Google Scholar]
- Gass G. H., Brown J., Okey A. B. Carcinogenic effects of oral diethylstilbestrol on C3H male mice with and without the mammary tumor virus. J Natl Cancer Inst. 1974 Nov;53(5):1369–1370. doi: 10.1093/jnci/53.5.1369. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Good R. A. Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer. 1971 Jul;28(1):89–98. doi: 10.1002/1097-0142(197107)28:1<89::aid-cncr2820280117>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hemphill F. E., Kaeberle M. L., Buck W. B. Lead suppression of mouse resistance to Salmonella typhimurium. Science. 1971 Jun 4;172(3987):1031–1032. doi: 10.1126/science.172.3987.1031. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kersey J. H., Spector B. D., Good R. A. Primary immunodeficiency diseases and cancer: the immunodeficiency-cancer registry. Int J Cancer. 1973 Sep 15;12(2):333–347. doi: 10.1002/ijc.2910120204. [DOI] [PubMed] [Google Scholar]
- LAW L. W., TING R. C. IMMUNOLOGIC COMPETENCE AND INDUCTION OF NEOPLASMS BY POLYOMA VIRUS. Proc Soc Exp Biol Med. 1965 Jul;119:823–830. doi: 10.3181/00379727-119-30311. [DOI] [PubMed] [Google Scholar]
- Larsh J. E., Jr, Race G. J., Martin J. H., Weatherly N. F. Studies on delayed (cellular) hypersensitivity in mice infected with Trichinella spiralis. 8. Serologic and histopathologic responses of recipients injected with spleen cells from donors suppressed with ATS. J Parasitol. 1974 Feb;60(1):99–109. [PubMed] [Google Scholar]
- Loose L. D., DiLuzio N. R. Dose-related reticuloendothelial system stimulation by diethylstilbestrol. J Reticuloendothel Soc. 1976 Dec;20(6):457–460. [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Pittman K. A., Benitz K. F., Mueller W. Impaired host resistance to endotoxin and malaria in polychlorinated biphenyl- and hexachlorobenzene-treated mice. Infect Immun. 1978 Apr;20(1):30–35. doi: 10.1128/iai.20.1.30-35.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Dean J. H., Harris M. W., Luebke R. W., Padarathsingh M. L., Moore J. A. Examination of bone marrow, immunologic parameters and host susceptibility following pre- and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Int J Immunopharmacol. 1980;2(4):301–310. doi: 10.1016/0192-0561(80)90030-2. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Dean J. H., Luebke R. W., Lawson L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in immunological functions. J Reticuloendothel Soc. 1980 Dec;28(6):561–569. [PubMed] [Google Scholar]
- Luster M. I., Dean J. H., Boorman G. A. Cell-mediated immunity and its application in toxicology. Environ Health Perspect. 1982 Feb;43:31–36. doi: 10.1289/ehp.824331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean L. D. Host resistance in surgical patients. J Trauma. 1979 May;19(5):297–304. doi: 10.1097/00005373-197905000-00001. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Barnes D. W., Munson A. E. Relationship of molecular weight to antiviral and antitumor activities and toxic effects of maleic anhydride-divinyl ether (MVE) polyanions. Cancer Treat Rep. 1978 Nov;62(11):1797–1803. [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- PREHN R. T. FUNCTION OF DEPRESSED IMMUNOLOGIC REACTIVITY DURING CARCINOGENESIS. J Natl Cancer Inst. 1963 Oct;31:791–805. [PubMed] [Google Scholar]
- Penn I. Development of cancer in transplantation patients. Adv Surg. 1978;12:155–191. [PubMed] [Google Scholar]
- Penn I., Starzl T. E. A summary of the status of de novo cancer in transplant recipients. Transplant Proc. 1972 Dec;4(4):719–732. [PubMed] [Google Scholar]
- Stjernswärd J. Effect of noncarcinogenic and carcinogenic hydrocarbons on antibody-forming cells measured at the cellular level in vitro. J Natl Cancer Inst. 1966 Jun;36(6):1189–1195. [PubMed] [Google Scholar]
- Theiss J. C., Shimkin M. B., Poirier L. A. Induction of pulmonary adenomas in strain A mice by substituted organohalides. Cancer Res. 1979 Feb;39(2 Pt 1):391–395. [PubMed] [Google Scholar]
- Thigpen J. E., Faith R. E., McConnell E. E., Moore J. A. Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect Immun. 1975 Dec;12(6):1319–1324. doi: 10.1128/iai.12.6.1319-1324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso P., Gengozian N. Depressed humoral immunity and increased tumor incidence in mice following in utero exposure to benzo[alpha]pyrene. J Toxicol Environ Health. 1980 May;6(3):569–576. doi: 10.1080/15287398009529874. [DOI] [PubMed] [Google Scholar]
- Vos J. G. Immune suppression as related to toxicology. CRC Crit Rev Toxicol. 1977 May;5(1):67–101. doi: 10.3109/10408447709101342. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Kreeftenberg J. G., Kruijt B. C., Kruizinga W., Steerenberg P. The athymic nude rat. II. Immunological characteristics. Clin Immunol Immunopathol. 1980 Feb;15(2):229–237. doi: 10.1016/0090-1229(80)90033-1. [DOI] [PubMed] [Google Scholar]


