Abstract
We have shown recently that by acting on the thyroid-stimulating hormone (TSH) receptor (TSHR), TSH negatively regulates osteoclast differentiation. Both heterozygotic and homozygotic TSHR null mice are osteopenic with evidence of enhanced osteoclast differentiation. Here, we report that the accompanying elevation of TNFα, an osteoclastogenic cytokine, causes the increased osteoclast differentiation. This enhancement in TSHR−/− and TSHR+/− mice is abrogated in compound TSHR−/−/TNFα−/− and TSHR+/−/TNFα+/− mice, respectively. In parallel studies, we find that TSH directly inhibits TNFα production, reduces the number of TNFα-producing osteoclast precursors, and attenuates the induction of TNFα expression by IL-1, TNFα, and receptor activator of NF-κB ligand. TSH also suppresses osteoclast formation in murine macrophages and RAW-C3 cells. The suppression is more profound in cells that overexpress the TSHR than those transfected with empty vector. The overexpression of ligand-independent, constitutively active TSHR abrogates osteoclast formation even under basal conditions and in the absence of TSH. Finally, IL-1/TNFα and receptor activator of NF-κB ligand fail to stimulate AP-1 and NF-κB binding to DNA in cells transfected with TSHR or constitutively active TSHR. The results suggest that TNFα is the critical cytokine mediating the downstream antiresorptive effects of TSH on the skeleton.
Keywords: bone remodeling, osteoclast, macrophage, cytokine
Anterior pituitary hormones have long been thought just to stimulate the secretion of master hormones from target endocrine glands, except for our recent demonstration of direct effects of thyroid-stimulating hormone (TSH) and follicle-stimulating hormone on the skeleton (1). Thus, until recently, TSH was considered solely to regulate thyroid follicular cell differentiation and thyroid hormone secretion by binding to a seven transmembrane, glycosylated G protein-coupled receptor, the TSH receptor (TSHR). Previous studies had identified TSHRs in other tissues and cells, including the pituitary, thymus, testes, kidney, brain, lymphocytes, adipocytes, and fibroblasts (2, 3), but their functional significance has remained uncertain.
Gene ablation studies in mice revealed that TSHR haploinsufficiency did not affect thyroid gland development or function, whereas the total absence of the TSHR expectedly disrupted thyroid follicular structure (4). However, bone mass was reduced not only in homozygote mice but also in the haploinsufficient heterozygotes (5). That TSHR haploinsufficiency, in the absence of a thyroid defect, resulted in osteoporosis established a primary role for the TSHR in bone metabolism. Furthermore, supplementation of TSHR−/− mice with thyroid extract to render them euthyroid corrected all hypothyroid abnormalities, including runting, but not reductions in bone mass (5) or sodium-iodide symporter expression (4). The latter observation confirmed that the osteoporosis arose from TSHR deficiency rather than altered thyroid hormone levels. Consistent with this notion, the genetic ablation of the α1/β thyroid hormone receptor has been shown not to result in a bone remodeling defect (6).
We found that the osteoporosis in TSHR knockout mice was the result of an enhancement in osteoclast differentiation. Consistent with the low bone mass, ex vivo cultures of bone marrow cell precursors from both heterozygote and homozygote mice showed increased osteoclast formation and the enhanced expression of an osteoclast marker tartrate-resistant acid phosphatase (TRAP) (5). This enhanced osteoclast formation was not associated with increased receptor activator of NF-κB ligand (RANKL) production but instead with a several-fold increase in the synthesis and release of TNFα, another osteoclastogenic cytokine (5). A blocking antibody to TNFα abrogated this increased osteoclastogenesis, suggesting that the osteoporosis in the TSHR−/− mice was TNFα-mediated.
Several studies have implicated elevated TNFα in the pathogenesis of various forms of osteoporosis (7). For example, TNFα is overexpressed in T lymphocytes in hypogonadal animal models and humans (7, 8), and T cell-deficient mice are resistant to hypogonadal osteoporosis. Likewise, the systemic osteoporosis and juxta-articular osteolysis that accompany rheumatoid arthritis are thought to be TNFα-mediated (9, 10). There is recent evidence indicating that the systemic and local bone loss can be abrogated by a TNFα antibody (11). The osteoporosis seen in hyperthyroidism is also associated with increased levels of various inflammatory cytokines, including TNFα, IL-1, and IL-6 (12).
To examine whether TNFα plays a critical role in mediating the skeletal effects of TSH, we (i) investigated whether the enhanced osteoclast formation can be rescued by abrogating TNFα in TSHR−/− and TSHR+/− mice and (ii) determined the mechanism by which TSH regulates TNFα production. For the former experiments, we measured osteoclast formation and TRAP expression in TSHR−/−/TNFα−/− and TSHR−/−/TNFα+/− mice, as well as in TSHR haploinsufficient mice that had one TNFα allele deleted. The increased osteoclastogenesis in both TSHR homozygote and heterozygote mice was rescued with graded reductions in the dosage of the TNFα gene. For the latter experiments, we constructed a constitutively active TSHR (caTSHR) with a single point mutation, Asp to Ala at 633 (13). This transmembrane ligand-independent, gain-of-function mutation has been identified in patients with autonomous thyroid adenomas and nonautoimmune hereditary hyperthyroidism. Overexpression of this mutant construct in primary murine macrophages and RAW-C3 cells demonstrated that TSH/TSHR regulates transcription of the TNFα gene and osteoclast formation.
Results
TNFα Gene Deletion Rescues the Enhanced Osteoclastogenesis in the TSHR-Null Mouse.
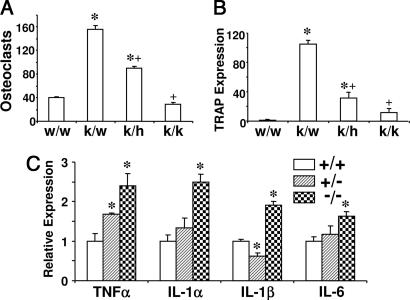
We have shown previously that TNFα mRNA and protein levels are increased in the bone marrow of TSHR−/− mice (5). We have further demonstrated that osteoclast formation is enhanced in TSHR−/− and TSHR+/− mice and that an antibody to TNFα abrogates this increase (5). To examine a cause–effect relationship, i.e., whether the increased TNFα expression causes the enhanced osteoclastogenesis, we crossed TSHR+/− mice with TNF+/− mice and examined osteoclast formation and TRAP expression in bone marrow cultures from the resulting genotypes. We confirmed an increase in osteoclast formation and TRAP expression in TSHR−/−/TNF+/+ and TSHR+/−/TNF+/+ mice compared with wild-type controls. These increases in osteoclastogenesis and TRAP mRNA expression were abrogated in TSHR−/−/TNFα−/− and TSHR+/−/TNFα+/− mice, respectively (Fig. 1 A and B and Fig. 8, which is published as supporting information on the PNAS web site). Consistent with a dose-dependent effect of TNFα, deletion of the TNFα gene from one allele only partly attenuated the increased osteoclastogenesis and TRAP expression in TSHR−/− mice. Overall, therefore, our finding that the deletion of the TNF gene fully rescues the increased osteoclastogenesis in TSHR null mice establishes that TNF mediates the skeletal effect of TSH.
Fig. 1.
Osteoclast formation and TRAP expression in the mice with TSHR and TNFα ablation, and cytokine expression in TSHR null mice. (A and B) Rescue experiments showing osteoclast formation (A) and TRAP mRNA expression (B) in bone marrow cell cultures isolated from various genotypes, namely TSHR+/+/TNF+/+ (w/w), TSHR−/−/TNF+/+ (k/w), TSHR−/−/TNF+/−(k/h), and TSHR−/−/TNF−/− (k/k) mice. (C) Real-time PCR measurements showing the expression of interleukin-1α (IL-1α), IL-1β, IL-6, and TNFα in bone marrow cell cultures from TSHR+/− (+/−) and TSHR−/− (−/−) mice, relative to TSHR+/+ (+/+) cultures. In both experimental protocols, bone marrow cell cultures were incubated with RANKL (60 ng/ml) and M-CSF (30 ng/ml) for up to 6 days. ∗, P < 0.05, comparisons with wild-type (w/w or +/+) mice in all cases; +, P < 0.05, compared with k/w mice.
In addition to the elevated TNF expression, we found that other proinflammatory cytokines were also elevated in TSHR−/− bone marrow cell cultures (Fig. 1C). However, TNF, but not IL-1 or IL-6, expression was significantly enhanced in heterozygotic TSHR+/− mice, suggesting that the effect of TNFα in mediating the skeletal effects of TSHR deletion was dominant. Nonetheless, we cannot rule out the role of other cytokines in the increased osteoclast formation caused by TSHR deletion, because all of the other cytokines of interest, including IL-6 and IL-1, are known to affect osteoclastogenesis (14).
TSH Signals Directly Inhibit TNFα Gene Expression and Osteoclastogenesis.
Gain-of-function studies were performed to determine whether the addition of TSH directly inhibited TNFα expression. We first tested the induction of TNFα expression in CD11b+ bone marrow macrophages and RAW-C3 cells by IL-1 or TNFα alone, or a combination of IL-1 and TNFα (IL-1/TNF) (Fig. 9, which is published as supporting information on the PNAS web site). A robust stimulation of TNFα expression was observed in response to IL-1 and TNFα together compared with each cytokine alone. Thus, we used IL-1 and TNFα in combination in the next experiments to examine TSH effect.
We found that IL-1/TNFα-induced TNFα mRNA expression was significantly attenuated in the presence of TSH both in RAW-C3 cells and CD11b+ bone marrow cells (Fig. 2 A and B). Consistent with the decreased TNFα mRNA expression, TSH also significantly reduced the level of TNFα secreted in response to murine recombinant IL-1/human recombinant TNFα, as measured by a murine-specific TNFα ELISA (Fig. 2 C and D). Similar to IL-1/TNFα, the addition of RANKL elicited a several-fold increase in TNFα mRNA expression (data not shown). Taken together, the findings indicate that TSH directly inhibits TNFα expression in osteoclast precursors.
Fig. 2.
RAW-C3 (A and C) and CD11b+ macrophages (B and D) were cultured with IL-1α (10 ng/ml) and TNFα (50 ng/ml) in the presence or absence of TSH (10 milliunits per ml). TNFα expression (A and B) or murine TNFα levels in the supernatants (C and D) were measured, respectively, by real-time PCR (after 2 h) and ELISA (after 1 day). Note that human recombinant TNFα was used for the ELISA experiments. ∗, P < 0.05, comparisons with respective controls; +, P < 0.05, compares TSH effects with IL-1/TNFα alone.
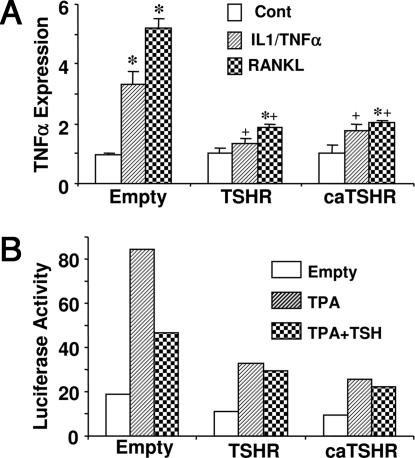
We have shown previously that the TSHR is expressed in bone marrow macrophages and RAW-C3 cells, and that its expression becomes elevated during osteoclast differentiation (5). Thus, we studied the effect of overexpressing the TSHR by stably transfecting RAW-C3 cells with the wild-type TSHR (TSHR-C3) or the constitutively active TSHR (caTSHR-C3) tagged with EGFP. TSHR mRNA expression measured by real-time PCR showed a 2.5- and 1.5-fold increase in TSHR-C3 and caTSHR-C3 cells, respectively. Consistent with this result, flow cytometery using MS-1, a hamster TSHR monoclonal antibody (15), revealed that 45% cells in empty-C3 and 73% and 55% in TSHR- and caTSHR-C3, respectively, were positive for the TSHR protein (nonspecific binding using control IgG at 27.0%, 28.5%, and 26.2%, respectively). Compared with previous CHO cell experiments (15), TSHR overexpression in RAW-C3 cells was relatively low. As intensely fluorescent cells became large and flat and stopped proliferation, cells expressing high levels of TSHR or caTSHR were likely eliminated during the selection procedure. This analysis was performed on pooled cells to minimize clone-to-clone variation.
Empty-C3, TSHR-C3, and caTSHR-C3 cells showed no difference in basal TNFα expression (Fig. 3A). However, the overexpression of the TSHR or caTSHR significantly attenuated the increased TNFα mRNA expression triggered by IL-1/TNFα or RANKL. We then performed promoter-reporter assays to determine whether the inhibitory effect of TSH on TNFα was because of a direct effect on gene expression. A 197-bp TNFα promoter-luciferase construct was transfected into empty-C3, TSHR-C3, and caTSHR-C3 cells, and the protein kinase C agonist PMA (phorbol 12-myristate 13-acetate) was used to activate TNFα gene transcription. Although basal luciferase activity did not differ between the three groups, the PMA-induced stimulation of TNFα gene transcription was substantially reduced in cells transfected with either TSHR or caTSHR (Fig. 3B). Recombinant TSH attenuated the response to PMA in empty-C3 cells, but not in TSHR- or caTSHR-infected cells (Fig. 3B). This result indicates TSHR overexpression could regulate TNFα expression in osteoclast progenitors. It remains unclear how TSHR, in the absence of ligand, reduces TNFα expression. It is possible that high, nonphysiological levels of TSHR expression could trigger specific signaling in those cells.
Fig. 3.
Regulation of TNFα mRNA expression and TNFα promoter activity. (A) TNFα mRNA levels quantitated by real-time PCR in RAW-C3 cells overexpressing the TSHR or the constitutively active TSHR (caTSHR) after treatment with IL-1α (10 ng/ml) and TNFα (50 ng/ml) (IL-1/TNFα) or RANKL (100 ng/ml) for 2 h. ∗, P < 0.05, comparisons with respective controls; +, P < 0.05, compares the IL-1/TNFα and RANKL effects of TSHR-C3 and caTSHR-C3 cells with those elicited in empty-C3 cells. (B) Empty-C3, TSHR-C3, or caTSHR-C3 transformants were transfected with the TNFα promoter (−197 to +115 bp) luciferase fragment. Luciferase activity was measured in duplicate 24 h after treatment with PMA (3 × 10−8 M) or PMA plus TSH (10 milliunits per ml).
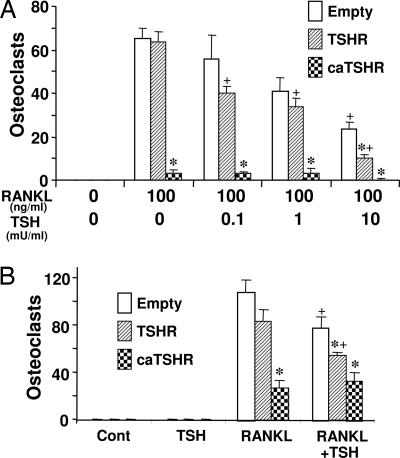
We next examined the effects of TSH on the TRAP-positive osteoclast formation induced by RANKL. The osteoclast formation was inhibited strongly in caTSHR-C3 cells, even in the absence of TSH (Fig. 4A). Additionally, TSH caused a further concentration-dependent decrease in osteoclastogenesis from empty-C3 or TSHR-C3 cells, and this decrement was expectedly greater in TSHR-C3 cells (Fig. 4A). Fig. 4B likewise shows that bone marrow cells infected transiently with a retrovirus containing caTSHR or TSHR displayed a significant attenuation of osteoclastogenesis compared with vector-infected cells. Finally, TSH did not reduce osteoclastogenesis in cells infected with caTSHR.
Fig. 4.
Osteoclast formation in TSHR or caTSHR overexpressing macrophages. (A) RAW-C3 cells stably transfected with empty vector, TSHR, or caTSHR were cultured with 100 ng/ml RANKL with or without human TSH (0.1–10 milliunits per ml) for 6 days. TRAP-positive osteoclasts were counted. ∗, P < 0.05 compares TSHR and caTSHR transformants with Empty-C3 cells at each concentration of TSH; +, P < 0.05, compares the effect of each concentration of TSH against zero-TSH in empty-C3 cells. (B) Bone marrow macrophages were transiently infected with either an empty retrovirus (empty) or a retrovirus containing either the TSHR or caTSHR construct. The infected cells were treated with RANKL (60 ng/ml) and M-CSF (30 ng/ml) for 6 days and examined for TRAP-positive osteoclast formation. ∗, P < 0.05, compares the effect of RANKL or RANKL plus TSH in TSHR-C3 and caTSHR-C3 cells versus empty-C3 cells; +, P < 0.05, compares the effect of RANKL plus TSH with that of RANKL alone (control) for each transformant.
Note that TSHR overexpression inhibits TNFα but not osteoclastogenesis in the absence of TSH, whereas caTSHR inhibits both (cf. Figs. 3A and 4A). The different action in TSHR and caTSHR cells may arise from the distinct sensitivities of gene expression and osteoclast formation. Thus, even at relatively high levels of TSHR, the expression of a single gene, such as TNFα, may be inhibited, whereas osteoclast formation may not, because the latter depends on a full program of gene expression. In contrast, more profound signaling by caTSHR may inhibit the other TSH-sensitive genes causing osteoclast formation to decline.
TSH Inhibits Osteoclast Precursor Proliferation and Downstream Signaling.
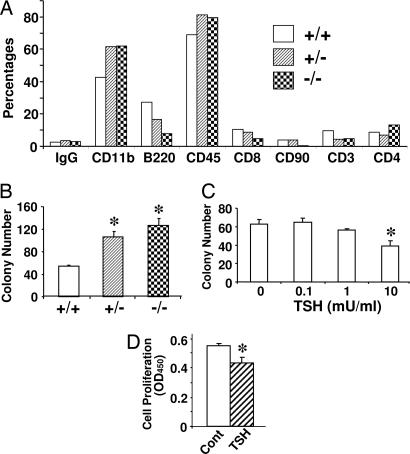
As TNFα is produced mainly in hematopoetic precursors and because total bone marrow TNFα levels depend on the relative abundance of TNFα-producing cells, we examined whether there were significant differences in the populations of CD11b+(macrophages), CD45+ (leukocytes), CD8+, CD90+, CD4+, CD3+ (T lymphocytes), and B220+ B cells in the TSHR genotypes. We found that CD11b+ and CD45+ precursor populations were significantly increased in both TSHR−/− and TSHR+/− mice, whereas the B220+ cell population was reduced (Fig. 5A). This phenomenon is similar to that seen in oc/oc mice (16). No significant increases were noted in the relatively minor populations of CD4+, CD8+, CD3+, and CD90+ T cells (Fig. 5A).
Fig. 5.
Increased macrophage population in bone marrow of TSHR null mice and inhibition of macrophage proliferation by TSH. (A) Cell types present in fresh bone marrow from TSHR+/+ (+/+), TSHR+/− (+/−), and TSHR−/− (−/−) mice analyzed by specific antibodies using flow cytometry and expressed as percentages of positive cells. (B) Fresh bone marrow cells from TSHR+/+ (+/+), TSHR+/− (+/−), and TSHR−/− (−/−) mice were cultured with M-CSF (30 ng/ml) in Metcalf for 7 days, and colony numbers were determined. (C) Fresh bone marrow cells from TSHR+/+ mice were similarly cultured with M-CSF and TSH (0.1–10 milliunits per ml), and colony numbers were determined. (D) Isolated and precultured CD11b+ cells were treated with M-CSF alone or M-CSF plus TSH (10 milliunits per ml) for 3 days. Proliferating cells were measured by BrdU incorporation (expressed as absorbance at 450 nm). ∗, P < 0.05, comparison with TSHR+/+ mice (B) or with zero-TSH (C and D).
To confirm the increased osteoclast precursor populations, we quantitated M-CSF-dependent macrophage numbers in colony forming units–macrophage (CFU-M) assays. There was a significant increase in CFU-M formation both in TSHR+/− and TSHR−/− mice compared with wild-type littermates (Fig. 5B). Furthermore, TSH significantly reduced CFU-M formation in a concentration-dependent manner (Fig. 5C). Importantly, BrdU assays demonstrated a clear effect of TSH in reducing the proliferation of CD11b+ osteoclast precursors (Fig. 5D).
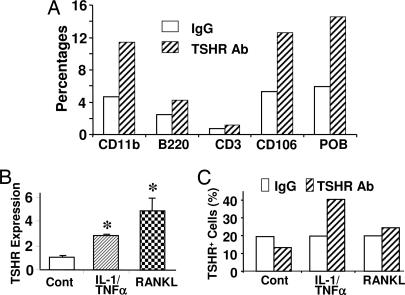
We next attempted to (i) determine which specific bone marrow cell populations expressed the TSHR and (ii) study the mechanism through which the expression of TSHRs is regulated. Flow cytometry was used to examine TSHR expression on isolated CD11b+, B220+, CD3+, CD106+ (stromal cells), and primary calvarial osteoblasts (POB). Fig. 6A shows that B220+ and CD3+ cells exhibited low TSHR expression compared with CD11b+ or CD106+ cells or osteoblasts. Furthermore, TSHR mRNA and protein expression in CD11b+ cells was enhanced by not only RANKL but also by IL-1/TNFα (Fig. 6 B and C). In contrast, TSHR expression in B220+ and CD3+ cells was insensitive to IL-7 and Con A, respectively (data not shown). Together, the results indicate that TSHR is expressed in TNFα-expressing osteoclast and osteoblast progenitors but not in B or T lymphocytes. Furthermore, it is clear that TNF production and TSHR expression are both regulated by IL-1, TNF, and RANKL during osteoclast activation and formation.
Fig. 6.
TSHR expression in primary macrophages and its increase by cytokines and RANKL. (A) CD11b+, B220+, and CD106+ cells were isolated from fresh bone marrow, CD3+ cells were obtained from spleen, and primary osteoblasts (POB) were obtained from neonatal calvaria. The cells were incubated with a TSHR antibody, RSR-1 (mouse IgG, negative control), followed by a second incubation with FITC-labeled anti-mouse IgG antibody. Both nonspecific and specific binding are shown as percentages. (B and C) Bone marrow macrophages were treated with IL-1 (10 ng/ml) and TNFα (50 ng/ml) (IL-1/TNFα) or with RANKL (60 ng/ml) in the presence of M-CSF (30 ng/ml) for 24 h. TSHR expression was assessed by real-time PCR (B) or flow cytometry (using MS-1 antibody) (C). Please refer to Materials and Methods for details on the antibodies. ∗, P < 0.05, compares IL-1/TNFα and RANKL against untreated (control).
We next tested the hypothesis that TSH interferes with downstream signals evoked by RANKL and IL-1/TNF. AP-1 and NF-κB binding to DNA were thus studied in empty-C3, TSHR-C3, and caTSHR-C3 cells using EMSA. In empty-C3 cells, at both 40 and 60 min post-RANKL, and 60 min post-IL-1/TNF exposure, there was a significant increase in binding of AP-1 (Fig. 7). However, there was an unexpected increase in basal AP-1- and NF-κB-DNA binding in TSHR-C3 and caTSHR-C3 cells without treatment and RANKL or IL-1/TNFα failed to further increase in the DNA binding in those cells (Fig. 7). These data suggest that the TSHR signaling interferes with downstream AP-1- and NF-κB-DNA binding to account for the reduced TNFα expression and osteoclastogenesis. We are aware that in other TSH-responsive cells, such as thyroid cells or CHO cells overexpressing TSHR, there is a stimulation of cAMP (15, 17). However, we did not find the same phenomenon in RAW-C3 cells even after transfected with TSHR or caTSHR (Fig. 10 and Supporting Text, which are published as supporting information on the PNAS web site).
Fig. 7.
Electrophoretic mobility shift assay (EMSA) for AP-1 and NF-κB binding of TSHR or caTSHR overexpressing RAW-C3 cells. RAW-C3 cells transfected with empty plasmid, TSHR, or caTSHR were treated with IL-1α (10 ng/ml) and TNFα (50 ng/ml) (IL-1/TNFα) or with RANKL (100 ng/ml) for 20, 40, or 60 min. Nuclear fractions prepared from each group were incubated with biotin-labeled AP-1 or NF-κB for DNA binding studies.
Discussion
Proinflammatory cytokines such as TNFα, IL-1, and IL-6 stimulate osteoclast formation, activation, and survival (14). These cytokines are increased in various osteoporoses and are thought to contribute to the bone loss. Most notably, enhanced TNFα production from T cells has been implicated in the genesis of postmenopausal osteoporosis (7, 8). Additionally, various clinical features of hyperthyroidism, including the high turnover osteoporosis, have been attributed to elevated levels of cytokines such as TNF and their soluble receptors (12).
We showed previously that an elevated TNFα production accompanies the osteopenia and increased osteoclastogenesis of TSHR deficiency (5). Preliminary studies had also indicated that this increased osteoclast formation was abrogated with an anti-TNFα blocking antibody. Here, we provide unequivocal genetic evidence that TNFα mediates the increased osteoclastogenesis resulting from TSHR deficiency. The ex vivo phenotype in TSHR−/− and TSHR+/− mice was abrogated in compound TSHR−/−TNF−/− and TSHR+/−TNF+/− mutants, respectively. Furthermore, although all three cytokines, TNFα, IL-1, and IL-6, were elevated in TSHR−/− cultures, only TNFα was elevated in the cultures from heterozygote mice, indicating a dominant effect of TNFα. Together, the data strongly support our conclusion that enhanced TNFα expression mediates the increased osteoclastogenesis and bone loss of TSHR deficiency.
We next studied the cellular mechanism through which TSH regulates TNFα expression and osteoclast formation. That TSH acts solely through CD11b+ osteoclast progenitors was attested by the following observations. First, TNFα-producing CD11b+ positive cells were increased in bone marrow isolated freshly from TSHR+/− and TSHR−/− mice. Second, M-CSF-dependent macrophage colony formation was increased in TSHR+/− and TSHR−/− mice, and was decreased with recombinant TSH. Finally, TSH attenuated cytokine-induced TNFα mRNA and protein expression only in CD11b+ cells via a transcriptional effect involving AP-1 and NFκB activation.
Similarly to TSHR-null mice, selective enhancements in TNFα-producing CD11b+ cells have also been reported in oc/oc and TNFα transgenic mice, as well as with TNFα administration (16, 18, 19). As with TSHR−/− mice, enhanced CD11b+ cells in oc/oc mice are accompanied by reduced B220+ cells and enhanced osteoclastogenesis, albeit with dysfunctional osteoclasts. It is thus possible that TNFα stimulates the common progenitor toward CD11b+ formation, as suggested in ref. 20.
In contrast to TSHR deficiency, estrogen deficiency is characterized by an increase in the population of TNFα-producing T lymphocytes (7). This enhancement is considered primary and of pathophysiological significance in mediating hypogonadal bone loss. During estrogen deficiency, T cell-derived TNFα is enhanced through the actions of IL-7, IFN-γ, and TGFβ (7). The apparent difference between the cellular effects of estrogen and TSHR deficiency may be the consequence of the distinct patterns of estrogen and TSH receptor localization. Estrogen receptors are present on T cells, B cells, and, to some extent, macrophages (21), whereas only CD11b+ macrophages in bone marrow express TSHRs.
Besides inhibiting TNFα expression in CD11b+ cells, TSH paradoxically increases TNFα expression in certain cell types including CD11b− bone marrow cells (22), CD106+ stromal cells, and primary calvarial osteoblasts (data not shown). In addition, TNFα attenuates osteoblast differentiation similarly to TSH (23). We therefore speculate that the TSH-induced attenuation in osteoblast differentiation that we have noted earlier is also mediated via enhanced TNFα expression.
To explore the mechanism of the TSH effect on osteoclast inhibition, we measured NF-κB- and AP-1-DNA binding activity and cAMP levels in RAW-C3 cells. We found that RANKL and IL-1/TNFα failed to stimulate NF-κB- and AP-1-DNA binding in cells overexpressing TSHR or caTSHR. This result suggests that the TSHR interferes with downstream AP-1 and NF-κB signaling. The finding also supports our view that high, nonphysiologic levels of recombinant TSHR expression, in the absence of ligand, mimic the caTSHR in interfering with IL-1/TNFα and RANKL signaling and TNFα mRNA expression. The precise molecular details underlying this apparently ligand-less signaling by the wild-type receptor remains to be determined.
Another interesting observation was that TSH did not increase cAMP levels in RAW-C3 cells, although forskolin increased some levels of cAMP in those cells (Fig. 10). In contrast, thyroid cells or CHO cells overexpressing the TSHR do respond to both TSH and forskolin with a marked stimulation of cAMP. The reason for this distinct action is unclear but may be somewhat explained by the differential, cell-specific coupling of the TSHR to a distinct G protein. Similar findings have been reported by us whereby the osteoclast follicle-stimulating hormone receptor couples to a Gi2α, instead of a Gsα, as reported in ovarian cells (24). Alternatively, TSH may transduce nontraditional, non-cAMP-dependent signals, as observed in preadipocytes and thyrocytes (25, 26) that include Ca2+ transients, MAP kinases, p70 S6K, and protein kinase B/Akt.
Finally, the question remains whether the skeletal effects of TSH mediated through TNFα are applicable to the osteoporosis and high fracture rates seen in hyperthyroidism. Indeed, there is a strong correlation between reduced TSH levels and fracture risk at both vertebral and nonvertebral sites (27). Furthermore, this high remodeling bone loss is reminiscent of cytokine-induced osteoporosis, and there is growing evidence that thyrotoxic states in both humans and animals are associated with elevated IL-1β, IL-6, and TNFα, as well as their soluble receptors (28–30). There is also evidence that TNFα polymorphisms are associated with Graves’ disease (31). Thus, the osteoporosis in human hyperthyroidism may also be TNF-driven, but this hypothesis needs clinical testing.
Materials and Methods
Animals and Chemicals.
Recombinant murine (m) IL-1α, murine or human (h) TNFα, hRANKL, hM-CSF, and hTSH were purchased from R & D Systems (Minneapolis, MN), Peprotech (Rocky Hill, NJ), Sigma–Aldrich (St. Louis, MO), or Fitzgerald Industries (Concord, MA). TSHR+/+, TSHR+/−, and TSHR−/− mice were generated and maintained in-house by using standard protocols approved by Institutional Animal Care and Use Committee at Mount Sinai School of Medicine. TNFα-null animals were obtained from The Jackson Laboratory (Bar Harbor, ME). Compound TSHR/TNFα mutant mice were generated by crossing TSHR+/− and TNFα+/− animals.
Osteoclast Formation.
TSHR and caTSHR cDNA plasmids were provided by G. Vassert (Universite Libre de Bruxelles, Bruxelles, Belgium) (13, 32). The plasmids were subcloned into the expression vector with EGFP (CLONTECH, Mountain View, CA) and transfected into RAW-C3 cells by using Mirus–Neural (Mirus, Madison, WI) to establish stable transformants through G418 (geneticin) selection. Pooled cells were used for TNFα expression, NF-κB- and AP-1 binding, and osteoclast formation experiments to minimize clone–clone variation. In the separate experiments, TSHR-EGFP or caTSHR-EGFP DNA fragments were also subcloned into a retroviral vector (pLXSN; CLONTECH) and transfected into a packaging cell line Plat-E (provided by I. Kitamura, Tokyo Medical Institute, Tokyo University, Tokyo, Japan) (33) by using Fugene 6 (Roche Applied Science, Indianapolis, IN). The supernatants were concentrated and used for transient infection into bone marrow macrophages in the presence of 4 μg/ml polybrene to examine osteoclast formation.
For osteoclastogenesis assays, RAW-C3 cells with an empty vector (Empty-C3) or stable transformants overexpressing TSHR or caTSHR (TSHR-C3 or caTSHR-C3) were cultured with RANKL (100 ng/ml) for 6 days. Bone marrow macrophages prepared by Ficoll-Plus (Amersham Pharmacia, Piscataway, NJ) were precultured with M-CSF (30 ng/ml) and then infected with the respective virus and cultured with RANKL (60 ng/ml) and M-CSF (30 ng/ml) for 6 days (5). The osteoclasts formed either in RAW-C3 or bone marrow cell cultures were stained for TRAP.
Flow Cytometry.
Cells in bone marrow were examined by flow cytometry using PE (phycoerythrin)-labeled CD11b, CD3, B220, CD90, CD45, CD4, and CD8 antibodies (PharMingen, San Diego, CA). TSHR expression was also detected by using RSR-1 (kindly provided by Bernard R. Smith; RSR Ltd., Cardiff, U.K.) (34) or MS-1 (15) antibodies. To avoid the binding of antibodies to Fc receptor, cells were preincubated with guinea pig serum and then incubated with TSHR antibodies or nonimmune IgG (isotype control). CD11b+, B220+, or CD106+ cells were isolated from fresh bone marrow, and CD3+ cells were obtained from spleen cells by using magnetic beads (PharMingen).
ELISA and Luciferase Assays.
TNFα production from RAW-C3 cells or bone marrow macrophages was measured by an ELISA kit (PharMingen), per manufacturer’s protocol. For ELISA, murine IL-1α and human TNFα were added in RAW-C3 cells or CD11b+ macrophages for 24 h, and the supernatant was collected to measure murine TNFα levels. The antimurine TNFα antibody did not cross-react with recombinant human TNFα.
For promoter assays, the murine TNFα promoter was PCR-amplified, and its sequence was confirmed by matching to the known sequence provided by the National Center for Biotechnology Information. The cDNA obtained was then cloned into pGL3 basic luciferase plasmid (Promega, Madison, WI), and the resulting construct was transfected into RAW-C3 cells by using Mirus–Neural. Luciferase activity was measured by using a dual luciferase reporter assay system (Promega) with Renilla luciferase (Promega) to normalize transfection efficiency. PMA (Sigma–Aldrich), a potent inducer of TNFα promoter activity (35), was used to stimulate TNFα transcription.
Gene Expression and M-CSF-Dependent Cell Proliferation.
TNFα, IL-1α, IL-1β, IL-6, TRAP, and TSHR mRNA expression were quantified by real-time PCR by using specific primer sets and iTaq SYBR green Supermix with a ROX kit (Bio-Rad, Hercules, CA). For cell proliferation studies, fresh bone marrow cells from TSHR+/+, TSHR+/−, and TSHR−/− mice were cultured in the presence of 30 ng/ml M-CSF in MethoCult (Stemcell Technologies, Vancouver, BC, Canada) for 7 days, and colonies containing >50 cells were counted. Proliferating CD11b+ macrophages were identified by the Cell Proliferation BrdU kit (Roche) at OD450.
EMSA.
Nuclear fractions of nonstimulated and stimulated RAW-C3 cells were prepared as reported in ref. 5. The nuclear fractions obtained were incubated with biotin-labeled consensus oligonucleotides prepared by using a biotin 3′-end DNA labeling kit (Pierce, Rockford, IL). The resulting oligonucleotide-transcription factor complexes were applied on a 6% acrylamid gel containing 0.5× TBE and 2.5% glycerol. Consensus DNA sequences used for AP-1 and NF-κB binding were CGCTTGATGAGTCAGGCCGGAA and AGTTGAGGGGACTTTCCCAGGC, respectively. Italicized nucleotide sequences are essential for DNA-protein binding.
Statistical Analyses.
Statistically significant differences were analyzed by using one-way ANOVA test (P < 0.05), and data were presented as the mean ± SEM.
Supplementary Material
Acknowledgments
E.A., T.F.D., and M.Z. thank the Department of Veterans Affairs for Merit Award support. This work was supported by National Institutes of Health Grants AR 052258 (to E.A.); AG14917, DK70526, and AG23176 (to M.Z.); and DK52464 (to T.F.D.).
Abbreviations
- RANKL
receptor activator of NF-κB ligand
- PMA
phorbol 12-myristate 13-acetate
- TRAP
tartrate-resistant acid phosphatase
- TSH
thyroid-stimulating hormone
- TSHR
TSH receptor
- caTSHR
constitutively active TSHR.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zaidi M. Cell Metab. 2005;1:219–221. doi: 10.1016/j.cmet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Szkudlinski M. W., Fremont V., Ronin C., Weintraub B. D. Physiol. Rev. 2002;82:479–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 3.Davies T., Marians R., Latif R. J. Clin. Invest. 2002;110:161–164. doi: 10.1172/JCI16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marians R. C., Ng L., Blair H. C., Unger P., Graves P. N., Davies T. F. Proc. Natl. Acad. Sci. USA. 2002;99:5776–5781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe E., Marians R. C., Yu W., Wu X. B., Ando T., Li Y., Iqbal J., Eldeiry L., Rajendren G., Blair H. C., et al. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 6.Gothe S., Wang Z., Ng L., Kindblom J. M., Barros A. C., Ohlsson C., Vennstrom B., Forrest D. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitzmann N. M., Pacifici R. Immunol. Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamada M., Irahara M., Maegawa M., Ohmoto Y., Murata K., Yasui T., Yamano S., Aono T. Gynecol. Obstet. Invest. 2001;52:82–88. doi: 10.1159/000052948. [DOI] [PubMed] [Google Scholar]
- 9.Nanes M. S. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 10.O’Gradaigh D., Ireland D., Bord S., Compston J. E. Ann. Rheum. Dis. 2004;63:354–359. doi: 10.1136/ard.2003.008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann M., Maini R. N. Annu. Rev. Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 12.Pichler R., Maschek W., Hatzl-Griesenhofer M., Huber H., Crespillo-Gomez C., Berg J. Horm. Metab. Res. 2003;35:427–433. doi: 10.1055/s-2003-41624. [DOI] [PubMed] [Google Scholar]
- 13.Govaerts C., Lefprt A., Costagliola S., Wodak S. J., Ballesterps J. A., van Sande J., Pardo L., Vassart G. J. Biol. Chem. 2001;276:22991–22999. doi: 10.1074/jbc.M102244200. [DOI] [PubMed] [Google Scholar]
- 14.Kwan-Tat S., Padrines M., Theoleyre S., Heymann D., Fortun Y. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Ando T., Latif R., Pritsker A., Moran T., Nagayama Y., Davies T. F. J. Clin. Invest. 2002;110:1667–1674. doi: 10.1172/JCI16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blin-Wakkach C., Wakkach A., Sexton P. M., Rochet N., Carle G. F. Leukemia. 2004;18:1505–1511. doi: 10.1038/sj.leu.2403449. [DOI] [PubMed] [Google Scholar]
- 17.Dremier S., Coulonval K., Perpete S., Vandeput F., Fortemaison N., van Keymeulen A., Deleu S., Ledent C., Clement S., Schurmans S., et al. Ann. N.Y. Acad. Sci. 2002;968:106–121. doi: 10.1111/j.1749-6632.2002.tb04330.x. [DOI] [PubMed] [Google Scholar]
- 18.Boyce B. F., Yao Z., Zhang Q., Badell I. R., Schwarz E. M., O’Keefe R. J., Xing L. Keio J. Med. 2005;54:127–131. doi: 10.2302/kjm.54.127. [DOI] [PubMed] [Google Scholar]
- 19.Yao Z., Li P., Zhang Q., Schwarz E. M., Keng P., Arbini A., Boyce B. F., Xing L. J. Biol. Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 20.Xie H., Ye M., Graf T. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 21.Maret A., Coudert J. D., Garidou L., Foucras G., Gourdy P., Krust A., Dupont S., Chambon P., Druet P., Bayard F., et al. Eur. J. Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 22.Wang H.-C., Dragoo J., Zhou Q., Klein J. R. Blood. 2003;101:119–123. doi: 10.1182/blood-2002-02-0544. [DOI] [PubMed] [Google Scholar]
- 23.Kaneki H., Guo R., Chen D., Yao Z., Schwarz E. M., Zhang Y. E., Boyce B. F., Xing L. J. Biol. Chem. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L., Peng Y., Sharrow A., Iqbal J., Zhang Z., Dionysios J., Papachristou D. J., Zaidi S., Zhu L.-L., Yaroslavskiy B. B., et al. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Bell A., Gagnon A., Dods P., Papineau D., Tiberi M., Sorisky A. Am. J. Physiol. 2002;283:C1056–C1064. doi: 10.1152/ajpcell.00058.2002. [DOI] [PubMed] [Google Scholar]
- 26.Rivas M., Santisteban P. Mol. Cell. Endocrinol. 2003;213:31–45. doi: 10.1016/j.mce.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Bauer D. C., Ettinger B., Nevitt M. C., Stone K. L. Ann. Intern. Med. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 28.Diez J. J., Hernanz A., Medina S., Bayon C., Iglesias P. Clin. Endocrinol. (Oxford) 2002;57:515–521. doi: 10.1046/j.1365-2265.2002.01629.x. [DOI] [PubMed] [Google Scholar]
- 29.Simsek G., Karter Y., Aydin S., Uzun H. Chin. J. Physiol. 2003;46:181–186. [PubMed] [Google Scholar]
- 30.Senturk T., Kozaci L. D., Kok F., Kadikoylu G., Bolaman Z. Clin. Invest. Med. 2003;26:58–63. [PubMed] [Google Scholar]
- 31.Simmonds M. J., Heward J. M., Howson J. M., Foxall H., Nithiyananthan R., Franklyn J. A., Gough S. C. Genes Immun. 2004;5:267–273. doi: 10.1038/sj.gene.6364066. [DOI] [PubMed] [Google Scholar]
- 32.Perret J., Ludgate M., Libert F., Gerard C., Dumont J. E., Vassart G., Parmentier M. Biochem. Biophys. Res. Commun. 1990;171:1044–1050. doi: 10.1016/0006-291x(90)90789-p. [DOI] [PubMed] [Google Scholar]
- 33.Morita S., Kojima T., Kitamura T. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 34.Sanders J., Jeffreys J., Depraetere H., Richards T., Evans M., Kiddie A., Brereton K., Groenen M., Oda Y., Furmaniak J., et al. Thyroid. 2002;12:1043–1050. doi: 10.1089/105072502321085135. [DOI] [PubMed] [Google Scholar]
- 35.Rhoades K. L., Golub S. H., Economou J. S. J. Biol. Chem. 1992;267:22102–22107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.