Abstract
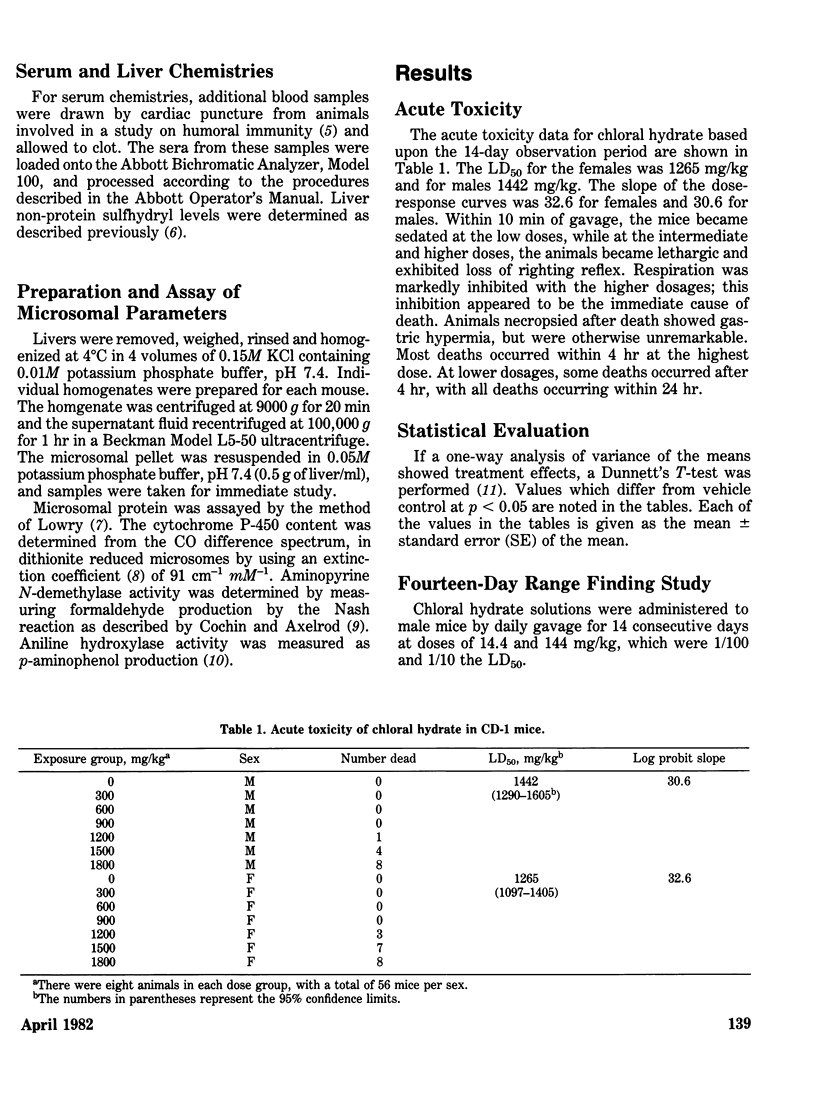
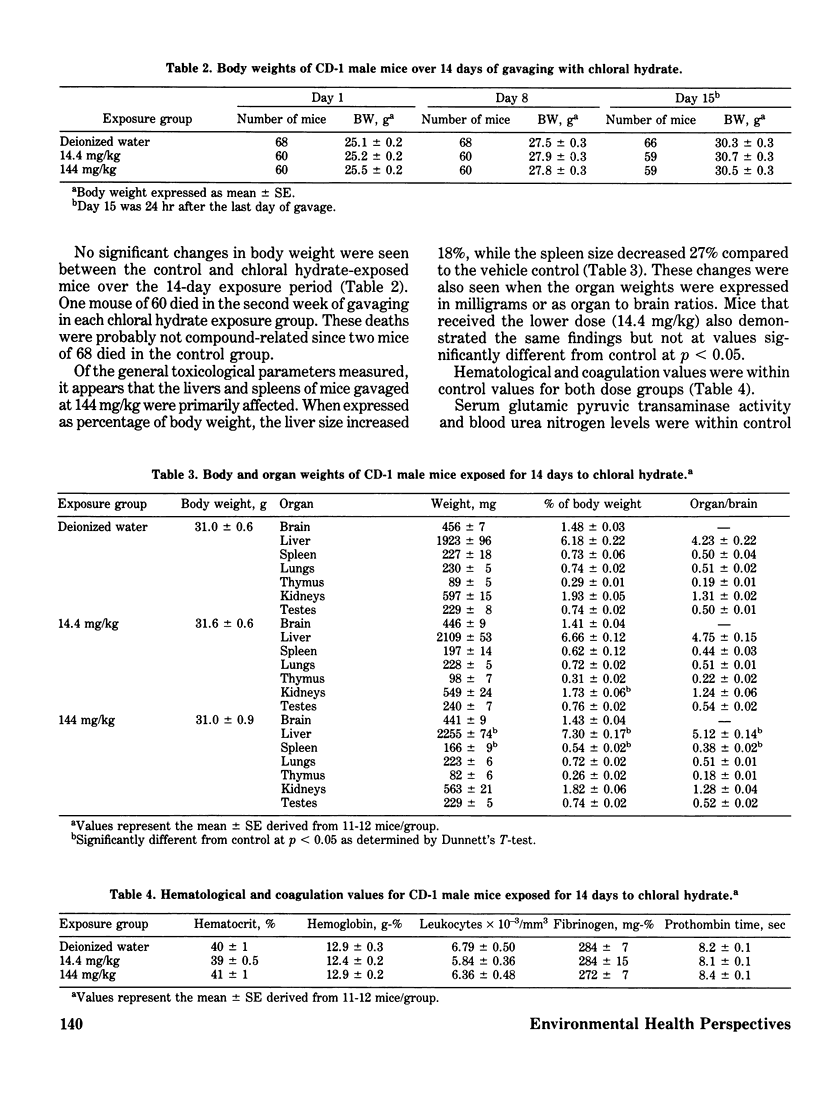
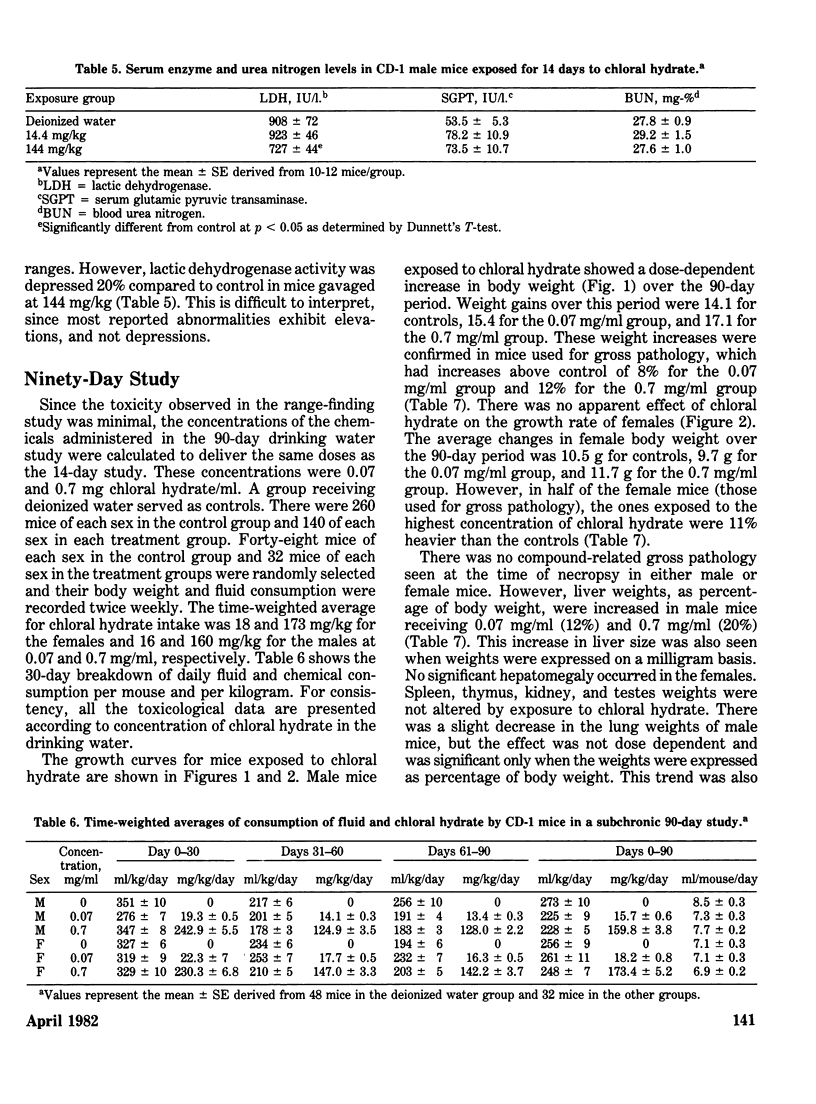
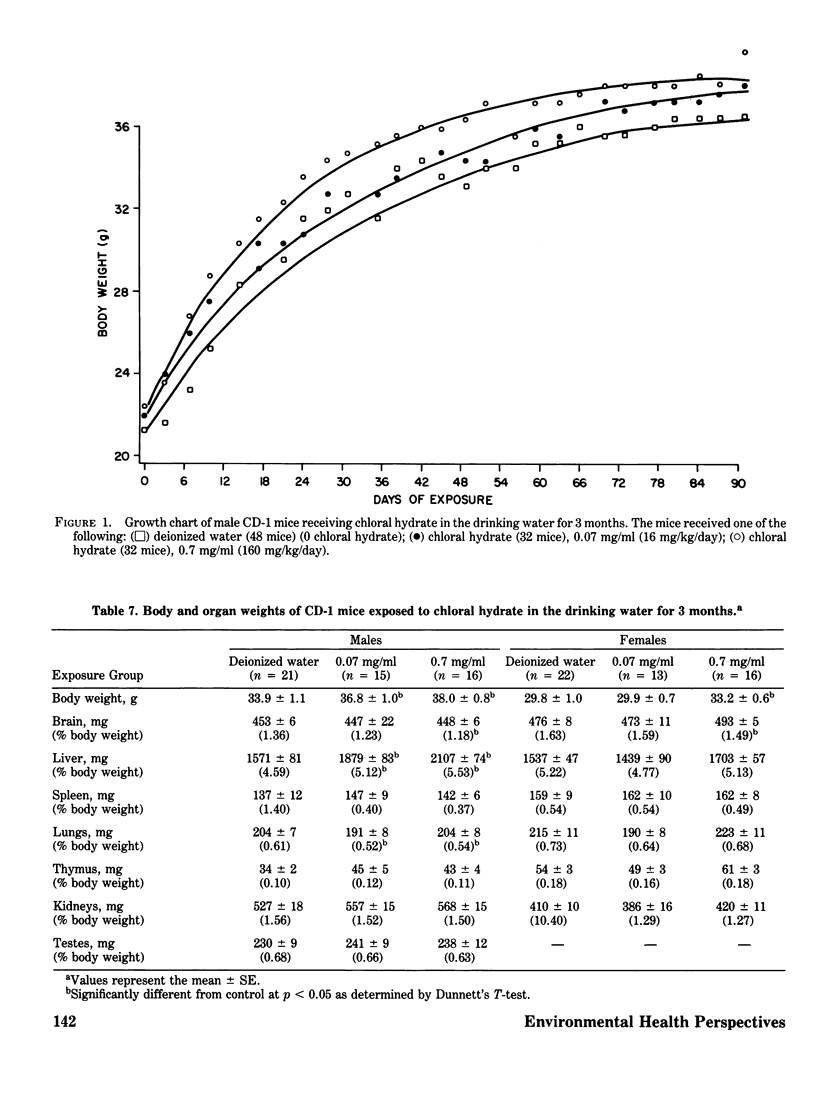
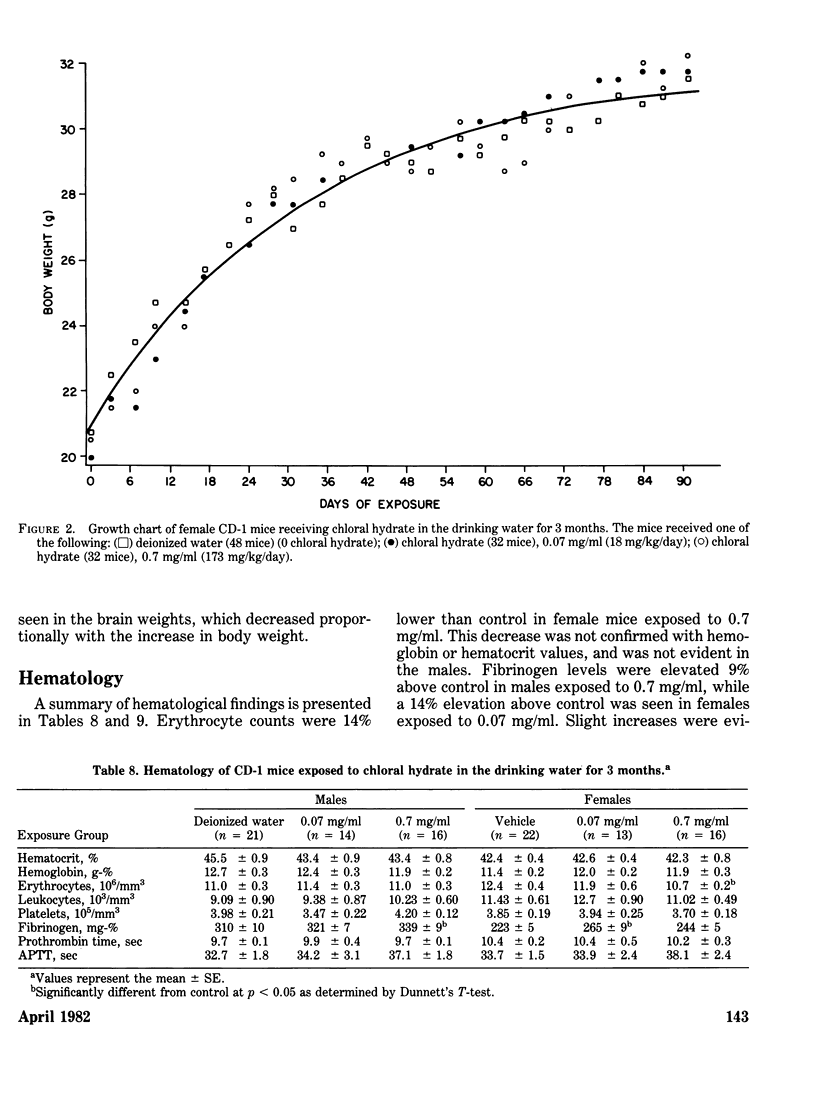
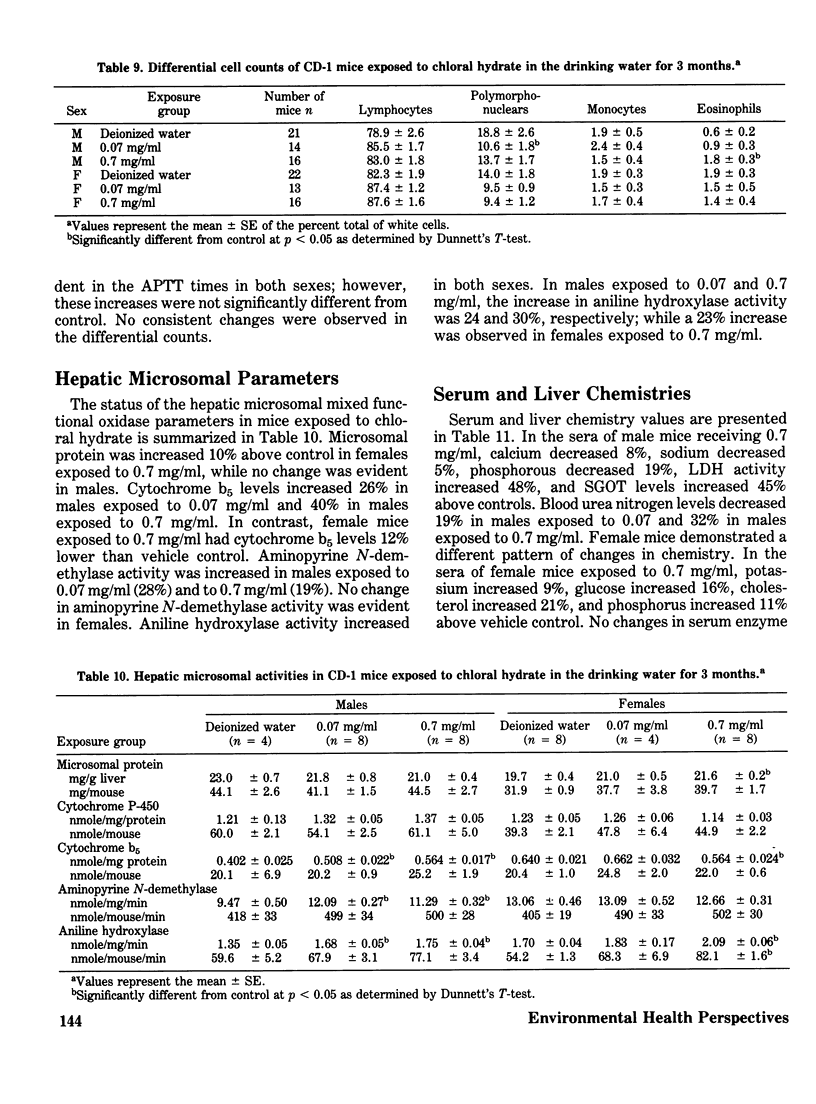
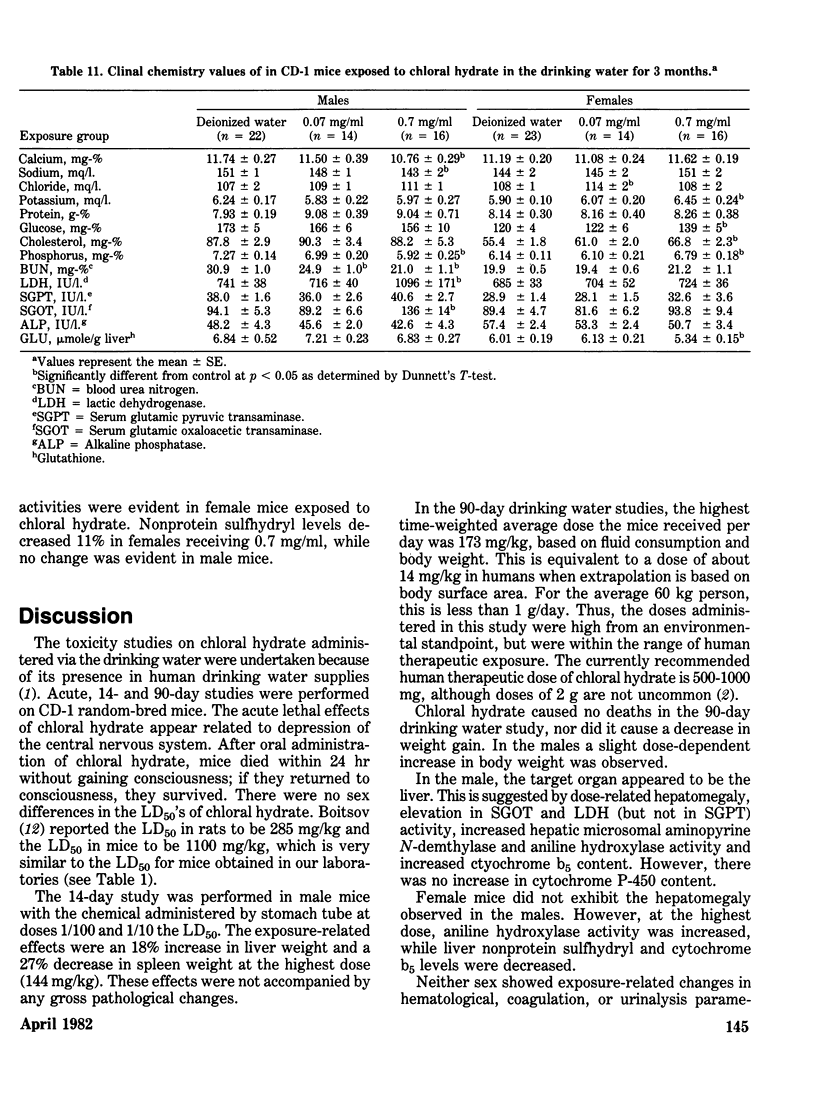
Chloral hydrate has been found in our drinking water supplies at levels up to 5 micrograms/1. The purpose of this study was to evalute the acute and subchronic toxicology of chloral hydrate in the random-bred CD-1 mouse, to provide data for risk assessment. The acute oral LD50 of this compound was 1442 and 1265 mg/kg in male and female mice, respectively. Acute toxicity appeared to be related to depression of the central nervous system. Fourteen-day exposure by gavage in male mice at doses 1/10 and 1/100 the LD50 caused an increase in liver weight and a decrease in spleen weight at the highest dose level. Based on the data derived from 14 days of exposure, a 90-day study was performed. The compound was delivered via the drinking water; levels of the compound delivered per day were equivalent to those dosed in the 14-day study. The target organ in both sexes appeared to be the liver, with the males most affected. Male mice demonstrated a dose-related hepatomegaly accompanied by significant changes in serum chemistries and hepatic microsomal parameters. The females did not demonstrate the hepatomegaly observed in males, but did show alterations in hepatic microsomal parameters. No other significant toxicological changes were observed in either sex following 90 days of exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W., Morahan P. S., Loveless S., Munson A. E. The effects of maleic anhydride-divinyl ether (MVE) copolymers on hepatic microsomal mixed-function oxidases and other biologic activities. J Pharmacol Exp Ther. 1979 Mar;208(3):392–398. [PubMed] [Google Scholar]
- Boitsov A. N., Rotenberg Iu S., Mulenkova V. G. K voprosu o toksikologicheskoi otsenke khloralia v protsesse ego vydeleniia pri napylenii i zalivke penopoliuretanov. Gig Tr Prof Zabol. 1970 Jun;14(6):26–29. [PubMed] [Google Scholar]
- COCHIN J., AXELROD J. Biochemical and pharmacological changes in the rat following chronic administration of morphine nalorphine and normorphine. J Pharmacol Exp Ther. 1959 Feb;125(2):105–110. [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- Kauffmann B. M., White K. L., Jr, Sanders V. M., Douglas K. A., Sain L. E., Borzelleca J. F., Munson A. E. Humoral and cell-mediated immune status in mice exposed to chloral hydrate. Environ Health Perspect. 1982 Apr;44:147–151. doi: 10.1289/ehp.8244147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Waters E. M., Gerstner H. B., Huff J. E. Trichloroethylene. I. An overview. J Toxicol Environ Health. 1977 Jan;2(3):671–707. doi: 10.1080/15287397709529469. [DOI] [PubMed] [Google Scholar]


