Abstract
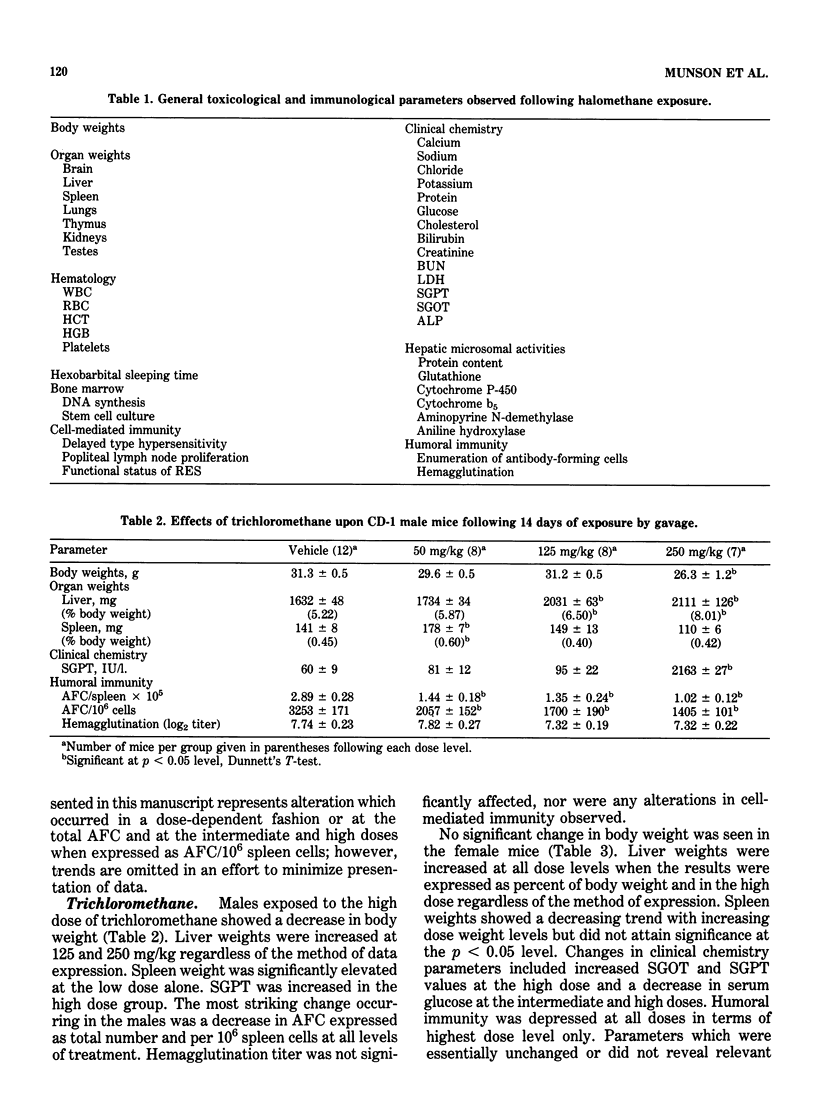
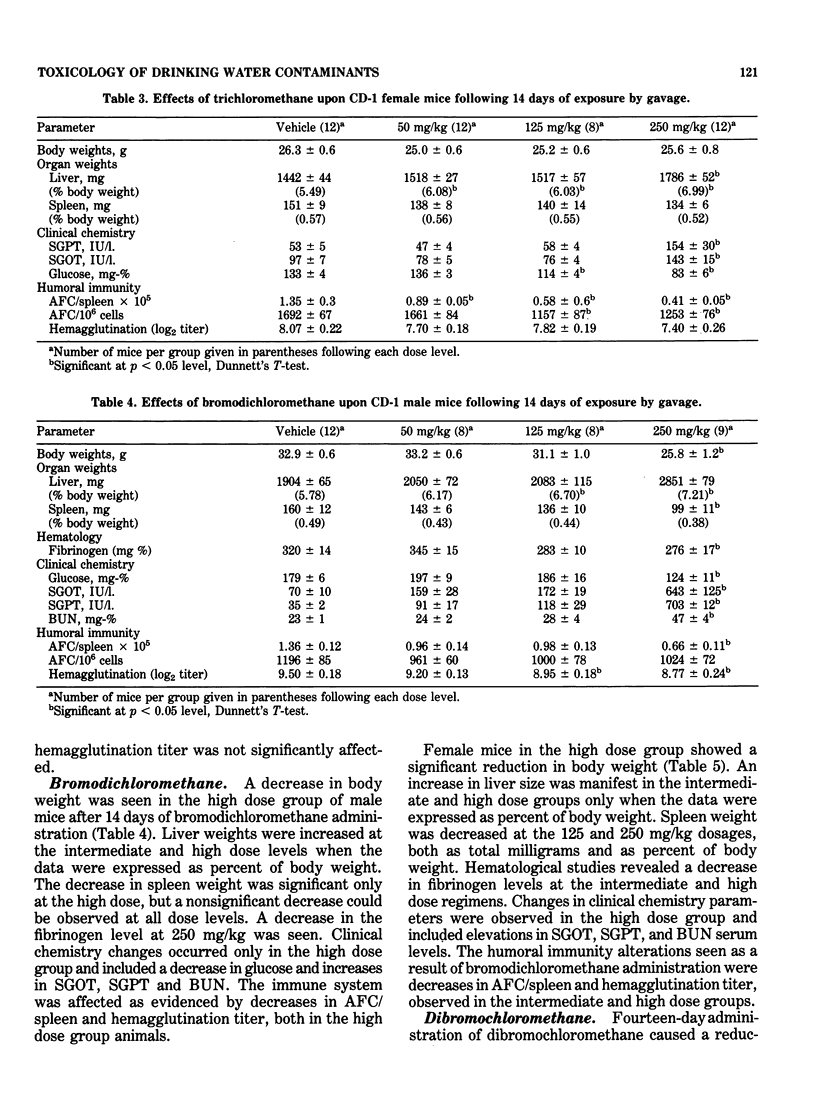
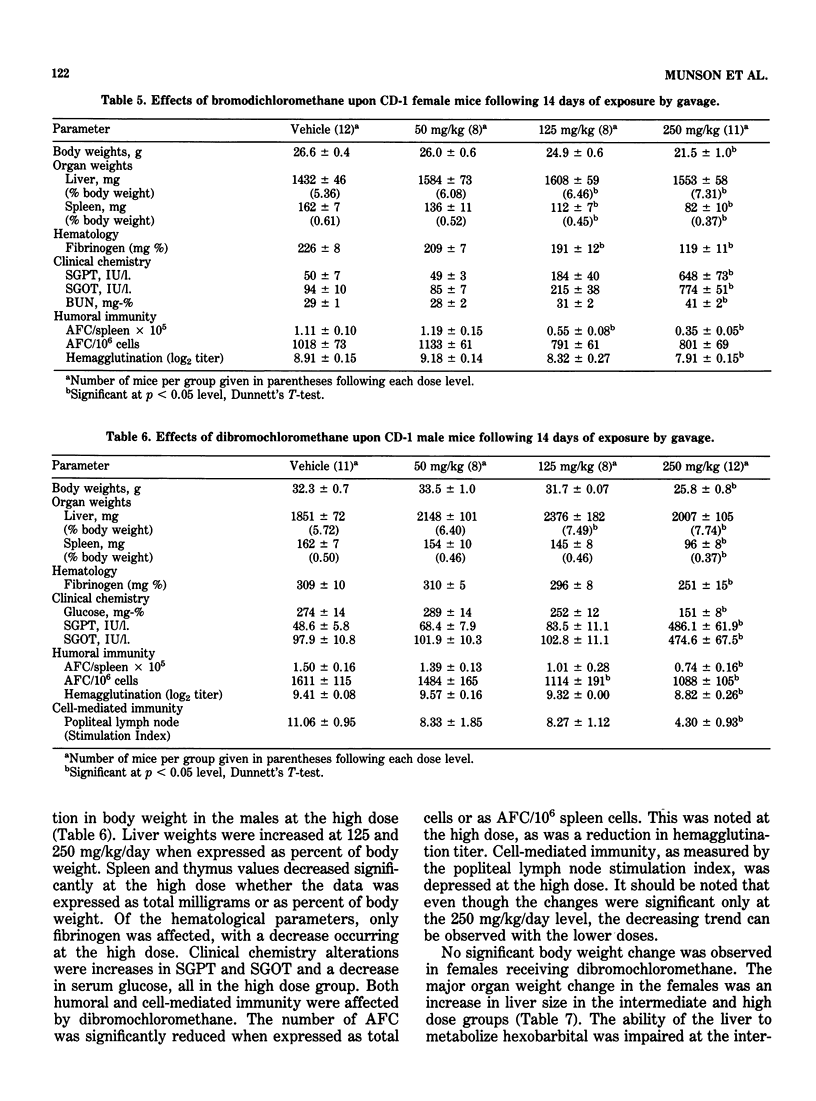
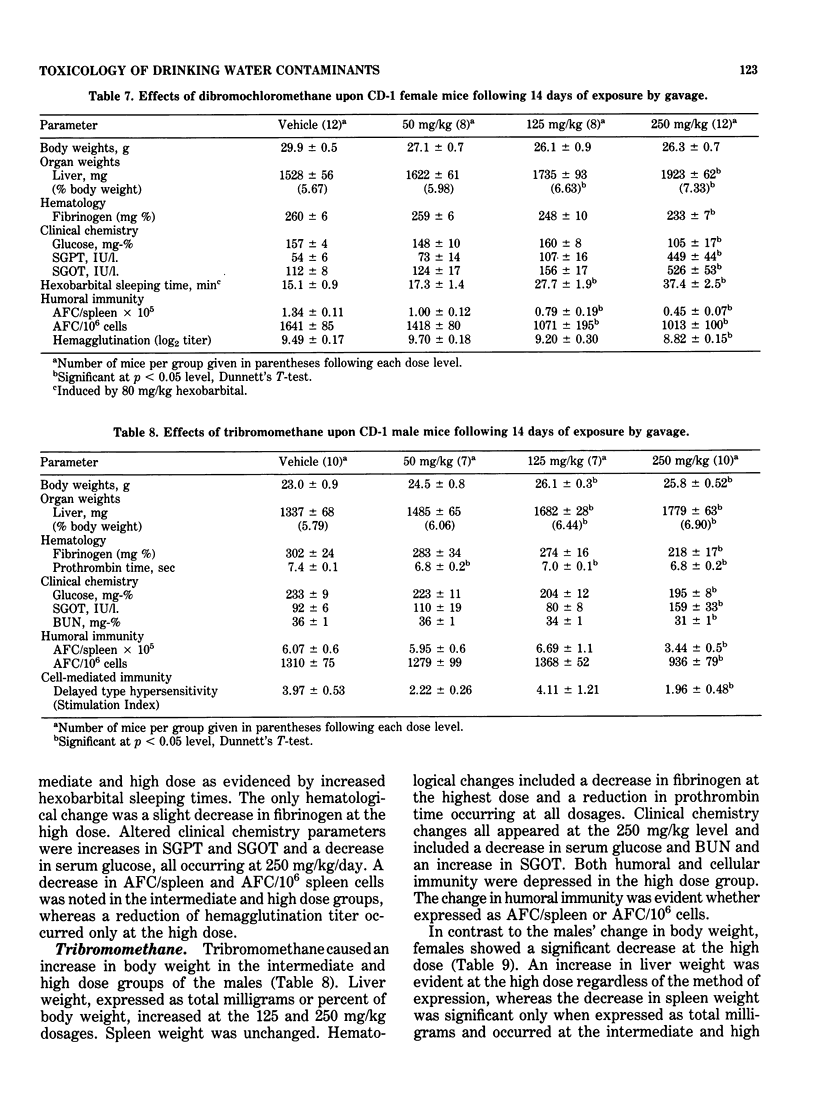
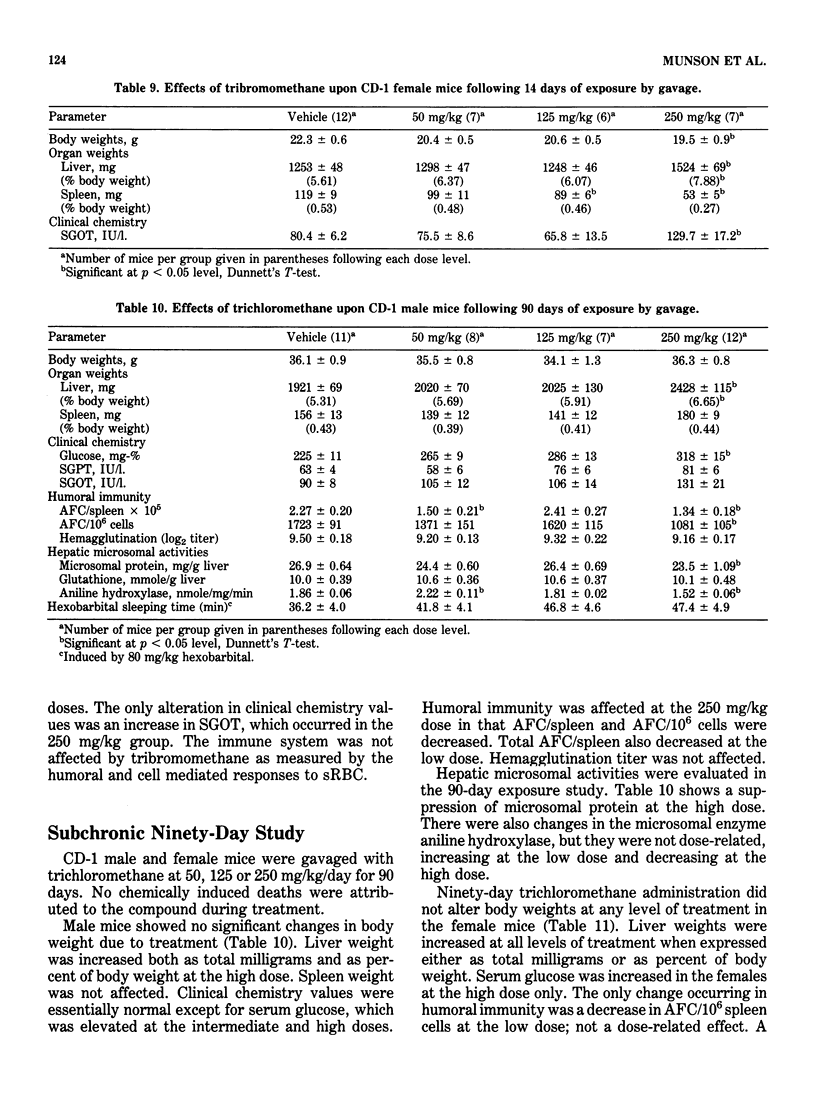
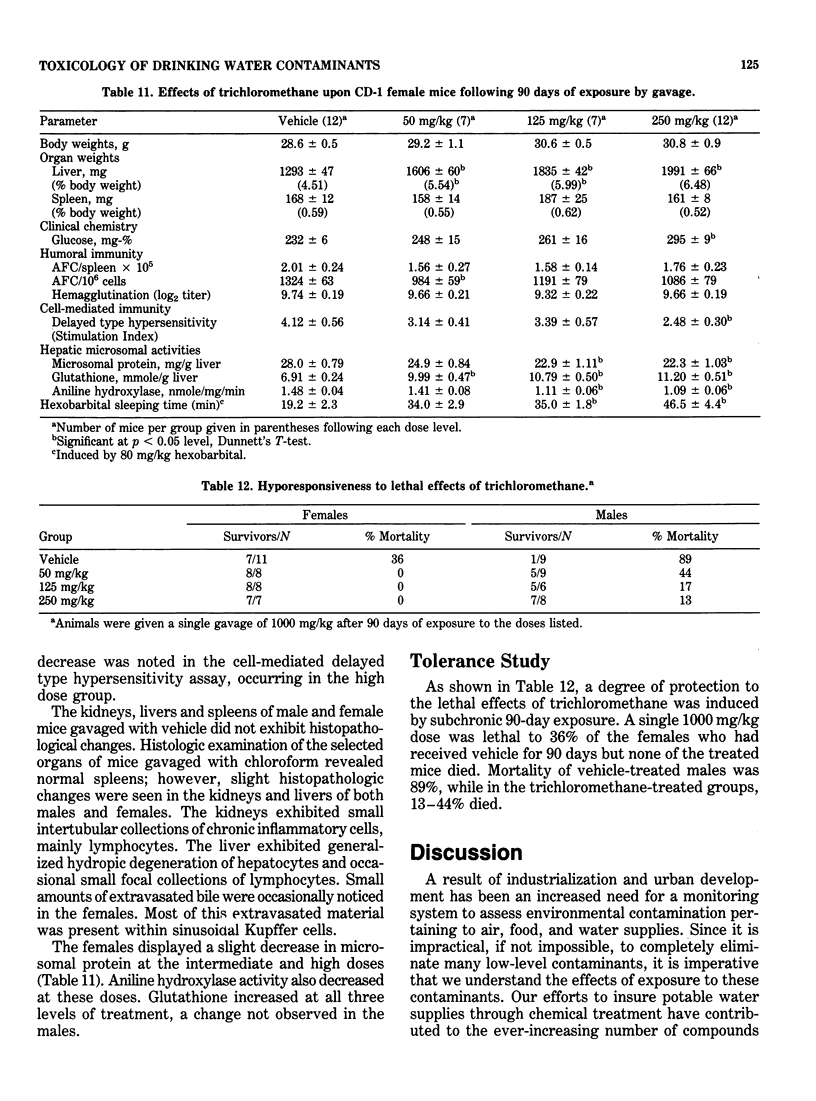
This study evaluated the subchronic toxicity of selected halomethanes which are drinking water contaminants. The compounds studied were trichloromethane, bromodichloromethane, dibromochloromethane and tribromomethane. Subchronic 14-day gavage studies were performed with the use of doses encompassing one-tenth the LD50 for the compounds. A 90-day gavage study of one of the compounds, trichloromethane, was also done. Parameters observed included body and organ weights, histopathology, hematology, clinical chemistries, and hepatic microsomal enzyme activities. Toxicity to the humoral immune system was assessed by measuring the number of splenic IgM antibody-forming cells and the serum antibody level to sheep erythrocytes. Cell-mediated immunity was evaluated by measuring the delayed type hypersensitivity response and popliteal lymph node proliferation response to sheep red blood cells. The functional activity of the reticuloendothelial system, as measured by the vascular clearance rate and tissue uptake of 51Cr sheep red blood cells was also determined. The major effects of the halomethanes were increased liver weights, elevations of SGPT and SGOT, decreased spleen weights and a decrease in the number of splenic IgM antibody-forming cells. The humoral immune system appeared to be an indicator of halomethane toxicity. There is evidence that subchronic 14-day exposure may be of greater value than long-term studies in determining the toxicity of these compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COCHIN J., AXELROD J. Biochemical and pharmacological changes in the rat following chronic administration of morphine nalorphine and normorphine. J Pharmacol Exp Ther. 1959 Feb;125(2):105–110. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson A. E., Regelson W., Wooles W. R. Tissue localization studies in evaluating the functional role of the RES. J Reticuloendothel Soc. 1970 Mar;7(3):366–374. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Paranjpe M. S., Boone C. W. Delayed hypersensitivity to simian virus 40 tumor cells in BALB-c mice demonstrated by a radioisotopic foot-pad assay. J Natl Cancer Inst. 1972 Feb;48(2):563–566. [PubMed] [Google Scholar]


