Abstract
Carcinogenesis bioassays of blocky (nonfibrous) tremolite and amosite asbestos alone or in combination with the intestinal carcinogen 1,2-dimethylhydrazine dihydrochloride (DMH) were conducted with male and female Fischer 344 rats. The minerals were administered at a concentration of 1% in pelleted diet for the entire lifetime of the rats starting with the dams of the test animals. One group of amosite rats also received chrysotile asbestos via gavage during lactation. Group sizes varied from 100 to 250 animals. The offspring from mothers exposed to tremolite or amosite asbestos were smaller at weaning than those from untreated mothers and remained smaller throughout their life. The administration of dimethylhydrazine (DMH) did not affect body weight gain, either in amosite-exposed or nonexposed animals. Survival was comparable in the tremolite and control groups. The amosite-exposed rats showed enhanced survival compared to the untreated controls. DMH exposure reduced survival by approximately one year, although the amosite plus DMH groups survived slightly better than the DMH alone groups. No toxicity or increase in neoplasia was observed in the tremolite-exposed rats compared to the controls. Significant increases (p less than 0.05) in the rates of C-cell carcinomas of the thyroid and monocytic (mononuclear cell) leukemia in male rats were observed in amosite-exposed groups. However, the biological significance of the C-cell carcinomas in relation to amosite asbestos exposure is discounted because of a lack of significance when C-cell adenomas and carcinomas were combined and the positive effect was not observed in the amosite plus preweaning gavage group. The biological significance of an increased incidence of mononuclear cell leukemia is questionable, because of a lack of statistical significance in the amosite group when evaluated using life table analysis, lack of significance when compared to the tremolite control group, and the fact that no toxic or neoplastic lesions were observed in the target organs, i.e., gastrointestinal tract and mesothelium. DMH caused a high rate of (62-74%) of intestinal neoplasia in amosite and nonamosite-exposed groups. Neither an enhanced carcinogenic nor protective effect was demonstrated by exposure to amosite asbestos.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coleman G. L., Barthold W., Osbaldiston G. W., Foster S. J., Jonas A. M. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977 May;32(3):258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. M., Olson G. F. Ingested mineral fibers: elimination in human urine. Science. 1979 Apr 13;204(4389):195–198. doi: 10.1126/science.219478. [DOI] [PubMed] [Google Scholar]
- Cunningham H. M., Moodie C. A., Lawrence G. A., Pontefract R. D. Chronic effects of ingested asbestos in rats. Arch Environ Contam Toxicol. 1977;6(4):507–513. doi: 10.1007/BF02097789. [DOI] [PubMed] [Google Scholar]
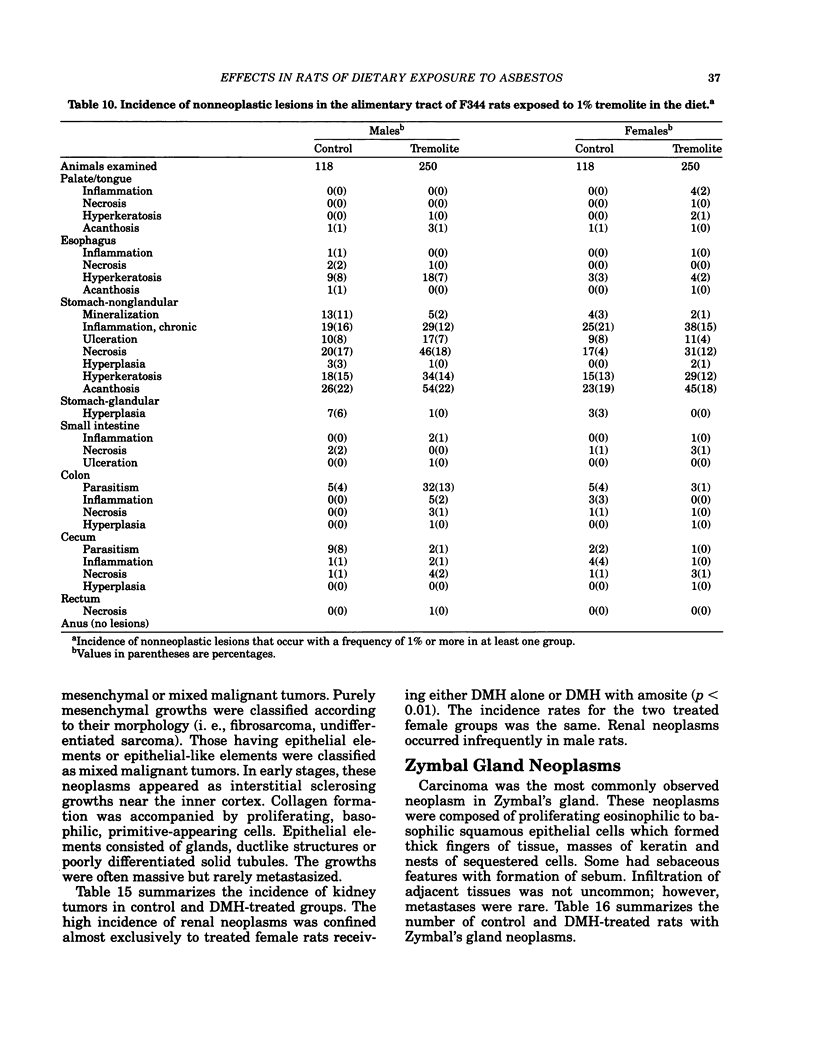
- Donham K. J., Berg J. W., Will L. A., Leininger J. R. The effects of long-term ingestion of asbestos on the colon of F344 rats. Cancer. 1980 Mar 15;45(5 Suppl):1073–1084. doi: 10.1002/1097-0142(19800315)45:5+<1073::aid-cncr2820451308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gart J. J., Chu K. C., Tarone R. E. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Inst. 1979 Apr;62(4):957–974. [PubMed] [Google Scholar]
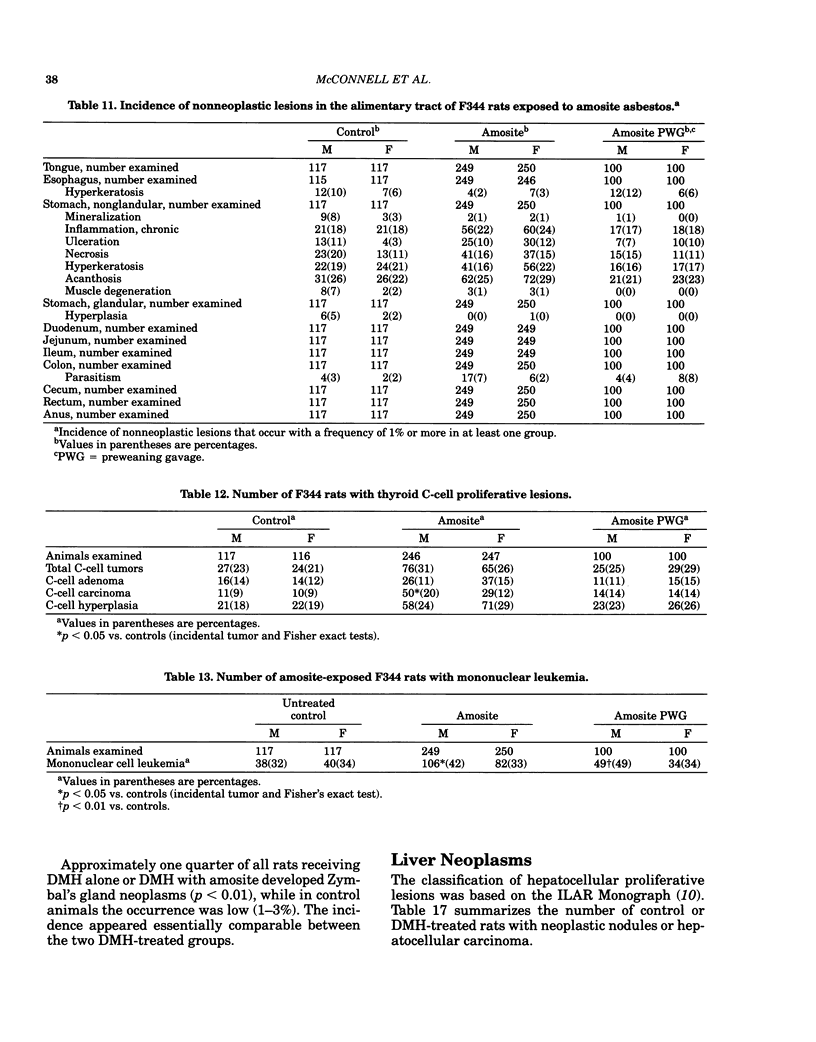
- Goodman D. G., Ward J. M., Squire R. A., Chu K. C., Linhart M. S. Neoplastic and nonneoplastic lesions in aging F344 rats. Toxicol Appl Pharmacol. 1979 Apr;48(2):237–248. doi: 10.1016/0041-008x(79)90029-2. [DOI] [PubMed] [Google Scholar]
- Gross P., Harley R. A., Swinburne L. M., Davis J. M., Greene W. B. Ingested mineral fibers. Do they penetrate tissue or cause cancer? Arch Environ Health. 1974 Dec;29(6):341–347. doi: 10.1080/00039896.1974.10666612. [DOI] [PubMed] [Google Scholar]
- Hilding A. C., Hilding D. A., Larson D. M., Aufderheide A. C. Biological effects of ingested amosite asbestos, taconite tailings, diatomaceous earth and Lake Superior water in rats. Arch Environ Health. 1981 Nov-Dec;36(6):298–303. doi: 10.1080/00039896.1981.10667641. [DOI] [PubMed] [Google Scholar]
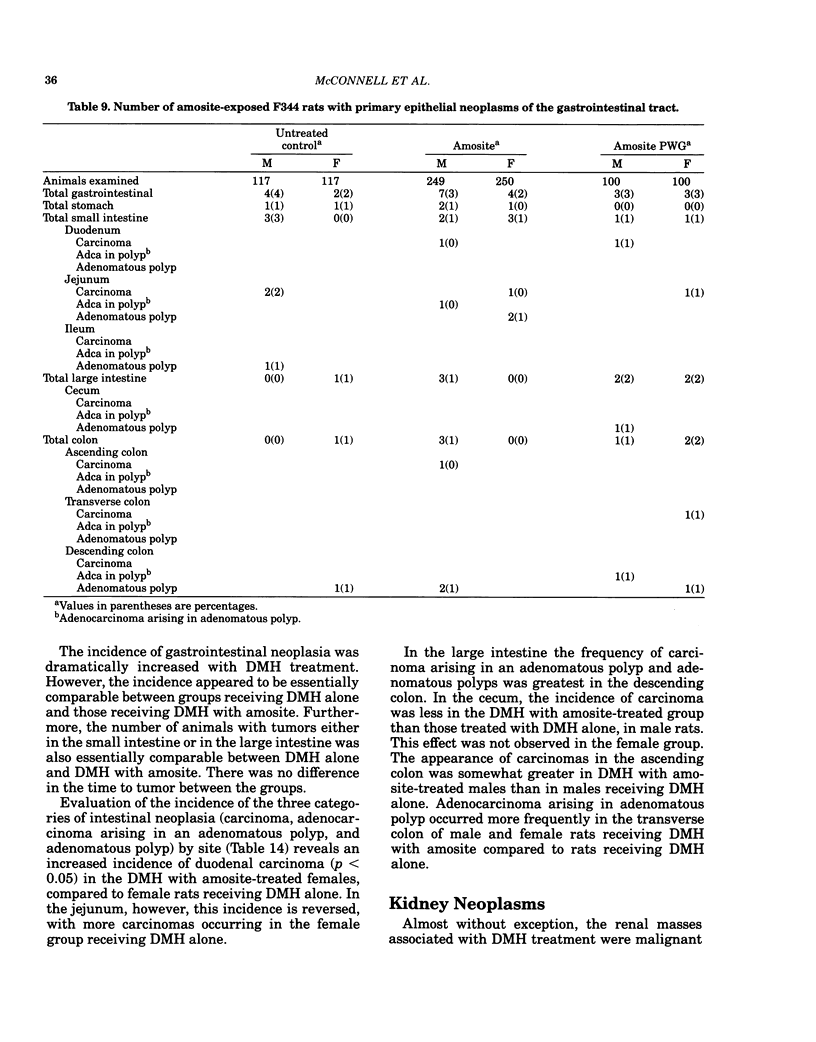
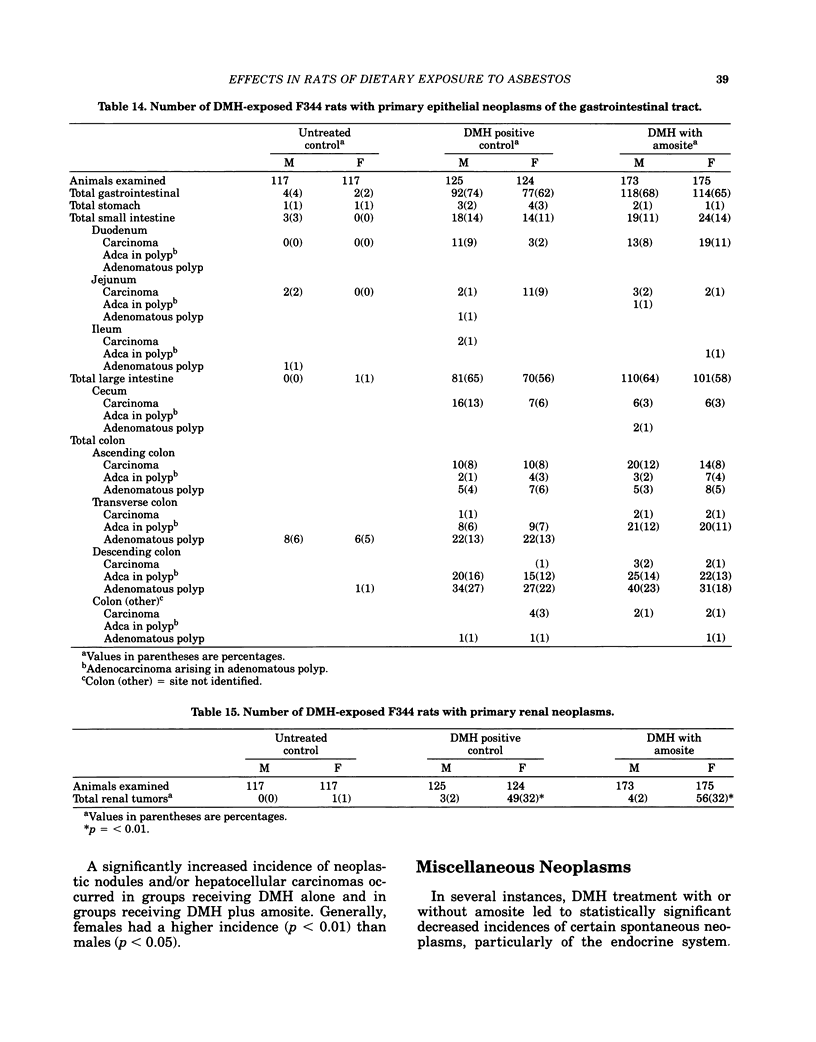
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- McConnell E. E., Wilson R. E., Moore J. A., Haseman J. K. Dose response of 1,2-dimethylhydrazine and methylazoxymethanol acetate in the F 344 rat. Cancer Lett. 1980 Jan;8(3):271–278. doi: 10.1016/0304-3835(80)90013-0. [DOI] [PubMed] [Google Scholar]
- Pozharisski K. M. Morphology and morphogenesis of experimental epithelial tumors of the intestine. J Natl Cancer Inst. 1975 May;54(5):1115–1135. doi: 10.1093/jnci/54.5.1115. [DOI] [PubMed] [Google Scholar]
- Sebastien P., Masse R., Bignon J. Recovery of ingested asbestos fibers from the gastrointestinal lymph in rats. Environ Res. 1980 Jun;22(1):201–216. doi: 10.1016/0013-9351(80)90132-2. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Layard M., Tegeris A., Miller E., May M., Morgan E., Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981 Nov;67(5):965–975. [PubMed] [Google Scholar]
- Ward J. M. Dose response to a single injection of azoxymethane in rats. Induction of tumors in the gastrointestinal tract, auditory sebaceous glands, kidney, liver and preputial gland. Vet Pathol. 1975;12(3):165–177. doi: 10.1177/030098587501200302. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Frank A. L., Wenk M., Devor D., Tarone R. E. Ingested asbestos and intestinal carcinogenesis in F344 rats. J Environ Pathol Toxicol. 1980 Jun-Jul;3(5-6):301–312. [PubMed] [Google Scholar]
- Yu B. P., Masoro E. J., Murata I., Bertrand H. A., Lynd F. T. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982 Mar;37(2):130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]


