Abstract
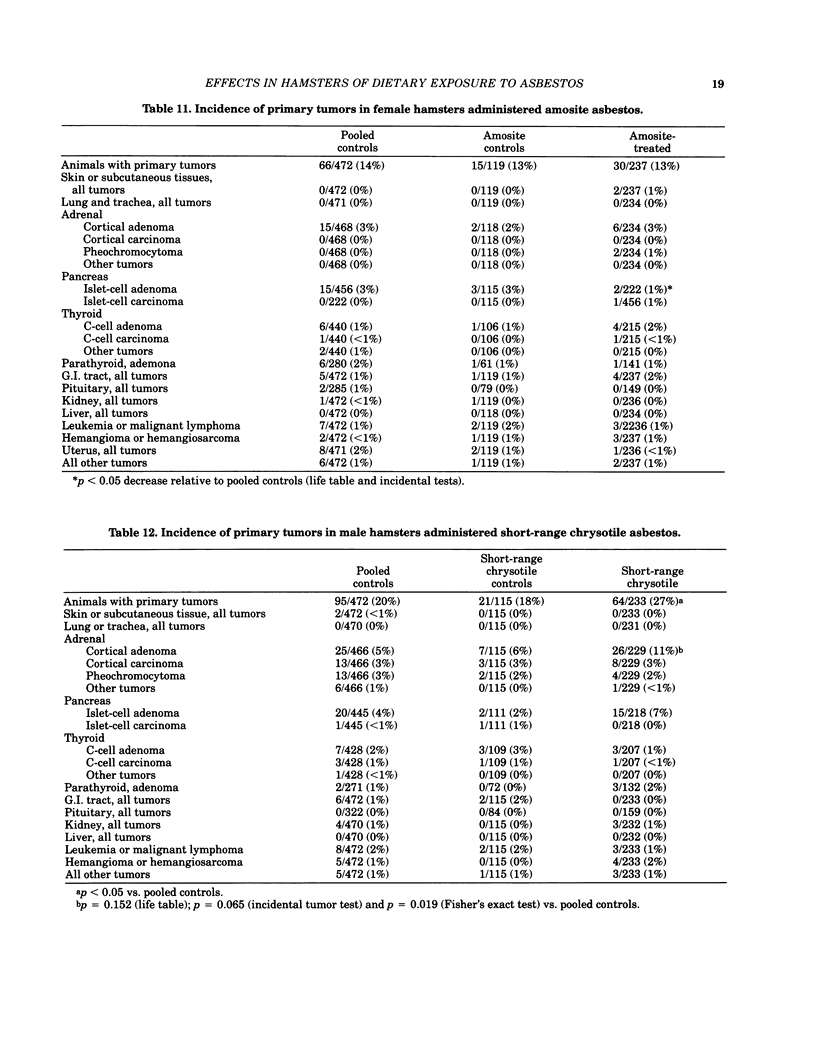
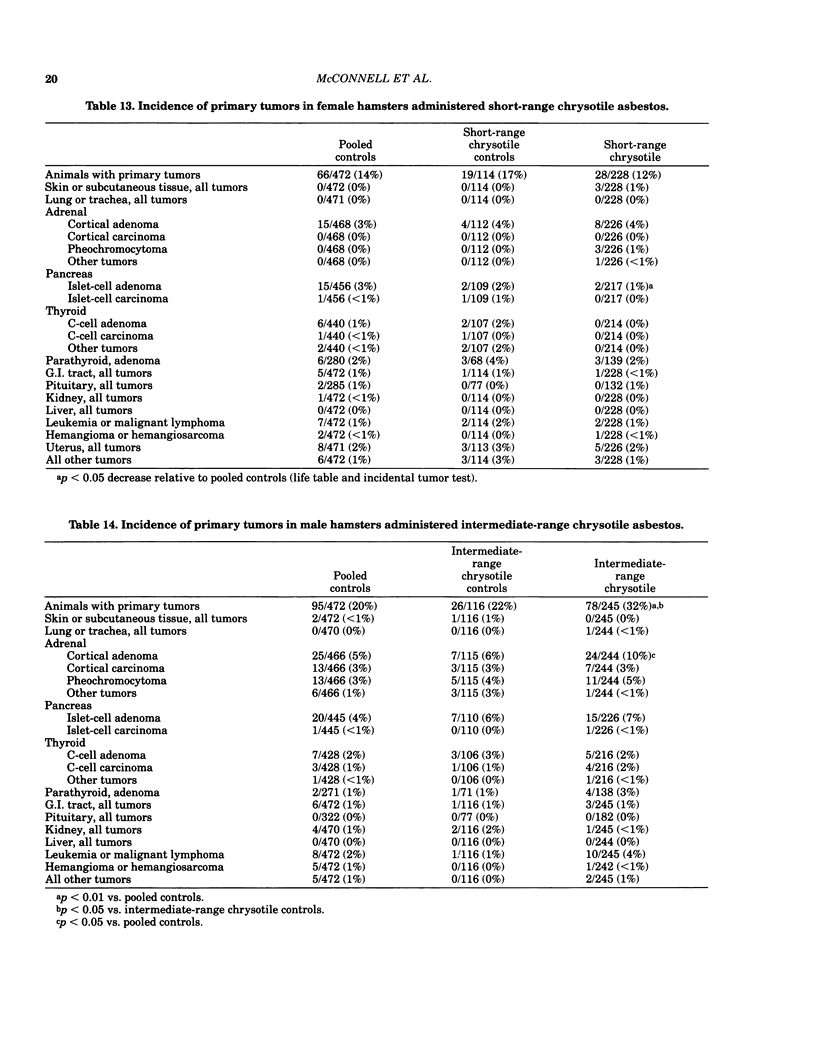
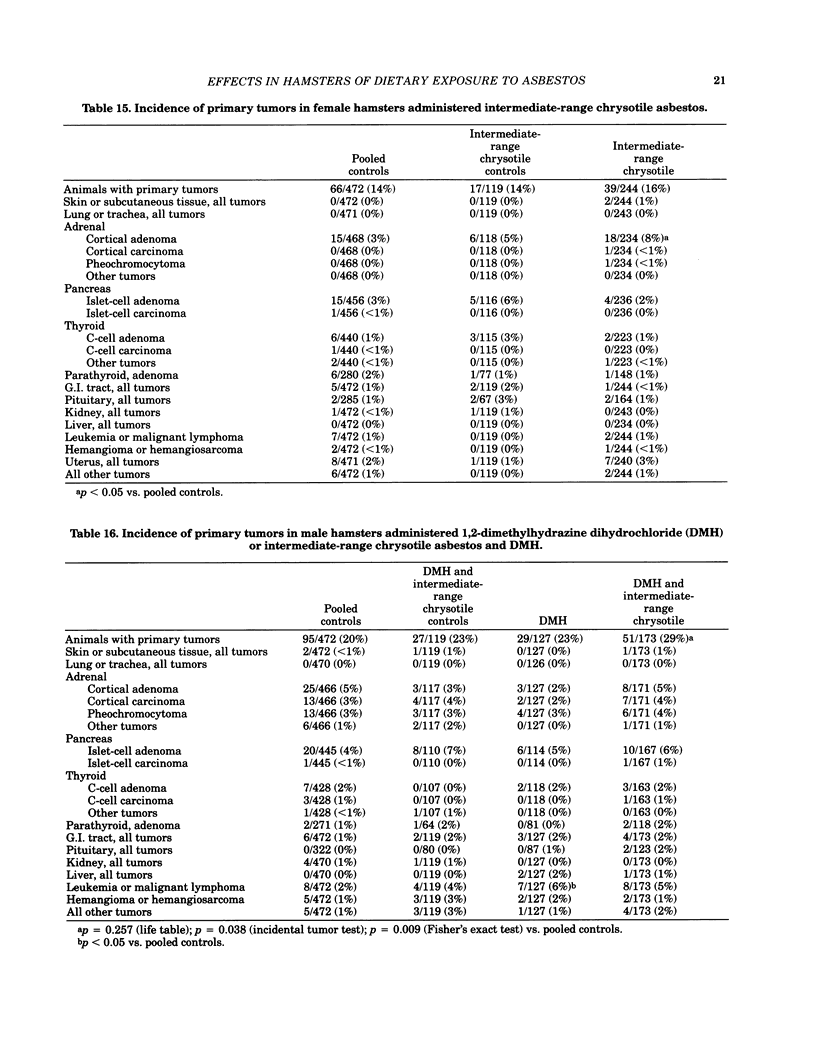
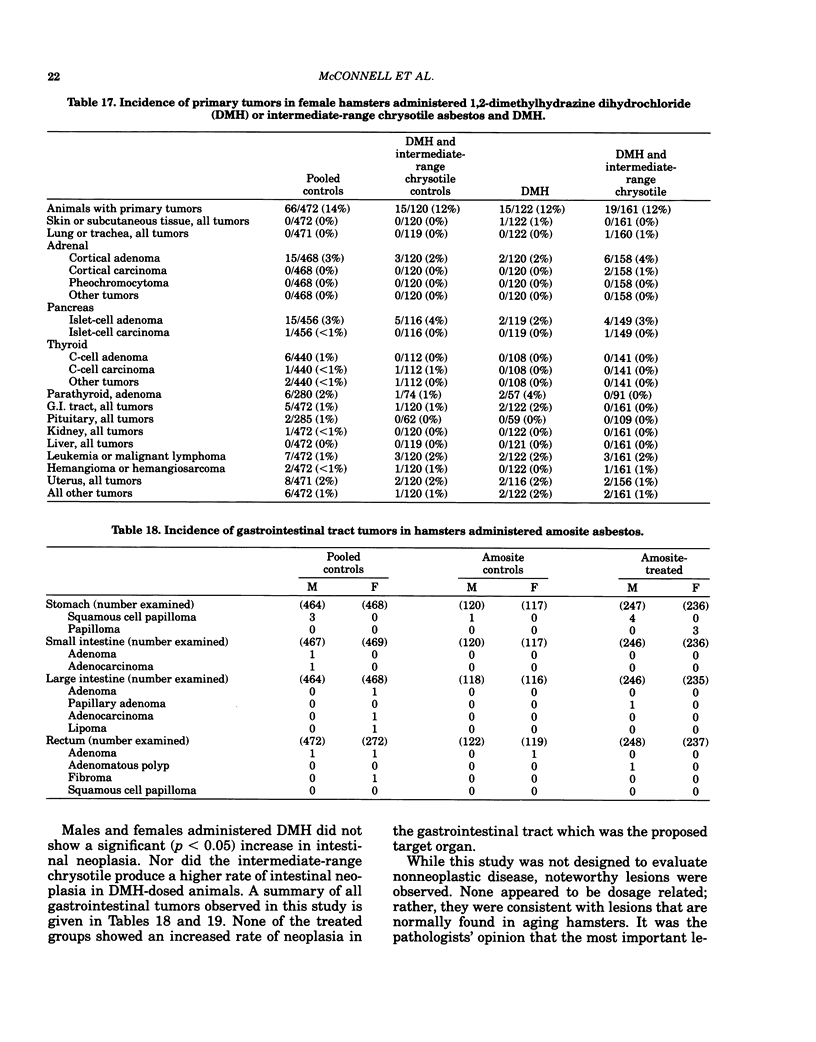
Bioassays of amosite, short-range (SR), intermediate-range (IR) or intermediate-range chrysotile asbestos in combination with the intestinal carcinogen 1,2-dimethylhydrazine dihydrochloride (DMH) were conducted with male and female Syrian golden hamsters. Amosite and both forms of chrysotile asbestos were administered at a concentration of 1% in pelleted diet for the entire lifetime of the hamsters starting with mothers of the test animals. Group sizes varied from 125-254. There was no adverse effect on body weight gain or survival by either type of asbestos or by IR chrysotile asbestos in combination with DMH. A significant increase (p less than 0.05) in adrenal cortical tumors was observed in male hamsters exposed to SR and IR chrysotile asbestos and in females treated with IR chrysotile asbestos when compared to the pooled control groups. However, statistical significance (p less than 0.05) was lost when these dosed groups were compared with temporal control groups. Neither of the male or female amosite asbestos groups showed increased neoplasia in any tissue or organ compared to the control groups. The cocarcinogen studies using IR chrysotile asbestos and 1,2-dimethylhydrazine dihydrochloride were considered inadequate because there was no increase in intestinal neoplasia in the DMH group.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook P. M., Olson G. F. Ingested mineral fibers: elimination in human urine. Science. 1979 Apr 13;204(4389):195–198. doi: 10.1126/science.219478. [DOI] [PubMed] [Google Scholar]
- Cunningham H. M., Moodie C. A., Lawrence G. A., Pontefract R. D. Chronic effects of ingested asbestos in rats. Arch Environ Contam Toxicol. 1977;6(4):507–513. doi: 10.1007/BF02097789. [DOI] [PubMed] [Google Scholar]
- Donham K. J., Berg J. W., Will L. A., Leininger J. R. The effects of long-term ingestion of asbestos on the colon of F344 rats. Cancer. 1980 Mar 15;45(5 Suppl):1073–1084. doi: 10.1002/1097-0142(19800315)45:5+<1073::aid-cncr2820451308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gart J. J., Chu K. C., Tarone R. E. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Inst. 1979 Apr;62(4):957–974. [PubMed] [Google Scholar]
- Gross P., Harley R. A., Swinburne L. M., Davis J. M., Greene W. B. Ingested mineral fibers. Do they penetrate tissue or cause cancer? Arch Environ Health. 1974 Dec;29(6):341–347. doi: 10.1080/00039896.1974.10666612. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- McConnell E. E., Wilson R. E., Moore J. A., Haseman J. K. Dose response of 1,2-dimethylhydrazine and methylazoxymethanol acetate in the F 344 rat. Cancer Lett. 1980 Jan;8(3):271–278. doi: 10.1016/0304-3835(80)90013-0. [DOI] [PubMed] [Google Scholar]
- Ward J. M. Dose response to a single injection of azoxymethane in rats. Induction of tumors in the gastrointestinal tract, auditory sebaceous glands, kidney, liver and preputial gland. Vet Pathol. 1975;12(3):165–177. doi: 10.1177/030098587501200302. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Frank A. L., Wenk M., Devor D., Tarone R. E. Ingested asbestos and intestinal carcinogenesis in F344 rats. J Environ Pathol Toxicol. 1980 Jun-Jul;3(5-6):301–312. [PubMed] [Google Scholar]


