Abstract
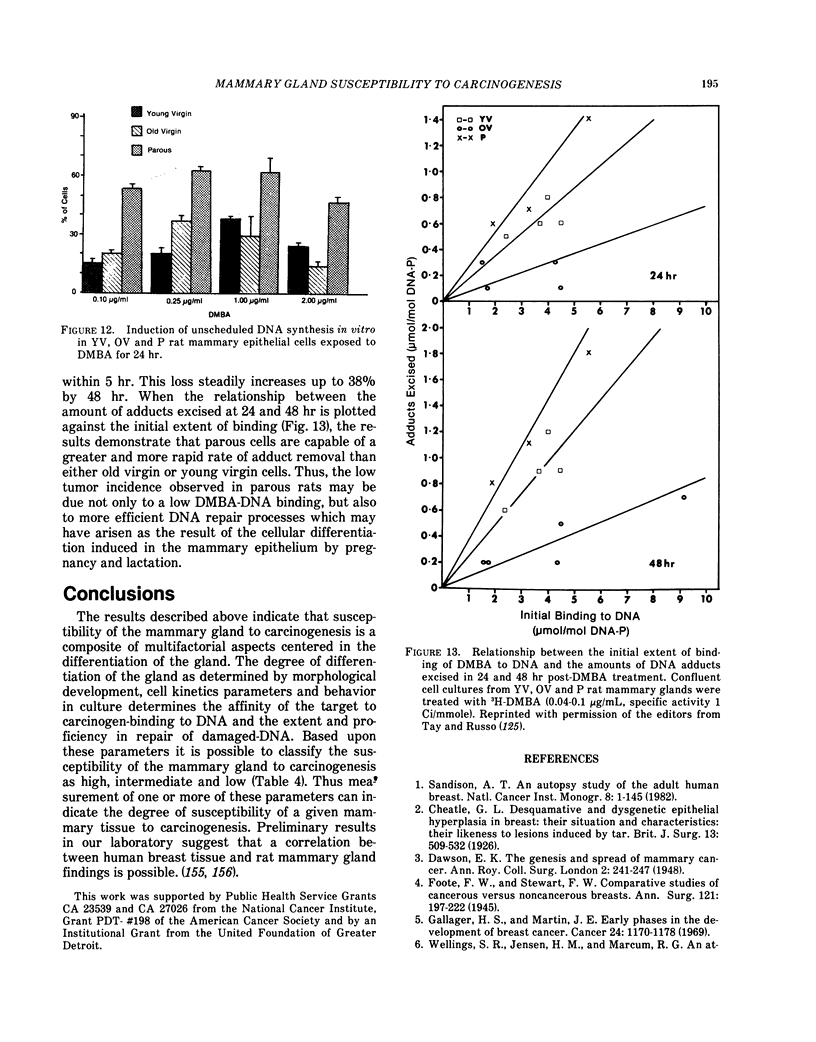
Mammary carcinomas induced by the administration of 7,12-dimethylbenz(a)anthracene (DMBA) to young virgin rats arise from undifferentiated terminal ductal structures called terminal end buds (TEBs). TEBs that normally differentiate into alveolar buds (ABs) and lobules under the influence of DMBA develop intraductal proliferations which progress to carcinoma. The high susceptibility of the young virgin rat TEBs to neoplastic transformation is due to its large proliferative compartment, with cells cycling every 10 hr, and to a higher 3H-DMBA uptake. Progressive differentiation of TEBs into ABs and lobules or their regression to terminal ducts (TDs) is seen with aging. Complete differentiation of the gland is attained only through pregnancy and lactation. The greater differentiation of the gland is manifested as permanent structural changes, consisting in the disappearance of TEBs and in a diminution of the number of TDs due to their differentiation into ABs and lobules. This greater differentiation results in a diminished or total refractoriness of the gland to the carcinogen because ABs and lobules have a lower proliferative compartment and a longer cell cycle than TEBs and TDs. Cells of parous rats have both in vivo and in vitro a lower DMBA-DNA binding capacity, a lower DNA synthesis and a greater ability to repair DMBA damaged DNA than cells of young virgin rats. The more efficient DNA repair capacity of the parous rat mammary gland is demonstrated by the induction of unscheduled DNA synthesis and a removal of DMBA-DNA adducts.
Full text
PDF
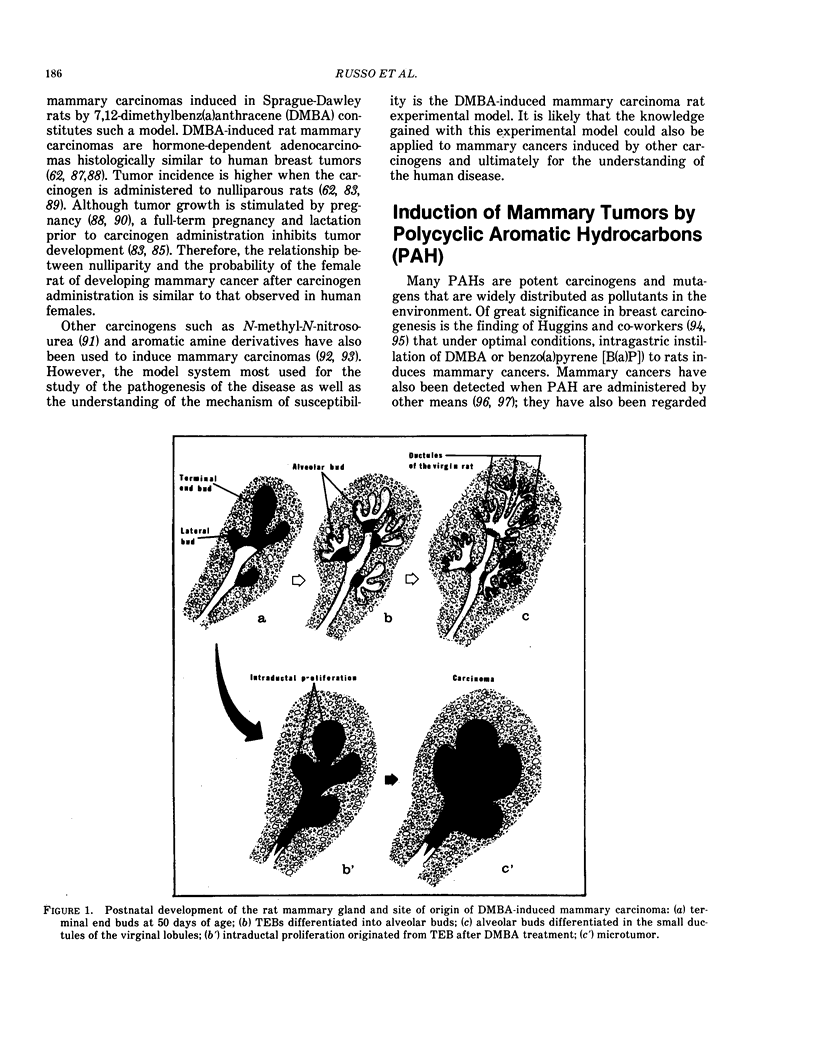
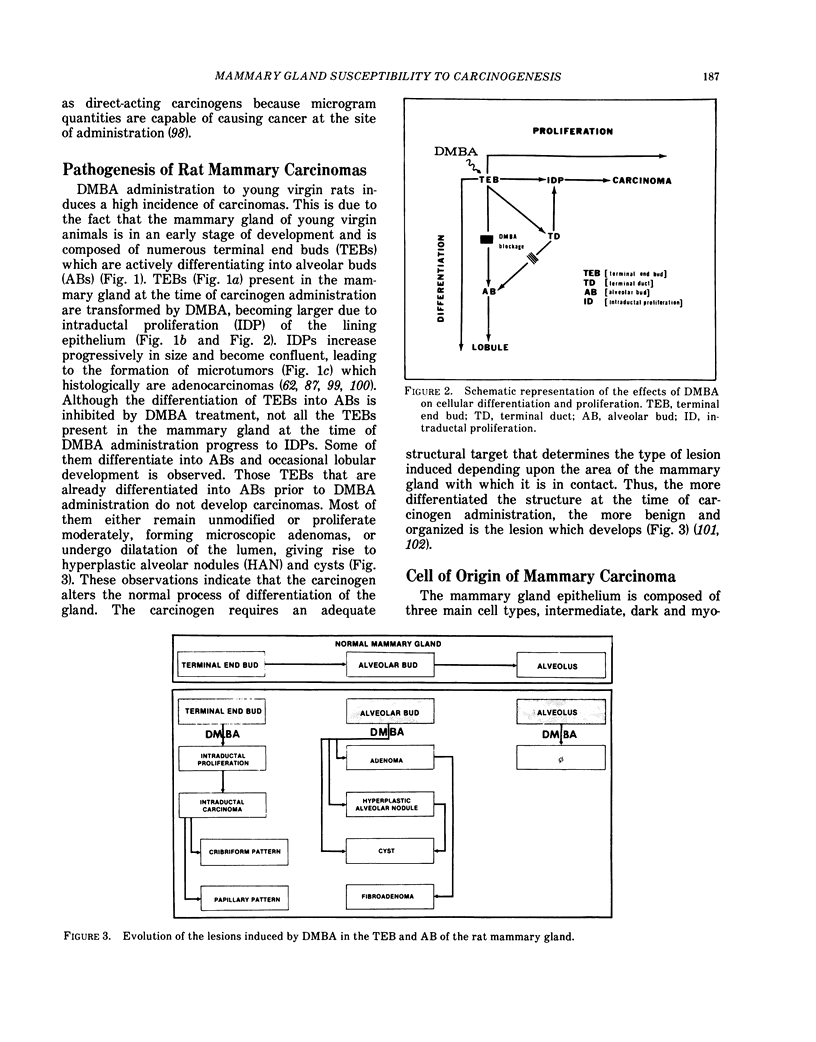

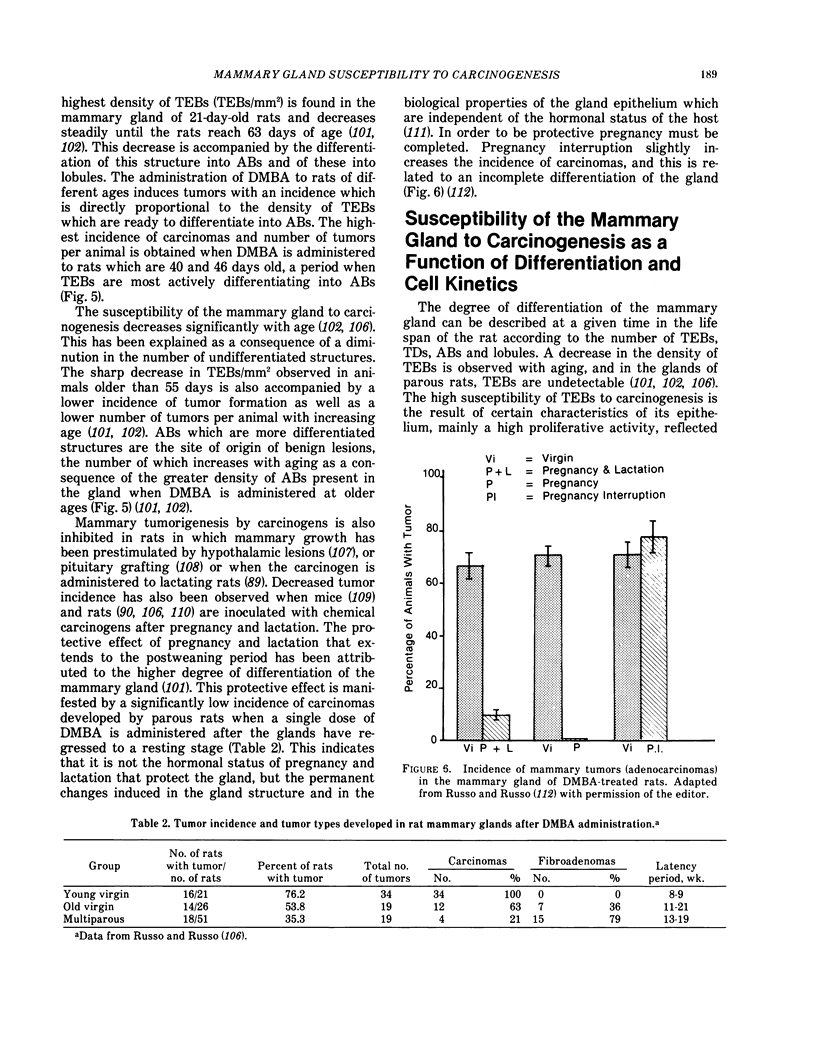




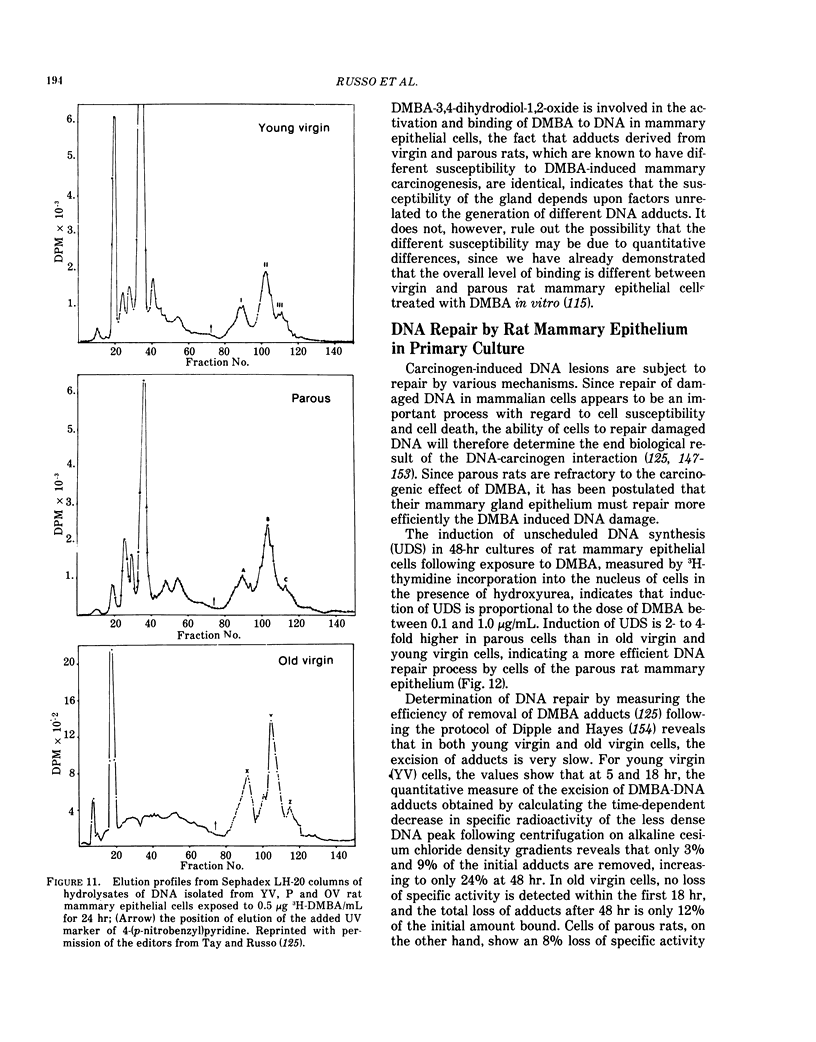




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. E. A genetic study of human breast cancer. J Natl Cancer Inst. 1972 Apr;48(4):1029–1034. [PubMed] [Google Scholar]
- Anderson D. E. Some characteristics of familial breast cancer. Cancer. 1971 Dec;28(6):1500–1504. doi: 10.1002/1097-0142(197112)28:6<1500::aid-cncr2820280623>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Armstrong B. Recent trends in breast-cancer incidence and mortality in relation to changes in possible risk factors. Int J Cancer. 1976 Feb 15;17(2):204–211. doi: 10.1002/ijc.2910170209. [DOI] [PubMed] [Google Scholar]
- BULBROOK R. D., HAYWARD J. L., SPICER C. C., THOMAS B. S. A comparison between the urinary steroid excretion of normal women and women with advanced breast cancer. Lancet. 1962 Dec 15;2(7268):1235–1237. doi: 10.1016/s0140-6736(62)92811-8. [DOI] [PubMed] [Google Scholar]
- BULBROOK R. D., HAYWARD J. L., THOMAS B. S. THE RELATION BETWEEN THE URINARY 17-HYDROXYCORTICOSTEROIDS AND 11-DEOXY-17-OXOSTEROIDS AND THE FATE OF PATIENTS AFTER MASTECTOMY. Lancet. 1964 May 2;1(7340):945–947. doi: 10.1016/s0140-6736(64)91741-6. [DOI] [PubMed] [Google Scholar]
- Baird W. M., Brookes P. Isolation of the hydrocarbon-deoxyribonucleoside products from the DNA of mouse embryo cells treated in culture with 7-methylbenz(a)anthracene-3H. Cancer Res. 1973 Oct;33(10):2378–2385. [PubMed] [Google Scholar]
- Boice J. D., Jr, Monson R. R. Breast cancer in women after repeated fluoroscopic examinations of the chest. J Natl Cancer Inst. 1977 Sep;59(3):823–832. doi: 10.1093/jnci/59.3.823. [DOI] [PubMed] [Google Scholar]
- Boyns A. R., Buchan R., Cole E. N., Forrest A. P., Griffiths K. Basal prolactin blood levels in three strains of rat with differing incidence of 7,12-dimethylbenz(a)anthracene induced mammary tumours. Eur J Cancer. 1973 Mar;9(3):169–171. doi: 10.1016/s0014-2964(73)80015-5. [DOI] [PubMed] [Google Scholar]
- Bulbrook R. D., Hayward J. L., Spicer C. C. Relation between urinary androgen and corticoid excretion and subsequent breast cancer. Lancet. 1971 Aug 21;2(7721):395–398. doi: 10.1016/s0140-6736(71)90113-9. [DOI] [PubMed] [Google Scholar]
- Ciocca D. R., Parente A., Russo J. Endocrinologic milieu and susceptibility of the rat mammary gland to carcinogenesis. Am J Pathol. 1982 Oct;109(1):47–56. [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Clemens J. A., Welsch C. W., Meites J. Effects of hypothalamic lesions on incidence and growth of mammary tumors in carcinogen-treated rats. Proc Soc Exp Biol Med. 1968 Mar;127(3):969–972. doi: 10.3181/00379727-127-32847. [DOI] [PubMed] [Google Scholar]
- Commoner B., Vithayathil A. J., Dolara P., Nair S., Madyastha P., Cuca G. C. Formation of mutagens in beef and beef extract during cooking. Science. 1978 Sep 8;201(4359):913–916. doi: 10.1126/science.567374. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Ribeiro O., Hewer A., Walsh C., Grover P. L., Sims P. Additional evidence for the involvement of the 3,4-diol 1,2-oxides in the metabolic activation of 7,12-dimethylbenz[a]anthracene in mouse skin. Chem Biol Interact. 1980 Mar;29(3):357–367. doi: 10.1016/0009-2797(80)90154-4. [DOI] [PubMed] [Google Scholar]
- DAMON A. Host factors in cancer of the breast and uterine cervix and corpus. J Natl Cancer Inst. 1960 Feb;24:483–516. [PubMed] [Google Scholar]
- DAO T. L., BOCK F. G., GREINER M. J. Mammary carcinogenesis by 3-methylcholanthrene. II. Inhibitory effect of pregnancy and lactation on tumor induction. J Natl Cancer Inst. 1960 Nov;25:991–1003. [PubMed] [Google Scholar]
- DAO T. L. CARCINOGENESIS OF MAMMARY GLAND IN RAT. Prog Exp Tumor Res. 1964;5:157–216. doi: 10.1159/000385999. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Scudiero D., Dimattina M. Excision repair by human fibroblasts of DNA damaged by r-7, t-8-dihyroxy-t-9,10-oxy-7,8,9,10- tetrahydrobenzo(a)pyrene. Mutat Res. 1978 Jun;50(3):383–394. doi: 10.1016/0027-5107(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Dipple A., Hayes M. E. Differential excision of carcinogenic hydrocarbon-DNA adducts in mouse embryo cell cultures. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1225–1231. doi: 10.1016/0006-291x(79)91198-7. [DOI] [PubMed] [Google Scholar]
- Dipple A., Lawley P. D., Brookes P. Theory of tumour initiation by chemical carcinogens: dependence of activity on structure of ultimate carcinogen. Eur J Cancer. 1968 Oct;4(5):493–506. doi: 10.1016/0014-2964(68)90005-4. [DOI] [PubMed] [Google Scholar]
- Dipple A., Nebzydoski J. A. Evidence for the involvement of a diol-epoxide in the binding of 7,12-dimethylbenz(a)anthracene to DNA in cells in culture. Chem Biol Interact. 1978 Jan;20(1):17–26. doi: 10.1016/0009-2797(78)90077-7. [DOI] [PubMed] [Google Scholar]
- Dipple A., Tomaszewski J. E., Moschel R. C., Bigger C. A., Nebzydoski J. A., Egan M. Comparison of metabolism-mediated binding to DNA of 7-hydroxymethyl-12-methylbenz(a)anthracene and 7, 12-dimethylbenz(a)anthracene. Cancer Res. 1979 Apr;39(4):1154–1158. [PubMed] [Google Scholar]
- Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968 Aug;41(2):315–329. [PubMed] [Google Scholar]
- Foote F. W., Stewart F. W. Comparative Studies of Cancerous Versus Noncancerous Breasts. Ann Surg. 1945 Feb;121(2):197–222. doi: 10.1097/00000658-194502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRATTAROLA R. THE PREMENSTRUAL ENDOMETRIAL PATTERN OF WOMEN WITH BREAST CANCER. A STUDY OF PROGESTATIONAL ACTIVITY. Cancer. 1964 Sep;17:1119–1122. doi: 10.1002/1097-0142(196409)17:9<1119::aid-cncr2820170904>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gallager H. S., Martin J. E. Early phases in the development of breast cancer. Cancer. 1969 Dec;24(6):1170–1178. doi: 10.1002/1097-0142(196912)24:6<1170::aid-cncr2820240615>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Grover P. L., MacNicoll A. D., Sims P., Easty G. C., Neville A. M. Polycyclic hydrocarbon activation and metabolism in epithelial cell aggregates prepared from human mammary tissue. Int J Cancer. 1980 Oct 15;26(4):467–475. doi: 10.1002/ijc.2910260412. [DOI] [PubMed] [Google Scholar]
- Grönroos M., Aho A. J. Estrogen metabolism in postmenopausal women with primary and recurrent breast cancer. Eur J Cancer. 1968 Oct;4(5):523–527. doi: 10.1016/0014-2964(68)90008-x. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Pettigrew H. M., Grantham F. H. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975 Feb;54(2):401–414. [PubMed] [Google Scholar]
- HUGGINS C., GRAND L. C., BRILLANTES F. P. Mammary cancer induced by a single feeding of polymucular hydrocarbons, and its suppression. Nature. 1961 Jan 21;189:204–207. doi: 10.1038/189204a0. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., YANG N. C. Induction and extinction of mammary cancer. A striking effect of hydrocarbons permits analysis of mechanisms of causes and cure of breast cancer. Science. 1962 Jul 27;137(3526):257–262. doi: 10.1126/science.137.3526.257. [DOI] [PubMed] [Google Scholar]
- HUMPHREY L. J., SWERDLOW M. Relationship to benign breast disease to carcinoma of the breast. Surgery. 1962 Dec;52:841–846. [PubMed] [Google Scholar]
- Haslam S. Z., Bern H. A. Histopathogenesis of 7,12-diemthylbenz(a)anthracene-induced rat mammary tumors. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4020–4024. doi: 10.1073/pnas.74.9.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Drewitt D., Freedman B., Killin E., Jenner D. A., Cameron E. H. Plasma hormone levels and the incidence of carcinogen-induced mammary tumours in two strains of rat. Br J Cancer. 1976 Nov;34(5):546–549. doi: 10.1038/bjc.1976.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflich R. H., Hazard R. M., Lommel L., Scribner J. D., Maher V. M., McCormick J. J. A comparison of the DNA binding, cytotoxicity and repair synthesis induced in human fibroblasts by reactive derivatives of aromatic amide carcinogens. Chem Biol Interact. 1980 Jan;29(1):43–56. doi: 10.1016/0009-2797(80)90085-x. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Huggins C., Grand L. C., Brillantes F. P. CRITICAL SIGNIFICANCE OF BREAST STRUCTURE IN THE INDUCTION OF MAMMARY CANCER IN THE RAT. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1294–1300. doi: 10.1073/pnas.45.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga M., Takebe H., Ishii Y. Excision repair of DNA base damage in human cells treated with the chemical carcinogen 4-nitroquinoline 1-oxide. Mutat Res. 1977 Jun;43(3):415–427. doi: 10.1016/0027-5107(77)90062-8. [DOI] [PubMed] [Google Scholar]
- Janss D. H., Ben T. L. Age-related modification of 7,12-dimethylbenz[a]anthracene binding to rat mammary gland DNA. J Natl Cancer Inst. 1978 Jan;60(1):173–177. doi: 10.1093/jnci/60.1.173. [DOI] [PubMed] [Google Scholar]
- Juret P., Couette J. E., Brune D., Vernhes J. C. L'âge à la première naissance: une variable à la signification simple, double ou triple en cancérologie mammaire. Bull Cancer. 1975 Apr-Jun;62(2):165–174. [PubMed] [Google Scholar]
- Juret P., Couette J. E., Mandard A. M., Carré A., Delozier T., Brune D., Vernhes J. C. Age at menarche as a prognostic factor in human breast cancer. Eur J Cancer. 1976 Sep;12(9):701–704. doi: 10.1016/0014-2964(76)90019-0. [DOI] [PubMed] [Google Scholar]
- Kaplan S. D., Acheson R. M. A single etiological hypothesis for breast cancer? J Chronic Dis. 1966 Nov-Dec;19(11):1221–1230. doi: 10.1016/0021-9681(66)90020-8. [DOI] [PubMed] [Google Scholar]
- Kern W. H., Brooks R. N. Atypical epithelial hyperplasia associated with breast cancer and fibrocystic disease. Cancer. 1969 Oct;24(4):668–675. doi: 10.1002/1097-0142(196910)24:4<668::aid-cncr2820240403>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kumaoka S., Sakauchi N., Abe O., Kusama M., Takatani O. Urinary 17-ketosteroid excretion of women with advanced breast cancer. J Clin Endocrinol Metab. 1968 May;28(5):667–672. doi: 10.1210/jcem-28-5-667. [DOI] [PubMed] [Google Scholar]
- LEVIN M. L., SHEEHE P. R., GRAHAM S., GLIDEWELL O. LACTATION AND MENSTRUAL FUNCTION AS RELATED TO CANCER OF THE BREAST. Am J Public Health Nations Health. 1964 Apr;54:580–587. doi: 10.2105/ajph.54.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILIENFELD A. M. THE EPIDEMIOLOGY OF BREAST CANCER. Cancer Res. 1963 Oct;23:1503–1513. [PubMed] [Google Scholar]
- LILIENFELD A. M. The relationship of cancer of the female breast to artificial menopause and martial status. Cancer. 1956 Sep-Oct;9(5):927–934. doi: 10.1002/1097-0142(195609/10)9:5<927::aid-cncr2820090510>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- LOGAN W. P. Marriage and childbearing in relation to cancer of the breast and uterus. Lancet. 1953 Dec 5;265(6797):1199–1202. doi: 10.1016/s0140-6736(53)90748-x. [DOI] [PubMed] [Google Scholar]
- Lemon H. M. Endocrine influences on human mammary cancer formation. A critique. Cancer. 1969 Apr;23(4):781–790. doi: 10.1002/1097-0142(196904)23:4<781::aid-cncr2820230407>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Lemon H. M., Wotiz H. H., Parsons L., Mozden P. J. Reduced estriol excretion in patients with breast cancer prior to endocrine therapy. JAMA. 1966 Jun 27;196(13):1128–1136. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Lin T. M., Chen K. P., MacMahon B. Epidemiologic characteristics of cancer of the breast in Taiwan. Cancer. 1971 Jun;27(6):1497–1504. doi: 10.1002/1097-0142(197106)27:6<1497::aid-cncr2820270634>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- MACKENZIE I. BREAST CANCER FOLLOWING MULTIPLE FLUOROSCOPIES. Br J Cancer. 1965 Mar;19:1–8. doi: 10.1038/bjc.1965.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHANT J. The inhibitory effect of continued lactation on the incidence of chemically-induced breast tumours in mice of the IF strain. Br J Cancer. 1958 Mar;12(1):55–61. doi: 10.1038/bjc.1958.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMORSTON J., CROWLEY L. G., MYERS S. M., STERN E., HOPKINS C. E. II. URINARY EXCRETION OF ESTRONE, ESTRADIOL, AND ESTRIOL BY PATIENTS WITH BREAST CANCER AND BENIGN BREAST DISEASE. Am J Obstet Gynecol. 1965 Jun 15;92:460–467. doi: 10.1016/s0002-9378(16)34835-9. [DOI] [PubMed] [Google Scholar]
- MCCORMICK G. M., MOON R. C. EFFECT OF PREGNANCY AND LACTATION ON GROWTH OF MAMMARY TUMOURS INDUCED BY 7,12-DIMETHLBENZ(A) ANTHRACENE (DMBA). Br J Cancer. 1965 Mar;19:160–166. doi: 10.1038/bjc.1965.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN C. W., Jr, SCHENKEN J. R., TAMISIEA J. X. A study of precancerous epithelial hyperplasia and noninvasive papillary carcinoma of the breast. Ann Surg. 1961 May;153:735–744. doi: 10.1097/00000658-196105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B., Cole P. Endocrinology and epidemiology of breast cancer. Cancer. 1969 Dec;24(6):1146–1151. doi: 10.1002/1097-0142(196912)24:6<1146::aid-cncr2820240612>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- MacMahon B., Cole P., Lin T. M., Lowe C. R., Mirra A. P., Ravnihar B., Salber E. J., Valaoras V. G., Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–221. [PMC free article] [PubMed] [Google Scholar]
- MacMahon B. Risk factors for endometrial cancer. Gynecol Oncol. 1974 Aug;2(2-3):122–129. doi: 10.1016/0090-8258(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Bendich A., Philips F. S., Hoffmann D. Binding of (G- 3 H)-7,12-dimethylbenz(a)anthracene to DNA of normal and of rapidly dividing hepatic cells of rats. Chem Biol Interact. 1971 Feb;3(1):1–11. doi: 10.1016/0009-2797(71)90021-4. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Sternberg S. S., Philips F. S. 7,12-dimethylbenz(a)anthracene and hepatic neoplasia in regenerating rat liver. Chem Biol Interact. 1970 Dec;2(4):401–403. doi: 10.1016/0009-2797(70)90060-8. [DOI] [PubMed] [Google Scholar]
- McGregor H., Land C. E., Choi K., Tokuoka S., Liu P. I., Wakabayashi T., Beebe G. W. Breast cancer incidence among atomic bomb survivors, Hiroshima and Nagasaki, 1950-69. J Natl Cancer Inst. 1977 Sep;59(3):799–811. doi: 10.1093/jnci/59.3.799. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Mechanisms of chemical carcinogenesis: nature of proximate carcinogens and interactions with macromolecules. Pharmacol Rev. 1966 Mar;18(1):805–838. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mirra A. P., Cole P., MacMahon B. Breast cancer in an area of high parity: São Paulo, Brazil. Cancer Res. 1971 Feb;31(2):77–83. [PubMed] [Google Scholar]
- Moon R. C. Relationship between previous reproductive history and chemically induced mammary cancer in rats. Int J Cancer. 1969 May 15;4(3):312–317. doi: 10.1002/ijc.2910040308. [DOI] [PubMed] [Google Scholar]
- Murad T. M., Von Haam E. Studies of mammary carcinoma induced by 7,12-dimethylbenz(a)anthracene administration. Cancer Res. 1972 Jul;32(7):1404–1415. [PubMed] [Google Scholar]
- Myrden J. A., Hiltz J. E. Breast cancer following multiple fluoroscopies during artificial pneumothorax treatment of pulmonary tuberculosis. Can Med Assoc J. 1969 Jun 14;100(22):1032–1034. [PMC free article] [PubMed] [Google Scholar]
- PARKS A. G. The micro-anatomy of the breast. Ann R Coll Surg Engl. 1959 Nov;25:235–251. [PMC free article] [PubMed] [Google Scholar]
- PAYNE S. The pathological effects of the intraperitoneal injection of 3:4-benzpyrene into rats and mice. Br J Cancer. 1958 Mar;12(1):65–74. doi: 10.1038/bjc.1958.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataki J., Huggins C. Molecular site of substituents of benz(a)anthracene related to carcinogenicity. Cancer Res. 1969 Mar;29(3):506–509. [PubMed] [Google Scholar]
- Petrakis N. L. Cerumen genetics and human breast cancer. Science. 1971 Jul 23;173(3994):347–349. doi: 10.1126/science.173.3994.347. [DOI] [PubMed] [Google Scholar]
- Petrakis N. L., Mason L., Lee R., Sugimoto B., Pawson S., Catchpool F. Association of race, age, menopausal status, and cerumen type with breast fluid secretion in nonlactating women, as determined by nepple aspiration. J Natl Cancer Inst. 1975 Apr;54(4):829–834. [PubMed] [Google Scholar]
- Pound A. W. Carcinogenesis and cell proliferation. N Z Med J. 1968 Jan;67(426 Suppl):88–99. [PubMed] [Google Scholar]
- Prodi G., Rocchi P., Grilli S. Binding of 7,12-dimethylbenz(a)anthracene and benzo(a)pyrene to nucleic acids and proteins of organs in rats. Cancer Res. 1970 Apr;30(4):1020–1023. [PubMed] [Google Scholar]
- Ravnihar B., MacMahon B., Lindtner J. Epidemiologic features of breast cancer in Slovenia, 1965-1967. Eur J Cancer. 1971 Aug;7(4):295–306. doi: 10.1016/0014-2964(71)90072-7. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Francis A. A., Dunn W. C., Hernandez O., Yagi H., Jerina D. M. Repair of DNA damaged by mutagenic metabolites of benzo(a)pyrene in human cells. Chem Biol Interact. 1978 Mar;20(3):279–287. doi: 10.1016/0009-2797(78)90106-0. [DOI] [PubMed] [Google Scholar]
- Russo I. H., Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1978 Dec;61(6):1439–1449. [PubMed] [Google Scholar]
- Russo J., Russo I. H. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst. 1978 Dec;61(6):1451–1459. [PubMed] [Google Scholar]
- Russo J., Russo I. H. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res. 1980 Aug;40(8 Pt 1):2677–2687. [PubMed] [Google Scholar]
- Russo J., Russo I. H. Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence. Am J Pathol. 1980 Aug;100(2):497–512. [PMC free article] [PubMed] [Google Scholar]
- Russo J., Tay L. K., Russo I. H. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- Russo J., Wilgus G., Russo I. H. Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am J Pathol. 1979 Sep;96(3):721–736. [PMC free article] [PubMed] [Google Scholar]
- Russo J., Wilgus G., Tait L., Russo I. H. Influence of age and parity on the susceptibility of rat mammary gland epithelial cells in primary cultures to 7,12-dimethylbenz(a)anthracene. In Vitro. 1981 Oct;17(10):877–884. doi: 10.1007/BF02618283. [DOI] [PubMed] [Google Scholar]
- Salber E. J., Trichopulos D., MacMahon B. Lactation and reproductive histories of breast cancer patients in Boston, 1965-66. J Natl Cancer Inst. 1969 Nov;43(5):1013–1024. [PubMed] [Google Scholar]
- Shapiro S., Strax P., Venet L., Fink R. The search for risk factors in breast cancer. Am J Public Health Nations Health. 1968 May;58(5):820–835. doi: 10.2105/ajph.58.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B. M., Korenman S. G. Inadequate corpus luteum function: a pathophysiological interpretation of human breast cancer epidemiology. Cancer. 1974 May;33(5):1306–1312. doi: 10.1002/1097-0142(197405)33:5<1306::aid-cncr2820330515>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shimkin M. B., Gruenstein M., Thatcher D., Baserga R. Tritiated thymidine labeling of cells in rats following exposure to 7,12-dimethylbenz(a)anthracene. Cancer Res. 1967 Aug;27(8):1494–1495. [PubMed] [Google Scholar]
- Shinohara K., Cerutti P. A. Excision repair of benzo[a]pyrene-deoxyguanosine adducts in baby hamster kidney 21/C13 cells and in secondary mouse embryo fibroblasts C57BL/6J. Proc Natl Acad Sci U S A. 1977 Mar;74(3):979–983. doi: 10.1073/pnas.74.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Fysh J. M., Lee M. S., Vaught J. B., King C. M. Relationship of metabolic activation of N-hydroxy-N-acylarylamines to biological response in the liver and mammary gland of the female CD rat. Cancer Res. 1981 Nov;41(11 Pt 1):4346–4353. [PubMed] [Google Scholar]
- Sinha D., Dao T. L. A direct mechanism of mammary carcinogenesis induced by 7,12-dimethyl-benz(alpha)anthracene. J Natl Cancer Inst. 1974 Sep;53(3):841–846. doi: 10.1093/jnci/53.3.841. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Selby F. W., Vanderlaan W. P. The natural history of prolactin and GH secretion in mice with high and low incidence of mammary tumors. Endocrinology. 1974 Mar;94(3):757–764. doi: 10.1210/endo-94-3-757. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Vlahakis G., Vanderlaan W. P. Serum, pituitary and urine concentrations of prolactin and growth hormone in eight strains of mice with varying incidence of mammary tumors. Int J Cancer. 1979 Oct 15;24(4):430–437. doi: 10.1002/ijc.2910240409. [DOI] [PubMed] [Google Scholar]
- Staszewski J. Age at menarche and breast cancer. J Natl Cancer Inst. 1971 Nov;47(5):935–940. [PubMed] [Google Scholar]
- Tanaka Y., Oota K. A stereomicroscopic study of the mastopathic human breast. I. Three-dimensional structures of abnormal duct evolution and their histologic entity. Virchows Arch A Pathol Pathol Anat. 1970;349(3):195–214. doi: 10.1007/BF00544572. [DOI] [PubMed] [Google Scholar]
- Tay L. K., Russo J. 7,12-dimethylbenz[a]anthracene-induced DNA binding and repair synthesis in susceptible and nonsusceptible mammary epithelial cells in culture. J Natl Cancer Inst. 1981 Jul;67(1):155–161. [PubMed] [Google Scholar]
- Tay L. K., Russo J. Formation and removal of 7,12-dimethylbenz[a]anthracene--nucleic acid adducts in rat mammary epithelial cells with different susceptibility to carcinogenesis. Carcinogenesis. 1981;2(12):1327–1333. doi: 10.1093/carcin/2.12.1327. [DOI] [PubMed] [Google Scholar]
- Thirty-second annual meeting of the Tissue Culture Association, 1981, Washington, D.C. Abstracts. In Vitro. 1981 Mar;17(3):201–266. [PubMed] [Google Scholar]
- Tominaga T., Dao T. L., Libby P. R. Effects of 7,12-dimethylbenz (a) anthracene on RNA polymerase in isolated mammary gland cell nuclei. Proc Soc Exp Biol Med. 1971 Mar;136(3):694–697. doi: 10.3181/00379727-136-35343. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Libby P. R., Dao T. L. An early effect of 7,12-dimethylbenz(a)anthracene on rat mammary gland DNA synthesis. Cancer Res. 1970 Jan;30(1):118–122. [PubMed] [Google Scholar]
- Tong C., Fazio M., Williams G. M. Cell cycle-specific mutagenesis at the hypoxanthine phosphoribosyltransferase locus in adult rat liver epithelial cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7377–7379. doi: 10.1073/pnas.77.12.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil D. V., Morgan R. W. Etiology of breast cancer. I. Genetic aspects. Can Med Assoc J. 1973 Jul 7;109(1):29–32. [PMC free article] [PubMed] [Google Scholar]
- Valaoras V. G., MacMahon B., Trichopoulos D., Polychronopoulou A. Lactation and reproductive histories of breast cancer patients in greater Athens, 1965-67. Int J Cancer. 1969 May 15;4(3):350–363. doi: 10.1002/ijc.2910040312. [DOI] [PubMed] [Google Scholar]
- WYNDER E. L., BROSS I. J., HIRAYAMA T. A study of the epidemiology of cancer of the breast. Cancer. 1960 May-Jun;13:559–601. doi: 10.1002/1097-0142(196005/06)13:3<559::aid-cncr2820130322>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wade A. P., Davis J. C., Tweedie M. C., Clarke C. A. Discriminants and breast cancer. Lancet. 1969 Jul 5;2(7610):54–54. doi: 10.1016/s0140-6736(69)92617-8. [DOI] [PubMed] [Google Scholar]
- Wade A. P., Tweedie M. C., Davis J. C., Clarke C. A., Haggart B. The discriminant function in early carcinoma of the breast. Lancet. 1969 Apr 26;1(7600):853–857. doi: 10.1016/s0140-6736(69)91899-6. [DOI] [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M., Marcum R. G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975 Aug;55(2):231–273. [PubMed] [Google Scholar]
- Welsch C. W., Clemens J. A., Meites J. Effects of multiple pituitary homografts or progesterone on 7,12-dimethylbenz[a]anthracene-induced mammary tumors in rats. J Natl Cancer Inst. 1968 Aug;41(2):465–471. [PubMed] [Google Scholar]
- Yuasa S., MacMahon B. Lactation and reproductive histories of breast cancer patients in Tokyo, Japan. Bull World Health Organ. 1970;42(2):195–204. [PMC free article] [PubMed] [Google Scholar]


