Abstract
Lymph node (LN) development depends on prenatal interactions occurring between LN inducer and LN organizer cells. We have distinguished defects in LN formation due to failure in embryonic development (aly/aly) from defects in postnatal maturation (Il2rγ−/−Rag2−/−). Both mutant strains form normal primordial LNs with differing fate. In aly/aly mice, the LN primordium dissipates irreversibly late in gestation; in contrast, Il2rγ−/−Rag2−/− LN anlage persists for a week after birth but disperses subsequently, a process reversible by neonatal transfer of WT IL7r+ TCR+ T or natural killer (NK) cells, suggesting a role for IL7/IL7r interactions. Thus, we reveal a unique stage of postnatal LN development during which mature lymphocytes and IL7/IL7r interactions may play an important role.
Keywords: imaging, lymphoid organogenesis, transgenic mice
Interactions between stroma and hematopoietic cells and consequent intracellular events are necessary for the proper development of lymph nodes (LNs). Embryonic events leading to the formation of LN anlage and its maturation in mice involve two major cellular contributors: LN inducer (LNi) cells with a CD45+ CD4+CD3−IL7rα+c-kit+lin− phenotype, and LN organizer (LNo) cells of mesenchymal origin that express, among other molecules, lymphotoxin β receptor (LTβr) and vascular cell adhesion molecule 1 (VCAM1) (for comprehensive reviews, see refs. 1–4). Temporally, there are three stages in LN organogenesis in the mouse. The first stage is characterized by the migration and aggregation of LNi cells to positions where future LNs will form. The second stage is characterized by LNi/LNo interactions leading to further maturation of the anlage. In the third stage, mature lymphocytes migrate into these embryonic structures to generate adult LNs.
Using transgenic and knockout mice has led to the identification of many molecules that are important in LN organogenesis. For example, deficiency in Ikaros (5), RORγ (6), Id2 (7), or TRANCE/TRANCER (8–10) genes affects LNi development or function, whereas defects in LTβr signaling affect LNo cells (11–14). Such mutations result in severe reduction in size and number (or complete absence) of LNs in adult life.
It is unclear at the moment how LNi cells migrate to the appropriate locations during early stages of LN organogenesis, although chemokines and their receptors have been implicated (15). In the subsequent stage, the interactions between the LNi and the LNo cells are governed mainly by three molecular loops: IL7rα-LTβr (16), TRANCE-TRANCEr (8, 17), and CXCL13-CXCR5 loops (18, 19), all of which are important for LN development (8, 20–22).
LTβr signaling is thought to be necessary for the differentiation of LNo cells within a narrow window of time (17, 20). In mice deficient in LT/LTβR interactions, LNi cells migrate to the right sites in the body, but the LNo stroma cells are unable to respond to the inductive signals from these cells (17). Accordingly, LTα mutant mice fail to develop LN, but treatment of developing embryos with an agonist LTβr antibody between days 15.5 and 18.5 postcoitum (p.c.) leads to LN formation that persists in adult life despite the continuing absence of LTβr signaling (20). aly/aly carry a mutation in NFκB-inducing kinase (NIK), which is essential for LTβr signaling in the stroma cells; these mice develop no peripheral LNs, even though mature T and B cells are found in their spleen and blood (23, 24, 25). In addition, in aly/aly mice LNo fail to up-regulate vascular cell adhesion molecule 1 (VCAM1) during LN development (26).
Common γ chain-deficient mice (Il2rγ−/−) have small LNs (27, 28). Interestingly, mice doubly deficient in Il2rγ and Rag2 genes (Il2rγ−/−Rag2−/−) lack peripheral LNs (apart from a small mesenteric and sacral LN) (29). So far, the stage at which LN development fails in these mice has not been defined; thus, adult mice may lack lymphoid organs either because they fail to develop a lymphoid anlage during embryonic life or because they fail to maintain the embryonic lymphoid structures into adult life because of lack of lymphocytes.
To distinguish among these possibilities and to identify the events that lead to defective formation of LNs, we examined the behavior of LNi cells during embryonic life. For this, we used mice carrying a GFP cDNA under the control of the human CD2 promoter and locus control region (LCR) (30) and thus expressing GFP in T,B, and natural killer (NK) cells (31). Here, we show that GFP is also expressed in embryonic hemopoietic cells, including the LNi population. These mice are a powerful tool to image development, maturation, and maintenance of LNs and demonstrated that LNi cells migrate and aggregate normally in the correct sites in embryos of Rag1−/−, Il2rγ−/−, Il2rγ−/−Rag2−/−, and aly/aly mutant mice. The primordial structures formed in the aly/aly mice survive up to late stages in gestation and degenerate by birth. In contrast, in the Il2rγ−/−Rag2−/− mice, LN anlagen persist up to a week after birth, during which period it is possible to rescue LN formation by transferring WT TCR+ T and NK1.1+ cells into these mice, whereas B cells are almost incapable of such rescue. The capacity of lymphoid cells to rescue neonatal LN structure seems to be closely associated with Il7r expression because Il7r− lymphoid cells are incapable of such rescue. These results show that, within the first week after birth, mature lymphocytes are necessary for the maturation of LNs and their maintenance in adult life, defining a new developmental window in LN organogenesis distinct in cellular and molecular requirements from the preceding late gestation stage and one in which Il7/Il7r interactions may play an important role.
Results
Visualization of Lymphoid Organs in Adult WT and Mutant Adult Mice.
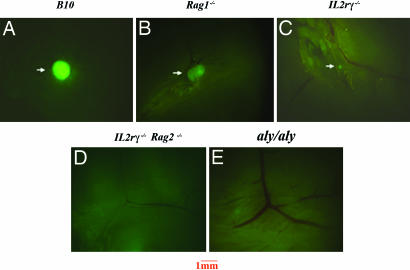
Several mutations have been described that affect LN organogenesis; however, the exact developmental point or why such mutant mice lack LNs have not always been clear. In aly/aly mice, LN absence has been attributed to defective development of LNo cells in fetal life whereas, in Il2rγ−/− mice, it has been attributed to the absence of B and T cells. To address the cellular mechanisms involved in LN organogenesis in these mice, we used mice transgenic for the GFP gene under the control of the hCD2 promoter and locus control region (LCR) (30). Adult mice of this strain express high levels of GFP in T cells and NK cells and low levels in mature B cells (31). The expression of GFP in lymphoid cells permits stereomicroscopic analysis of very small lymphoid structures and scarce cells that would be invisible otherwise. For our studies, hCD2-GFP transgenic mice were bred into B10, Rag1−/−, Il2rγ−/−, Il2rγ−/−Rag2−/−, and aly/aly genetic backgrounds. GFP+ peripheral LNs were readily visualized in adult B10.hCD2-GFP/Rag1+/+ (Fig. 1A) and B10.hCD2-GFP/Rag1−/− (Fig. 1B) mice because of the presence of T, B, and NK in Rag1+/+, or NK cells alone in Rag1−/− mice.
Fig. 1.
Analysis of peripheral LNs in mutant mouse strains crossed to the human CD2 GFP transgenic mice. Inguinal LNs from B10 (A), Rag1−/− (B), Il2rγ−/− (C), Il2rγ−/−Rag2−/− (D), and aly/aly (E) mice transgenic for GFP. Results are representative of 5–10 experiments. Mice were analyzed at 6–8 weeks of age. (Magnification: ×16.)
In adult hCD2-GFP/Il2rγ−/− mice, very small LNs were detected in all of the expected LN sites populated by the few lymphoid cells found in these mice (Fig. 1C). In contrast, most of the peripheral LNs, such as cervical, inguinal, brachial, and axillary, were undetectable by fluorescence microscopy in adult hCD2-GFP/Il2rγ−/−Rag2−/− (Fig. 1D) and hCD2-GFP/aly/aly (Fig. 1E) mice. Small mesenteric and sacral LN structures were seen in both strains (data not shown). Adult hCD2-GFP/aly/aly mice also showed small infiltrates of GFP+ cells in a number of different organs, characteristic of the autoimmune pathology seen in these mice (data not shown).
Visualization of Lymphoid Organs in WT Mouse Embryos.
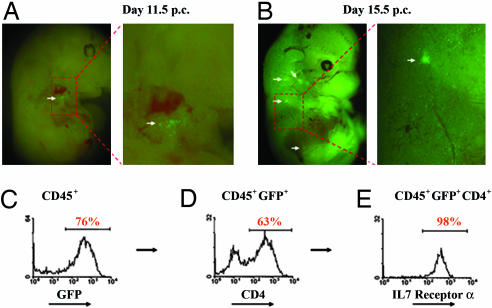
We used hCD2-GFP mice to carry out a systematic study of embryonic LN development. CD45+GFP+ hemopoietic cells were detected in the fetal liver, blood, intestines, and LN anlagen in embryos from day 11.5 p.c. onward before the appearance of mature T, B, and NK cells (data not shown and Fig. 2A and B). These cells form discreet organized aggregations in sites described before (17) marking LN anlagen. Microdissection of the GFP+ structures from these embryos and flow cytometric analysis of the GFP+ cells therein revealed the presence of LNi cells characterized by the expression of CD4, IL-7 receptor (Fig. 2 C–E), and c-kit and by the absence of CD3 or CD19 and other lineage markers, confirming that GFP is not expressed in macrophages or dendritic cells (see Fig. 8, which is published as supporting information on the PNAS web site, and ref. 31).
Fig. 2.
Characterization of human CD2 GFP transgenic mice. (A) GFP-expressing cells first appear at sites of LN development (white arrow) at day 11.5 p.c. (B) GFP-expressing cells are found in the developing LN anlagen (cervical, brachial, and inguinal; white arrows) at day 15.5 p.c. (C) Flow cytometric analysis of GFP expression on gated CD45+ cells in developing LN anlagen (day 15.5). (D) CD4 expression on CD45+GFP+ cells. (E) IL7 receptor expression on CD45+GFP+CD4+ cells. Results are representative of 5–10 experiments.
Visualization of Lymphoid Organs in Mutant Embryos.
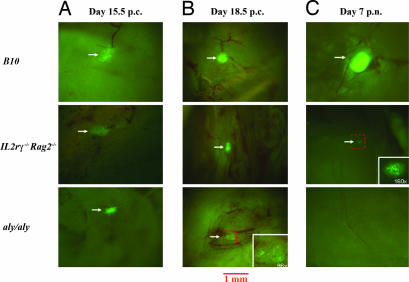
Adult aly/aly mice lack LNs because of defects in embryonic LN organogenesis, but it is not clear whether this is true also for Il2rγ−/−Rag2−/−. Using hCD2-GFP mice, we examined the LN organogenesis in Rag1−/−, Il2rγ−/−Rag2−/−, and aly/aly embryos. Interestingly, analysis of day 15.5 p.c. embryos showed that, in all three strains, aggregates containing LNi cells were formed in the expected sites, suggesting that embryonic migration and homing of these cells are not affected in these mutants (Fig. 3A). These structures were still clearly visible at day 18.5 p.c. in hCD2-GFP/Il2rγ−/−Rag2−/− embryos; in contrast, in hCD2-GFP/aly/aly embryos, only a few scattered GFP+ cells within the LN sites could be detected by this stage (Fig. 3B). FACS analysis of the GFP+ cells recovered from these sites in day 15.5 and day 18.5 p.c. mutant embryos showed a similar phenotype to the one seen in WT embryos (Fig. 9, which is published as supporting information on the PNAS web site). Analysis of 1-week-old Il2rγ−/−Rag2−/− and aly/aly mice for the presence of peripheral LNs showed that, in the Il2rγ−/−Rag2−/− mice, small GFP+ structures were still detectable at each LN site; in contrast, aly/aly mice showed no such structures after birth (Fig. 3C). In control hCD2-GFP-B10 mice LNs were already populated with mature GFP+ lymphocytes at this point (Fig. 3C).
Fig. 3.
Analysis of developing LN anlagen from hCD2 GFP, Il2rγ−/−Rag2−/−, and aly/aly mice. (A) Day 15.5 p.c. LN anlage. (B) Day 18.5 p.c. LN anlage. (C) Day 7 p.n. LN. (Magnification: ×32.) (Insets) Images are at magnifications as indicated. Results are representative of 4–10 experiments.
We conclude that migration and aggregation of LNi cells at the appropriate sites proceed normally in these mutants up to day 15.5 p.c. However, in aly/aly mice, LN primordia dissipate by day 18.5 p.c. whereas, in Il2rγ−/−Rag2−/−, they survive for at least 7 days post birth, but disappear later. This finding was confirmed by the detection of vascular cell adhesion molecule 1 (VCAM1)+ structures at LN sites in neonates, but their complete absence from adult Il2rγ−/−Rag2−/− mice (Fig. 10, which is published as supporting information on the PNAS web site).
Rescuing of LN Structures in Neonate Mice by Adoptive Transfer of Lymphoid Cells.
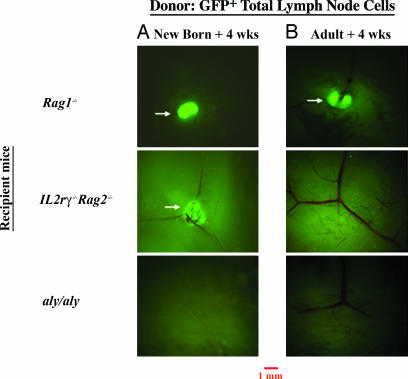
As shown in Fig. 3, in Il2rγ−/−Rag2−/− mice, LN structures were formed in embryonic life and persisted for at least 7 days after birth. This finding suggested a postnatal (p.n.) period of LN stroma maturation requiring lymphoid cells, absent in Il2rγ−/−Rag2−/− mice. To test this hypothesis, we transferred 3–4 × 106 total LN cells from 6-week-old B10.hCD2-GFP mice into newborn and adult Il2rγ−/−Rag2−/− mice. As controls, we transferred cells into Rag1−/− and aly/aly mice. Fig. 4 shows the analysis of inguinal LNs in recipients 4 weeks posttransfer. Transferred cells were readily detectable in the spleens of all groups of recipient mice at this time (Fig. 11, which is published as supporting information on the PNAS web site).
Fig. 4.
Rescuing of LN formation by adoptive transfer of lymphocytes in mutant mice. Peripheral LN cells from hCD2-GFP/B6 mice were transferred to into newborn recipient mice (+4 weeks) (A) and adult recipient mice (+4 weeks) (B) of the following genotype: Rag1−/−, Il2rγ−/−Rag2−/−, and aly/aly mice. Analysis of the inguinal LNs is shown 4 weeks after reconstitution. Results are representative of 8–10 experiments. (Magnification: ×16.)
Lymphoid cell transfer into Il2rγ−/−Rag2−/− neonates led to substantial rescue of LN structures persisting into adulthood (Fig. 4A), demonstrating that colonization of embryonic LN anlagen by lymphocytes during neonatal life is critical for the maturation and maintenance of LNs. Both T and B cells were recovered from the rescued nodes (Fig. 12, which is published as supporting information on the PNAS web site). Control newborn Rag1−/− recipient mice showed efficient colonization of the LNs by transferred cells. In contrast, transfer of cells into aly/aly neonates failed to rescue any LNs (Fig. 4A).
To test how long after birth it was possible to rescue the LNs in Il2rγ−/−Rag2−/− mice, ≈3 × 106 WT LN GFP+ cells were transferred into 6-week-old adults, and, as controls, into Rag1−/− and aly/aly mice of the same age. Fig. 4B shows the analysis of inguinal LNs in recipients 4 weeks posttransfer. No structures populated by the transferred WT GFP+ lymphocytes were detected in adult Il2rγ−/−Rag2−/− recipient mice (Fig. 4B), despite large numbers of transferred cells seen in the spleen of these mice (Fig. 11). Transferred GFP+ lymphocytes in aly/aly adult mice also failed to rescue LN structures (Fig. 4B), consistent with experiments where WT bone marrow transfer failed to restore LNs in aly/aly mice (32). Similar results were found for all of the other peripheral LNs examined (Table 1, which is published as supporting information on the PNAS web site). In contrast, we detected replenished LN structures in posttransfer Rag1−/− adult recipient mice, suggesting that the organ had formed in these mice and had been maintained into adult life (Fig. 4B).
These data taken together suggest that the absence of LNs in adult Il2rγ−/−Rag2−/− mice is due to failure to maintain p.n. the LN structures formed during embryonic life. This situation is corrected by providing mature lymphocytes during neonatal life but becomes irreversible by 6 weeks of age.
T and NK Cells Can Rescue Lymphoid Structures in Il2rγ−/−Rag2−/− Neonatal Mice.
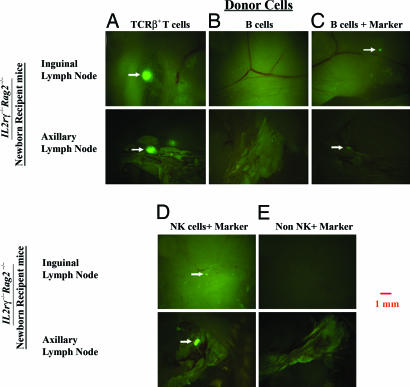
To determine which lymphoid population is capable of rescuing the LN structures, we transferred sorted lymphocyte populations in Il2rγ−/−Rag2−/− neonatal mice. Transfer of 3–4 × 106 purified TCR+GFP+ T cells into newborn Il2rγ−/−Rag2−/− recipient mice rescued peripheral LNs (Fig. 5A); in contrast, transfer of 4–5 × 106 purified B cells into newborn Il2rγ−/−Rag2−/− mice failed to do so (Fig. 5B), despite that the transferred B cells were detectable in the spleen of recipient animals 4 weeks later (Fig. 13B, which is published as supporting information on the PNAS web site). To test that the transferred purified GFP+ B cells could reach the appropriate sites, inguinal LNs of recipient mice were examined 24 h posttransfer. Significant numbers of the transferred cells were found in the LNs at that point (Fig. 14, which is published as supporting information on the PNAS web site). Therefore, a defect in migration of the transferred B cells into the LN structures is not the reason why these cells fail to rescue the neonatal LN maturation. However, B cells may not survive or expand as well as T cells during the 4-week experiment, and inability to do so would make the detection of potentially rescued LN structures difficult. To test this possibility, WT B cells were transferred into Il2rγ−/−Rag2−/− neonatal mice, and, 4 weeks later, carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled marker T cells were i.v. injected into these recipients. Examination of LNs in such mice 72 h later confirmed that B cells were very inefficient in rescuing the neonate LN structures (Fig. 5C).
Fig. 5.
Rescuing of LN formation by adoptive transfer of specific subsets of lymphocytes in mutant mice. Purified GFP+ T cells (A), YFP+ B cells (B and C), NK cells (D), and non-T/B/NK cells (E) were transferred into newborn IL2rγ−/−Rag2−/− recipient mice. Three weeks later, CFSE-labeled LN cells were transferred i.v. in these mice 72 h before analysis. Images of inguinal and axillary LNs are shown at ×16 magnification. Results in the figure are representative of 3–10 experiments.
In adult Rag deficient mice, LNs develop normally but are small because of the absence of T or B cells; in contrast, in Il2rγ−/−Rag2−/− adults, LNs are undetectable. Both of these strains of mice lack T or B cells, but Il2rγ−/−Rag2−/− mice in addition lack NK cells (29). To test whether NK cells play a role in p.n. LN organogenesis, 1.5 × 106 purified NK1.1+ LN cells from B10.Rag1−/− mice were transferred into newborn Il2rγ−/−Rag2−/− mice. Injection of CFSE-labeled T cells 4 weeks after the NK1.1+ cell transfer and examination of the LN 3 days later confirmed that NK1.1+ cells rescued LNs in these recipients (Fig. 5D). In contrast, an NK1.1− cell fraction from B10.Rag1−/− mice failed to rescue LNs (Fig. 5 F and G). Interestingly, a CD4+ IL-7rα+ CD3− cell population described before in adult mice and included in the NK1.1− transferred population had failed to rescue neonatal LN structures (Fig. 15, which is published as supporting information on the PNAS web site, and ref. 33). Thus, we reveal here a role for NK1.1+ cells in neonatal LN maturation.
IL7/IL7r Interactions and the Maintenance of Lymphoid Structures p.n.
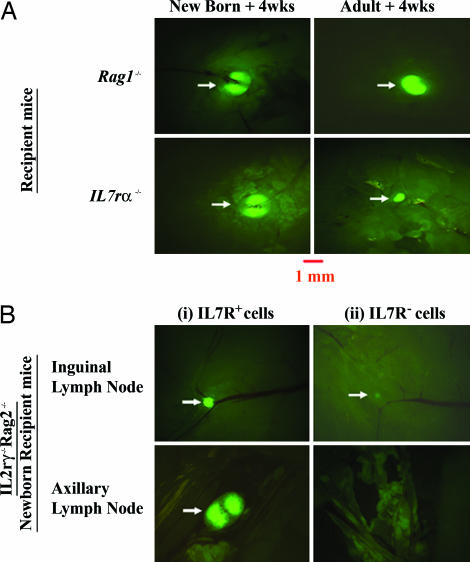
To address the mechanisms involved in the maintenance of lymphoid structures p.n., we turned our attention to the IL7/IL7r interactions, motivated by the fact that, whereas the transferred T cells express high levels of IL7r, mature B cells are devoid of this receptor (Fig. 15). Adult Il7−/− and Il7r−/−, as well as Il2rγ−/− mice, contain reduced numbers of lymphoid cells and small LNs. This finding indicates that the few lymphoid cells that exist in these adult mice can maintain to some extent the structures that have been formed during embryonic life. However, the small size of the LNs could be due to the paucity in lymphoid cells in these mice. To examine whether the small LN structures in the adult retain the same potential for growth as those in neonates, we transferred WT hCD2-GFP LN cells into neonatal and adult Il7r−/− mice. Transfer into neonates resulted in full-size LNs that were still apparent 4 weeks after transfer (Fig. 6A Left). In contrast, transfer of WT cells into adult Il7r−/− mice failed to rescue the size of the LNs (Fig. 6A Right). These data suggest that restoration of the IL7/IL7r interactions during the neonatal period in IL7r−/− mice may contribute to the full maturation and growth of the LNs. In contrast, restoring these interactions at later times fails to do so, indicating that the potential of LNs to grow has been lost in adult Il7r−/− mice.
Fig. 6.
IL7/IL7R interactions are necessary for p.n. rescuing of LNs. (A) Rescuing of LN size in IL7Rα−/− mice by mature WT lymphocytes. WT GFP-expressing LN cells were transferred into Rag1−/− and IL7rα−/− mice. Inguinal LNs are shown 4 weeks after reconstitution from newborn recipients (Left) and adult recipient mice (Right). (B) Rescuing of LN formation by IL7R+ cells from Rag−/− LNs: IL7R+ (Left) and IL7R− (Right) cells were transferred into newborn IL2rγ−/−Rag2−/− recipient mice. Three weeks later, CFSE-labeled LN cells were transferred i.v. 72 h before analysis. Images of LNs are shown at ×16 magnification. Results are representative of 10 experiments.
This interpretation was further supported by transfer experiments that compared the efficiency of different subsets of LN cells from Rag1-deficient mice to rescue LN structures in newborn Il2rγ−/−Rag2−/− recipient mice. Cells found in Rag 1−/− LNs can be divided into two fractions, an IL7r+ and an IL7r− (Fig. 15). Purification and transfer of these populations into Il2rγ−/−Rag2−/− mice revealed that the IL7R+ population was very efficient in restoring LNs in newborn Il2rγ−/−Rag2−/− recipient mice (Fig. 6B Left). In contrast, IL7R− cells were incapable of doing so (Fig. 6B Right). Because NK cells are the main population of lymphoid cells in Rag1−/− LNs, some of which express IL7r, we conclude that the latter are most likely responsible for the neonatal LN rescue in Il2rγ−/−Rag2−/− recipient mice. Rag1−/− LNs also have an NK1.1−/− population that includes IL7R+CD4+ cells that have been reported to be the equivalent of the embryonic LNi cells (33). However, we showed in Fig. 5E that these cells are not able to rescue neonatal LNs.
Taken together, these results indicate a possible role for IL7/IL7r interactions during the neonatal stages of LN structure maintenance and growth.
Discussion
Embryonic development of peripheral LN anlagen initiates between day 11.5 and day 12.5 p.c. in gestation associated with an influx of LNi cells in these sites (34). Using mice in which the embryonic LN anlage is marked by hemopoietic GFP+ cells, we studied the requirements for (i) the embryonic development of the LN anlagen, (ii) the maturation of LN structures in neonatal mice, and (iii) their maintenance in adult mice. For this study, an array of mutant mice with defects in LN development were analyzed.
Lack of Il2rγ signaling in Il2rγ−/− mice severely inhibits the maturation of lymphocytes (27, 28). As a result, these mice have few T, B, and NK cells and almost undetectable LNs. Il2rγ−/−Rag2−/− adult mice, on the other hand, lack T, B, and NK cells and show absence of LN structures apart from a small mesenteric and sacral LN (data not shown) (29, 35). It was unclear whether the absence of detectable LNs in Il2rγ−/−Rag2−/− adult mice was due to failure of lymphoid anlagen development or due to lack of lymphoid cells. hCD2-GFP/Il2rγ−/−Rag2−/− embryos showed no developmental defect in LN organogenesis; instead, their degeneration seems to be due to failure to populate the anlagen with mature lymphocytes during the neonatal period. Consistent with this interpretation, we were able to rescue the LNs permanently by transferring WT lymphoid cells during this neonatal period. The failure to rescue LN structures when T and NK cells were transferred into adult Il2rγ−/−Rag2−/− mice seems to be associated with the absence of stroma cells at this age with which mature lymphoid cells could interact and effect LN maintenance and growth. This finding suggests that signaling through cytokine receptors using the common γ chain may be dispensable for early LN development but becomes important for the maintenance of these organs p.n.
Although IL2rγ is used by other cytokines, such as IL15, IL2, etc., absence of these cytokines does not lead to the LN defects seen in IL7−/− mice, indicating that IL7 may be more important during this stage. Our data are consistent with the hypothesis that, after stimulation of incoming lymphocytes through their IL7r, downstream genes (chemokines, growth factors, etc.) are activated, and their products have a positive effect on the survival, growth, and maintenance of the LN stroma cells. However, in the absence of lymphocytes, either the neonatal stroma cells themselves or their ability to respond to these growth factors disappears, as indicated by the inability of transferred lymphocytes to restore LNs in adult Il2rγ−/−Rag2−/− or their size in adult Il7r−/− mice. Nevertheless, a degree of contribution by the other common γ chain cytokines in this process cannot be excluded at this point.
During the neonatal colonization of the LN anlagen by mature lymphoid cells, LN size increases due both to cell output from the bone marrow and thymus, as well as expansion of the incoming cells (36). It is well documented that transfer of adult T cells into neonates results in marked expansion of the transferred cells (37); B cells on the other hand seem to have limited capacity for expansion under similar circumstances (38). Therefore, the limited capacity of the B cells to expand may be the underlying reason why they are less capable of rescuing the neonatal stages of LN maturation. An additional factor for the differential potential of lymphoid subsets to promote the neonatal maturation of LNs may relate to their ability to signal through the IL7 receptor. Indeed, mature T cells and a proportion of NK cells express this receptor, whereas B cells are completely devoid of it (Fig. 15 and ref. 39).
In the absence of LTβr signaling (aly/aly mice), LNs also fail to develop (23, 24). However, migration and aggregation of LNi cells at the appropriate sites occur normally up to day 15.5 p.c. in development. This result is consistent with results reported by Yoshida et al. (17) for the LTα−/− mice. However, from day 15.5 p.c. onward, LN structures atrophy, leaving a small number of scattered GFP+ cells in sites of LN development. These data are consistent with defective steps described during the formation of Peyer's patches in aly/aly mice (22).
In aly/aly mice, absence of LTβr signaling leads to irreversible degeneration of LN stroma between day 15.5 and day 18.5 p.c., confirming that anlagen failure in the embryo is the main reason for the absence of LN structures in adult aly/aly mice. Significantly, the development of peripheral LNs in LTα-deficient mice has previously been shown to be rescued by means of a single treatment with an agonist antibody for LTβr during the same window in fetal life, but not later (20).
Taken together, the data indicate that the absence of LNs in the adult mice of these mutant strains is not due to deficiencies in migration and homing of LNi cells to the right sites in the embryo. This finding confirms results from LTα-deficient mice, suggesting that LTβr signaling is dispensable for these stages of LN development, but is important for the immediately subsequent step (17). Thus, absence of LTβr signaling leads to atrophy of the anlage by day 18.5 p.c. and failure of further LN development, despite the appearance later of T, B, and NK cells in aly/aly mice. These data also indicate that, in the Il2rγ−/−Rag2−/− mice, an intact LTβr signaling potential is not sufficient for these structures to persist into adult life and that other molecular interactions may become crucial.
In conclusion, the combined and additional results from lymphoid cell transfers into Il2rγ−/−Rag2−/− and aly/aly mice presented in our article have allowed the dissociation of the LTβ-dependent late embryonic development of LN anlagen from the subsequent neonatal maturation of the LN stroma. Thus, we have revealed a window around birth, during which lymphoid cells are necessary for the continuing maturation of the LNs and during which the cellular and molecular requirements for LN development are different from those during late embryogenesis.
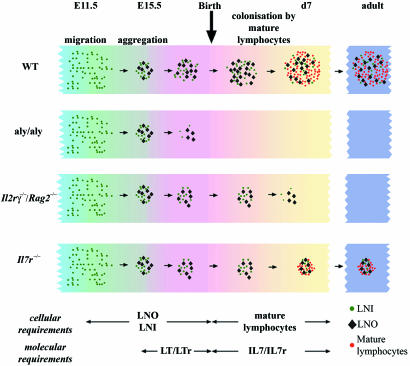
Taking into account previously published data and the additional results reported in this article, we would like to propose a model that encompasses a previously uncharacterized stage of LN development during the p.n. period in the mouse (Fig. 7). The prenatal period is marked by an absolute requirement for LTβr signaling within the stroma cells without which the LN anlage degenerates by day 18.5 p.c. This stage depends on the presence of LNi cells. Postnatally, the requirements for hemopoietic cells switch from the LNi cells to newly immigrating T and NK1.1+ cells. In the absence of this influx, the developing LN degenerates irreversibly within the first week after birth, and IL7/IL7r interactions seem to play an important role during this stage.
Fig. 7.
A model of LN development during the perinatal period. Migrating hemopoietic cells including LNi cells aggregate with LNo cells to form the LN anlage. In normal mice, the structure persists until after birth when it is colonized by mature lymphoid cells generated in the thymus and the bone marrow. In aly/aly mice, these structures degenerate near the end of pregnancy, so that there is no structure to be colonized by mature lymphoid cells after birth. In Il2rγ−/−Rag2−/− mice, these structures persist for a few days after birth, but, because of the complete absence of lymphoid cells, they degenerate within the first week after birth. In Il7r−/− mice, these structures are maintained by the few lymphoid cells that exist in these mice; however, the size of the LNs remains small throughout life.
Methods
Mice.
Human CD2 LCR GFP (hCD2-GFP) mice were crossed to a number of different strains. B6.Il7 receptor α−/− (40), B10.Rag1−/− (41), TCRα−/− (42), B10.Il2rγ−/−, B10.Il2rγ−/−Rag2−/− (28), B6.aly/aly (23), and B6.CD19 Cre R26R EYFP mice (43) were bred in a specific pathogen-free facility at the National Institute for Medical Research.
Cellular Analysis.
LN anlagen cells were isolated from adult or fetal LN anlagen by microdissection of fluorescent structures and collagenase H digestion (Roche, Penzberg, Germany). Cells were stained as described by using anti-TCRβ,H57.597 (eBioscience, San Diego, CA), anti-CD3,2C11 (eBioscience), anti-Thy-1.2,53-52.1 (eBioscience), anti-CD4,CT-CD4 (Caltag, Carlsbad, CA), anti-IL-7 receptor α,A7R34 (eBioscience), anti-CD45,30-F11 (eBioscience), anti-CD19 (Caltag), anti-NK1.1 (eBioscience), and anti-CD117 (eBioscience). Flow cytometric analysis was done on a FACSCalibur (Becton-Dickinson, San Jose, CA), and data analysis was done using WINMDI (Scripps, La Jolla, CA).
Cellular Transfers.
For adult recipient transfers, 3–4 × 106 hCD2-GFP cells were transferred i.v. 3–4 weeks before analysis. Adult aly/aly mice were given sublethal irradiation (500 R) before transfer. Newborn recipient mice received 3–4 × 106 cells i.p. <12 h after birth. T cells were purified by T cell enrichment column (R & D Systems, Boston, MA) and sorted (MoFlo/Dako-Cytomation, Fort Collins, CO) on the basis of GFP and TCR expression (>99% T cells). B cells were isolated from CD19cre R26R EYFP (>99%). For transfer experiments using tracer cells, CD19+NK1.1− B cells from TCRα−/−-deficient mice were sorted (>99%) and transferred i.p. into newborn Il2rγ−/−Rag2−/− mice. NK1.1+CD117− and NK1.1−CD117− cells from Rag1−/− mice were sorted to >99% purity and transferred i.p. into newborn Il2rγ−/−Rag2−/− mice. LN tracer cells (1 × 107) were labeled with CFSE (5 μM) and injected i.v. 72 h before analysis. IL7r+ and IL7r− cells were sorted (>99% purity) from Rag1−/− LNs and transferred i.p. into newborn IL2rγ−/−Rag2−/− recipient mice.
Microscopy.
Analysis of GFP/YFP expression was done on a Zeiss (Jena, Germany) M2Bio stereofluorescent microscope/Open Lab (Improvision, Warwick, U.K.). For confocal microscopy, tissues were fixed in 4% paraformaldehyde, stained with anti-VCAM1 (eBiosciences) and anti-rat Alexa Fluor 594 (Molecular Probes, Eugene, OR), and analyzed on a Leica (Vienna, Austria) SP2/Volocity (Improvision). To determine the presence or absence of peripheral LNs, we checked for the presence of GFP-expressing cells at the sites of cervical, brachial, axillary, and inguinal LNs by stereofluorescent microscopy. If GFP-expressing structures were >0.1 mm, the structures were scored as positive.
Supplementary Material
Acknowledgments
We thank Chris Atkins and Graham Preece for cell sorting and Drs. Gitta Stockinger [Division of Molecular Immunology, Medical Research Council (MRC), National Institute of Medical Research (NIMR), London, U.K.], Rose Zamoyska (Division of Molecular Immunology, MRC, NIMR, London, U.K.), and Anne O'Garra (Division of Immunoregulation, MRC, NIMR, London, U.K.) for animals and discussions. This work was supported by the MRC. H.V.-F. and K.E.F. were supported by European Union Molecular Imaging Grant LSHG-CT-2003-503259.
Abbreviations
- LN
lymph node
- LNi
LN inducer
- LNo
LN organizer
- LTβR
lymphotoxin β receptor
- CFSE
carboxyfluorescein diacetate-succinimidyl ester
- NK
natural killer
- p.c.
postcoitum
- p.n.
postnatal/postnatally
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nishikawa S., Honda K., Vieira P., Yoshida H. Immunol. Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 2.Cupedo T., Kraal G., Mebius R. E. Immunol. Rev. 2002;189:41–50. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- 3.Cupedo T., Mebius R. E. J. Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Mebius R. E. Nat. Rev. Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 5.Wang J. H., Nichogiannopoulou A., Wu L., Sun L., Sharpe A. H., Bigby M., Georgopoulos K. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 6.Kurebayashi S., Ueda E., Sakaue M., Patel D. D., Medvedev A., Zhang F., Jetten A. M. Proc. Natl. Acad. Sci. USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokota Y., Mansouri A., Mori S., Sugawara S., Adachi S., Nishikawa S., Gruss P. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 8.Kim D., Mebius R. E., MacMicking J. D., Jung S., Cupedo T., Castellanos Y., Rho J., Wong B. R., Josien R., Kim N., et al. J. Exp. Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougall W. C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M. E., Maliszewski C. R., et al. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., et al. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 11.Futterer A., Mink K., Luz A., Kosco-Vilbois M. H., Pfeffer K. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 12.Koni P. A., Sacca R., Lawton P., Browning J. L., Ruddle N. H., Flavell R. A. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 13.Banks T. A., Rouse B. T., Kerley M. K., Blair P. J., Godfrey V. L., Kuklin N. A., Bouley D. M., Thomas J., Kanangat S., Mucenski M. L. J. Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 14.De Togni P., Goellner J., Ruddle N. H., Streeter P. R., Fick A., Mariathasan S., Smith S. C., Carlson R., Shornick L. P., Strauss-Schoenberger J., et al. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 15.Kim C. H. Curr. Opin. Hematol. 2005;12:298–304. doi: 10.1097/01.moh.0000166496.18773.e3. [DOI] [PubMed] [Google Scholar]
- 16.Honda K., Nakano H., Yoshida H., Nishikawa S., Rennert P., Ikuta K., Tamechika M., Yamaguchi K., Fukumoto T., Chiba T., Nishikawa S. I. J. Exp. Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida H., Naito A., Inoue J., Satoh M., Santee-Cooper S. M., Ware C. F., Togawa A., Nishikawa S. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 18.Cyster J. G. Immunol. Rev. 2003;195:5–14. doi: 10.1034/j.1600-065x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Muller G., Hopken U. E., Lipp M. Immunol. Rev. 2003;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 20.Rennert P. D., James D., Mackay F., Browning J. L., Hochman P. S. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 21.Cupedo T., Mebius R. E. Semin. Immunol. 2003;15:243–248. doi: 10.1016/j.smim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Adachi S., Yoshida H., Kataoka H., Nishikawa S. Int. Immunol. 1997;9:507–514. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- 23.Miyawaki S., Nakamura Y., Suzuka H., Koba M., Yasumizu R., Ikehara S., Shibata Y. Eur. J. Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 24.Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. Nat. Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 25.Weih F., Caamano J. Immunol. Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M., Iwamasa K., Rennert P. D., Yamada T., Suzuki R., Matsushima A., Okabe M., Fujita S., Yokoyama M. J. Immunol. 1999;163:1584–1591. [PubMed] [Google Scholar]
- 27.Cao X., Shores E. W., Hu-Li J., Anver M. R., Kelsall B. L., Russell S. M., Drago J., Noguchi M., Grinberg A., Bloom E. T., et al. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 28.DiSanto J. P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman J. P., Blundell M. P., Lopes L., Kinnon C., Di Santo J. P., Thrasher A. J. Br. J. Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhumabekov T., Corbella P., Tolaini M., Kioussis D. J. Immunol. Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 31.de Boer J., Williams A., Skavdis G., Harker N., Coles M., Tolaini M., Norton T., Williams K., Roderick K., Potocnik A. J., Kioussis D. Eur. J. Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T., Mitani T., Yorita K., Uchida D., Matsushima A., Iwamasa K., Fujita S., Matsumoto M. J. Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 33.Lane P. J., Gaspal F. M., Kim M. Y. Nat. Rev. Immunol. 2005;5:655–660. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mebius R. E., Rennert P., Weissman I. L. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 35.Cupedo T., Vondenhoff M. F., Heeregrave E. J., De Weerd A. E., Jansen W., Jackson D. G., Kraal G., Mebius R. E. J. Immunol. 2004;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 36.Le Campion A., Bourgeois C., Lambolez F., Martin B., Leaument S., Dautigny N., Tanchot C., Penit C., Lucas B. Proc. Natl. Acad. Sci. USA. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min B., McHugh R., Sempowski G. D., Mackall C., Foucras G., Paul W. E. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 38.Freitas A. A., Rocha B. Annu. Rev. Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 39.Maraskovsky E., Teepe M., Morrissey P. J., Braddy S., Miller R. E., Lynch D. H., Peschon J. J. J. Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 40.Peschon J. J., Morrissey P. J., Grabstein K. H., Ramsdell F. J., Maraskovsky E., Gliniak B. C., Park L. S., Ziegler S. F., Williams D. E., Ware C. B., et al. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 42.Philpott K. L., Viney J. L., Kay G., Rastan S., Gardiner E. M., Chae S., Hayday A. C., Owen M. J. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 43.Rickert R. C., Roes J., Rajewsky K. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.