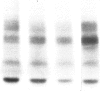
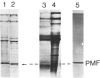
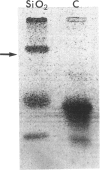
Abstract
A hypothesis is presented for the action of silica-treated macrophages on protein synthesis in fibroblasts and also a method for the isolation of silica-attached materials in lung tissue. The increased protein synthesis in the fibroblasts is due, at least partly, to an increase in mRNA. Silica prevents the suppressing "macrophage effect" of macrophage-originated ribonuclease on fibroblasts. However, under certain conditions, collagen synthesis is stimulated by silica-treated macrophage preparations to such an extent that the effect cannot be explained by the inhibition of macrophage ribonuclease alone. We therefore postulate the existence of a fibrogenic factor, which is released by the macrophages. This factor has been demonstrated and can be purified from lung homogenate of SiO2-treated rats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M., Kulonen E., Rönnemaa T., Sundström C., Vilpo J. Liberation of a fibrogenic factor from human blood monocytes, ascites cells, cultured histiocytes and transformed mouse macrophages by treatment with SiO2. Scand J Clin Lab Invest. 1980 Jun;40(4):311–318. doi: 10.3109/00365518009092649. [DOI] [PubMed] [Google Scholar]
- Aalto M., Potila M., Kulonen E. The effect of silica-treated macrophages on the synthesis of collagen and other proteins in vitro. Exp Cell Res. 1976 Jan;97:193–202. doi: 10.1016/0014-4827(76)90668-6. [DOI] [PubMed] [Google Scholar]
- Aalto M., Turakainen H., Kulonen E. Effect of SiO2-liberated macrophage factor on protein synthesis in connective tissue in vitro. Scand J Clin Lab Invest. 1979 May;39(3):205–213. doi: 10.1080/00365517909106095. [DOI] [PubMed] [Google Scholar]
- Aalto M., Viljanen M., Kulonen E. Neutralization of the fibrogenic silica-released macrophage factor by antiserum. Exp Pathol. 1982;22(3):181–184. doi: 10.1016/s0232-1513(82)80007-8. [DOI] [PubMed] [Google Scholar]
- Aho S., Kulonen E. Effect of silica-liberated macrophage factors on protein synthesis in cell-free systems. Exp Cell Res. 1977 Jan;104(1):31–38. doi: 10.1016/0014-4827(77)90065-9. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W., Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med. 1979 Dec 1;150(6):1421–1431. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
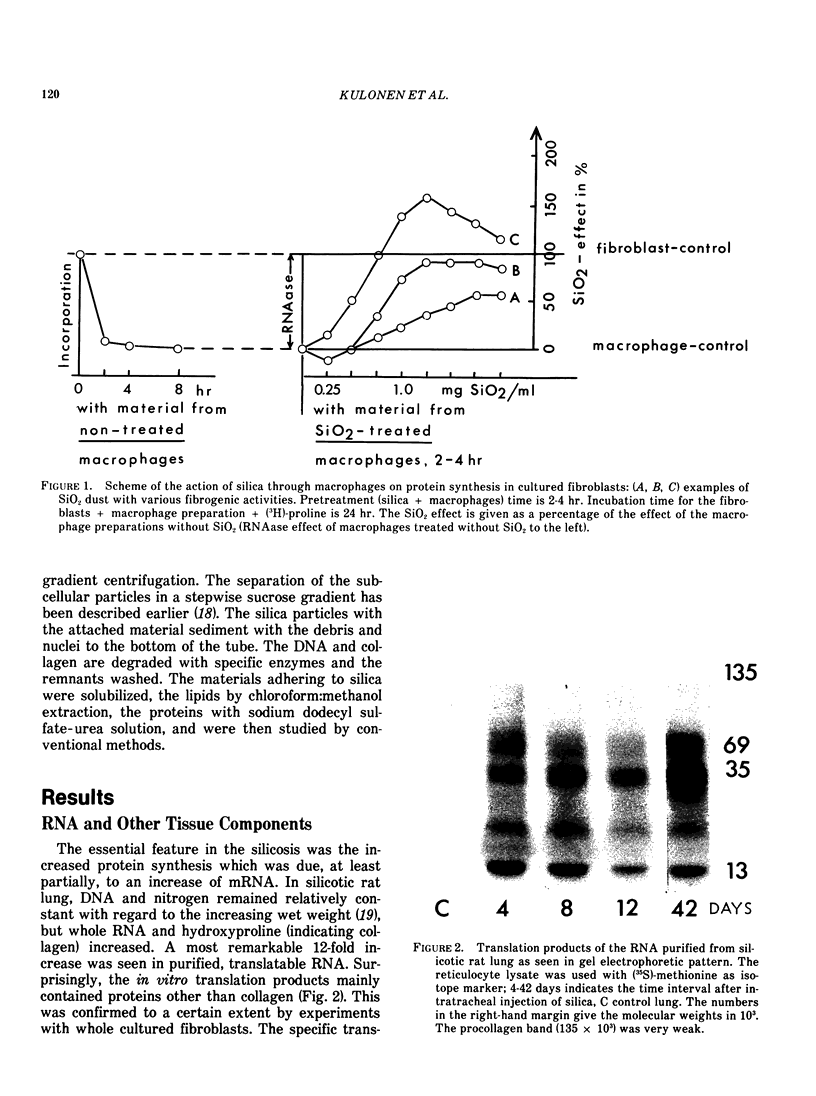
- Kulonen E., Aalto M., Aho S., Lehtinen P., Potila M. Increase of RNA and appearance of new protein in silicotic lung tissue. Ann Occup Hyg. 1982;26(1-4):463–471. [PubMed] [Google Scholar]
- Kulonen E., Aalto M., Aho S., Lehtinen P., Potila M. Recent investigations on macrophage proteins and of fibroblast RNA in silicotic lung fibrosis. Contrib Microbiol Immunol. 1983;7:204–211. [PubMed] [Google Scholar]
- Lehtinen P., Kulonen E. Preparation of subcellular fractions from granulation tissue by density gradient centrifugation. Acta Chem Scand A. 1979;B33(5):327–336. doi: 10.3891/acta.chem.scand.33b-0327. [DOI] [PubMed] [Google Scholar]
- Lehtinen P., Kulonen E. Subcellular targets of the soluble SiO2-liberated macrophage factors in experimental granulation tissue. Biochim Biophys Acta. 1979 Aug 29;564(1):132–140. doi: 10.1016/0005-2787(79)90194-1. [DOI] [PubMed] [Google Scholar]
- Liu D. K., Williams G. H., Fritz P. J. Alkaline ribonuclease and ribonuclease inhibitor in mammary gland during the lactation cycle and in the R3230AC mammary tumour. Biochem J. 1975 Apr;148(1):67–76. doi: 10.1042/bj1480067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Tolstoshev P., Berg R. A., Rennard S. I., Bradley K. H., Trapnell B. C., Crystal R. G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J Biol Chem. 1981 Mar 25;256(6):3135–3140. [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]