Abstract
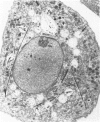
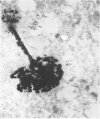
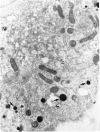
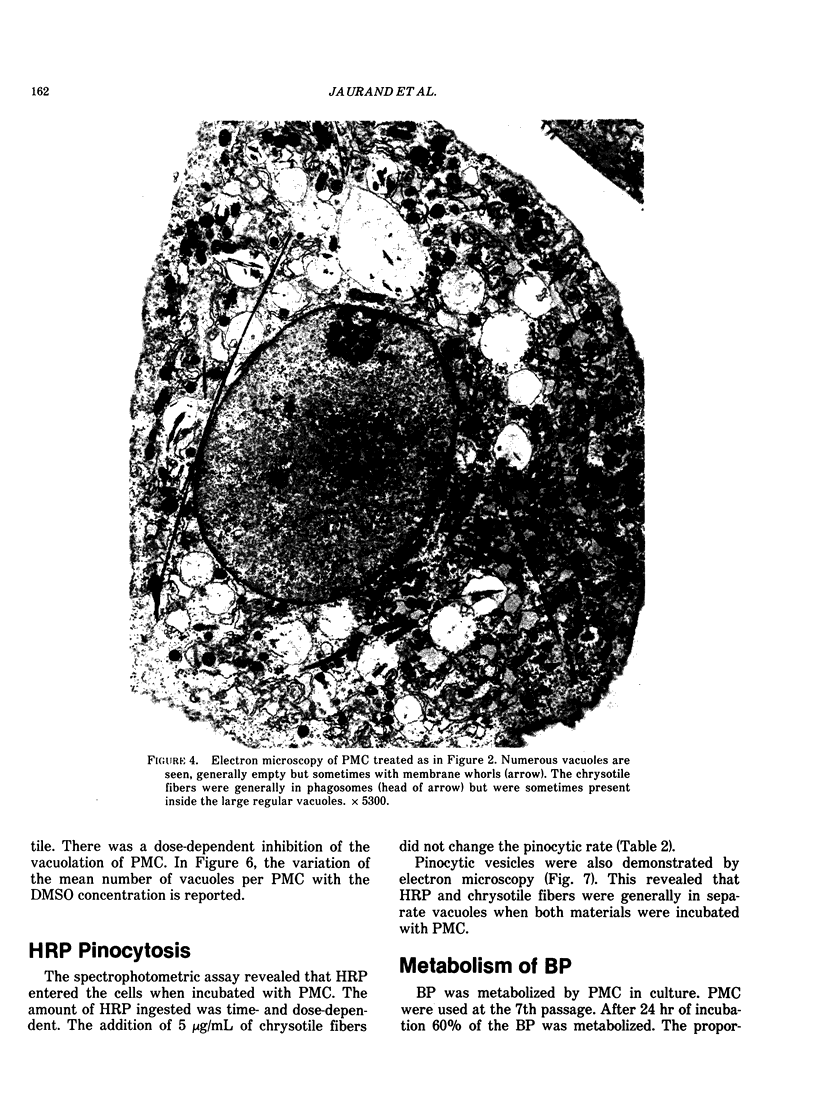
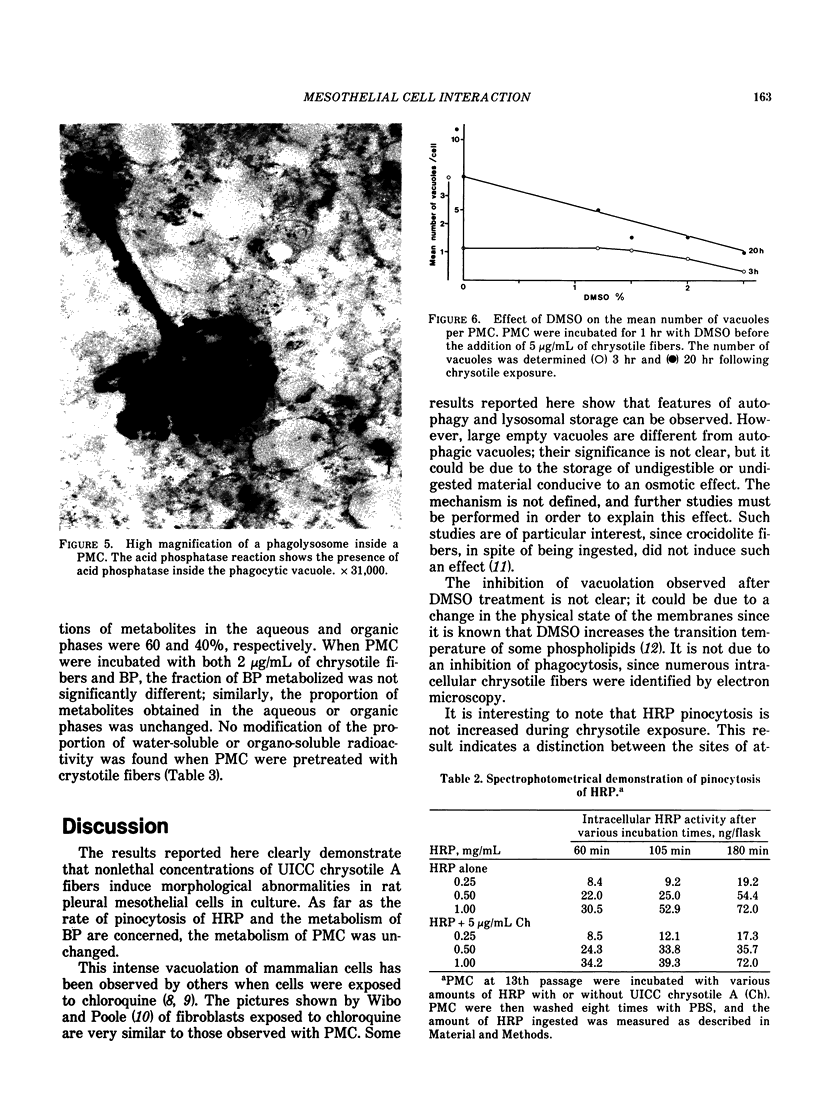
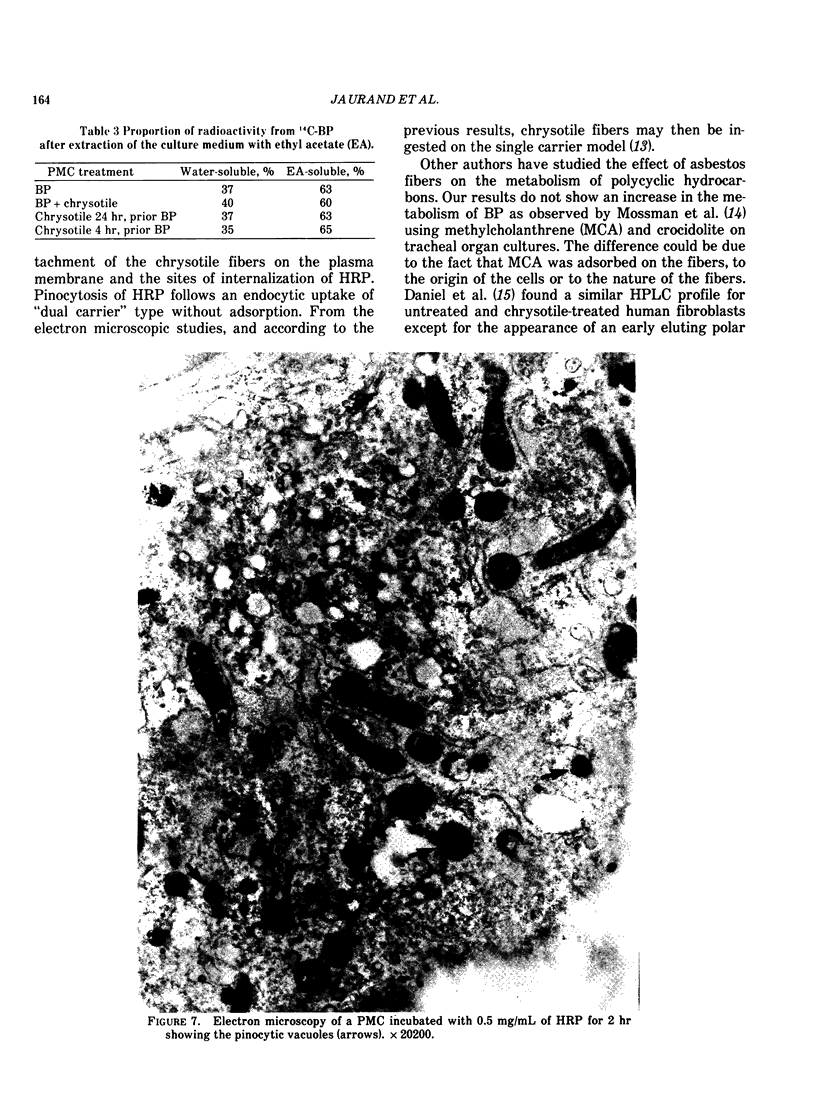
Cultures of rat pleural mesothelial cells (PMC) were exposed to nonlethal doses of UICC chrysotile A. The morphology was studied by optical and electron microscopy. The consequences of chrysotile ingestion on the rate of pinocytosis of horseradish peroxidase (HPR) metabolism and benzo-3-4-pyrene (BP) were studied. Nonlethal doses of chrysotile (5 micrograms/mL) induced a time-dependent vacuolation of PMC; a dose-dependent inhibition of the vacuolation was observed when PMC were pretreated with DMSO. The origin of the vacuoles is not clear, but some features of autophagy and lysosomal storage were observed. Chrysotile fibers did not modify the rate of pinocytosis of HRP. Similarly, the metabolism of BP was unchanged when BP and chrysotile were both added to the culture medium or when PMC were preincubated with the fibers 24 hr prior to the addition of BP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignon J., Jaurand M. C. Biological in vitro and in vivo responses of chrysotile versus amphiboles. Environ Health Perspect. 1983 Sep;51:73–80. doi: 10.1289/ehp.835173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G., Cohn Z. A. Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol. 1968 Aug;38(2):377–391. doi: 10.1083/jcb.38.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Bernaudin J. F., Renier A., Kaplan H., Bignon J. Rat pleural mesothelial cells in culture. In Vitro. 1981 Feb;17(2):98–106. doi: 10.1007/BF02618065. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Kaplan H., Thiollet J., Pinchon M. C., Bernaudin J. F., Bignon J. Phagocytosis of chrysotile fibers by pleural mesothelial cells in culture. Am J Pathol. 1979 Mar;94(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Jaurand M. C., Magne L., Boulmier J. L., Bignon J. In vitro reactivity of alveolar macrophages and red blood cells with asbestos fibres treated with oxalic acid, sulfur dioxide and benzo-3,4-pyrene. Toxicology. 1981;21(4):323–342. doi: 10.1016/0300-483x(81)90147-5. [DOI] [PubMed] [Google Scholar]
- Lyman G. H., Papahadjopoulos D., Preisler H. D. Phospholipid membrane stabilization by dimethylsulfoxide and other inducers of Friend leukemic cell differentiation. Biochim Biophys Acta. 1976 Oct 19;448(3):460–473. doi: 10.1016/0005-2736(76)90300-x. [DOI] [PubMed] [Google Scholar]
- MILLER F., PALADE G. E. LYTIC ACTIVITIES IN RENAL PROTEIN ABSORPTION DROPLETS. AN ELECTRON MICROSCOPICAL CYTOCHEMICAL STUDY. J Cell Biol. 1964 Dec;23:519–552. doi: 10.1083/jcb.23.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman B. T., Craighead J. E. Mechanisms of asbestos carcinogenesis. Environ Res. 1981 Aug;25(2):269–280. doi: 10.1016/0013-9351(81)90028-1. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]