Abstract
Bacterial cells are much smaller and have a much simpler overall structure and organization than eukaryotes. Several prominent differences in cell organization are relevant to the mechanisms of chromosome segregation, particularly the lack of an overt chromosome condensation/decondensation cycle and the lack of a microtubule-based spindle. Although bacterial chromosomes have a rather dispersed appearance, they nevertheless have an underlying high level of spatial organization. During the DNA replication cycle, early replicated (oriC) regions are localized towards the cell poles, whereas the late replicated terminus (terC) region is medially located. This spatial organization is thought to be driven by an active segregation mechanism that separates the sister chromosomes continuously as replication proceeds. Comparisons of various well-characterized bacteria suggest that the mechanisms of chromosome segregation are likely to be diverse, and that in many bacteria, multiple overlapping mechanisms may contribute to efficient segregation. One system in which the molecular mechanisms of chromosome segregation are beginning to be elucidated is that of sporulating cells of Bacillus subtilis. The key components of this system have been identified, and their functions are understood, in outline. Although this system appears to be specialized, most of the functions are conserved widely throughout the bacteria.
Keywords: chromosome segregation, bacterial cell cycle, Bacillus subtilis sporulation, soj–spo0J system, divIVA, racA
1. Differences between chromosome segregation in eubacteria and eukaryotes
The most prominent way in which bacterial cells differ from eukaryotes is in their small size. Bacteria have typical dimensions of 1–2 μm, about 1/10 the diameter of the nucleus of a mammalian cell. The bacterial chromosome is usually a single circular molecule with typical dimensions of about 4 Mbp, much smaller than most chromosomes in higher eukaryotes. It has a very high gene density (about one gene per kbp). Genes are relatively small, and introns are rare or absent. Bacterial chromosomes are not separated from the cytosol by a nuclear membrane, and the DNA has a relatively diffuse appearance, almost filling the cytoplasm in live cells (figure 1a). Fluorescence imaging of RNA polymerase or ribosomes, however, reveals that transcription and translation are spatially separated, in the main, with a similar topology to eukaryotes. Thus, transcription tends to occur in the central core of the cell, whereas translation occurs in the periphery (Lewis et al. 2000).
Figure 1.
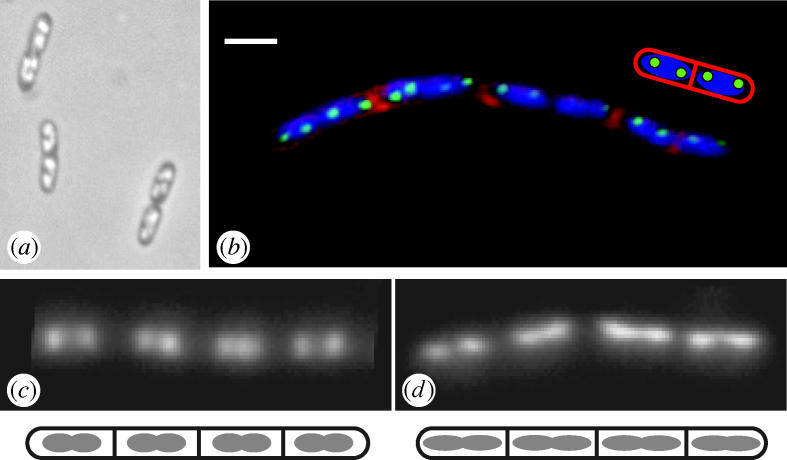
Examples of nucleoid morphology in bacterial cells. (a) Nucleoid arrangement in unstained living cells of E. coli, visualized by phase contrast microscopy. (Kindly supplied by H. Niki.) (b) Nucleoid arrangement in vegetative cells of B. subtilis, visualized by fluorescence microscopy after staining the DNA with DAPI (blue) and by observation of Spo0J–GFP (green). A chain of cells is shown, with the membranous outline of each cell and several intermediate division septa (red) visualized by staining with FM5-95. The cartoon shows an interpretation of the organization of the two right-most cells of the chain. Scale bar, 2 μm. (Kindly supplied by Alison Hunt.) (c,d) DAPI-stain fluorescence images showing the elongation of the nucleoid to form the axial filament during the onset of sporulation in B. subtilis. (c) Chain of vegetative cells, showing compact nucleoids. (d) Chain of early sporulating cells, showing the characteristic axial filament (elongated) nucleoid state, especially the nucleoids of the two cells to the right.
In view of the size and organizational differences between prokaryotic and eukaryotic cells, it is not surprising that the mechanics of chromosome segregation differ considerably. The most prominent general difference lies in the lack of a microtubule-based mitotic spindle. It seems possible that the spindle is not needed in bacteria because the distance the sister chromosomes need to be moved to achieve separation is much less (about 1 μm) than in eukaryotes (>10 μm). Another difference lies in the lack of an overt condensation/decondensation cycle during segregation, though some eukaryotes (e.g. yeast) also manage without such a process, perhaps because their chromosomes are relatively short. Finally, the circular topology of bacterial chromosomes is important because recombination between sister (circular) chromosomes can generate covalently connected dimeric structures, which need to be resolved by a chromosome dimer resolution system. This problem and the related one of removing catenanes (interlinks) between the daughter circles have been recently reviewed and will not be considered further here (Sherratt 2003; Wu 2004). We will also not deal with the question of chromosome organization by proteins that are thought to act by controlling or effecting chromosome condensation (e.g. SMC) or regulating DNA supercoiling (e.g. DNA gyrase). Although loss of this kind of protein can impair chromosome segregation, it is not clear whether these proteins should or should not be considered to function directly in segregation.
2. Observations of segregation in bacteria
Despite the lack of chromosome condensation and the mitotic spindle, many lines of evidence show that the bacterial chromosomes are nevertheless highly organized. Observations of sporulating cells of Bacillus subtilis provided the first evidence for a high level of organization (Wu & Errington 1994; see below). Subsequently, green fluorescent protein (GFP) tagging methods and fluorescence in situ hybridization (FISH) methods have provided ample evidence that the bacterial chromosome is highly organized and that any given site on the chromosome tends to have a preferred location within the cell (Niki & Hiraga 1998; Teleman et al. 1998; Niki et al. 2000; Roos et al. 2001; Viollier et al. 2004). Observations of cells in which the origin of chromosome replication (oriC) region has been labelled in various ways (example shown in figure 1b), have provided strong support for the notion that newly replicated sister chromosomes are actively segregated, probably involving mechanisms that act at or near the oriC region (Glaser et al. 1997; Webb et al. 1997; Niki & Hiraga 1998; Jensen & Shapiro 1999; Li et al. 2002; Lau et al. 2003). This is particularly evident from the work on Caulobacter crescentus, in which extreme bipolar separation of oriC regions occurs almost at the beginning of DNA replication (Viollier et al. 2004), and on sporulating cells of B. subtilis (see below). In Escherichia coli, the results are less clear cut because at least one group has reported that oriC separation occurs roughly in parallel with cell elongation (Roos et al. 1999, 2001). Nevertheless, in support of the active segregation idea, a small cis-acting site capable of directing adjacent chromosomal DNA to bipolar positions in the cell was recently characterized in E. coli (Yamaichi & Niki 2004). Identification of this sequence may be an important first step towards uncovering the mechanisms of active chromosome segregation in this organism. Unfortunately, deletion or translocation of this site seems to have little effect on overall segregation, suggesting that the sequence is either unimportant or functionally redundant. An analogous cis-acting site has also been found in B. subtilis, but further characterization is needed to establish the functional significance of the site (Kadoya et al. 2002).
3. Models for bacterial chromosome segregation
Several models for chromosome segregation have been described. Two general elements figure in most models for the segregation and or organization of bacterial chromosomes. First are spatially separated, usually bipolar ‘capture sites’ that sequester specific DNA sequences or regions at or near opposite poles of the cell. Second are motor proteins that drive the spatial separation of the sister chromosomes. The first capture sites were hypothetical membrane attachment sites for newly replicated sister oriC regions. It was proposed that growth of the cell envelope between sister oriC sites would then drive segregation (Jacob et al. 1963). It has subsequently become clear that growth of the cell envelopes of rod-shaped bacteria does not occur in the required zonal manner (Mobley et al. 1984; Woldringh et al. 1987). However, the situation has become more complicated by the discovery that in B. subtilis and probably many other rod-shaped bacteria, the pattern of wall synthesis is probably helical and governed by an underlying helical cytoskeleton of the actin homologue, MreB (Jones et al. 2001; Carballido-López & Errington 2003; Daniel & Errington 2003; Shih et al. 2003; Figge et al. 2004). Moreover, the ends of the helical filaments could, in principle, act as sites to separate chromosomes. Indeed, there is a well characterized plasmid segregation system that appears to operate in this manner using a plasmid-encoded MreB (actin) homologue (Møller-Jensen et al. 2002, 2003). There are also reports that mreB mutants are defective in chromosome segregation (Kruse et al. 2003; Defeu Soufo & Graumann 2004), and a possible membrane-associated effector protein, SetB, has been identified (Espeli et al. 2003). However, at least in B. subtilis, the segregation defect is likely to be an indirect consequence of mreB mutation on the cell shape and integrity (Formstone & Errington 2005), and the SetB protein does not seem to be conserved beyond close relatives of E. coli.
Possible motor proteins that might drive chromosome separation include the DNA polymerase holoenzyme (Lemon & Grossman 2001) and RNA polymerase (Dworkin & Losick 2002). In each of these cases, it is difficult to see how the directionality of segregation could be achieved and, moreover, because these macromolecular machines are essential for the majority of cellular processes, it is difficult to distinguish between direct and indirect effects. Also, hypothetical motor proteins might track along the MreB cytoskeleton. None of these putative effectors is mutually exclusive. A radically different kind of model supposes that a process called ‘transertion’ drives segregation. ‘Transertion’ refers to the process of coupled transcription, translation and secretion, pertaining to genes encoding membrane-associated proteins (Norris 1995). This coupling could impose restraints over the free diffusion of DNA, which could in principle drive the formation of separate DNA domains leading to spatial separation and then segregation of chromosomes (see Woldringh (2002) for a full discussion of this idea). Unfortunately, this kind of model is again relatively difficult to test experimentally. The theme that emerges from comparisons of various well characterized bacteria is that the mechanisms of chromosome segregation are likely to be diverse and specialized according to life style and cell architecture. Also, in many bacteria, overlapping and partly redundant mechanisms may contribute to efficient segregation.
4. Chromosome segregation during asymmetric cell division in Bacillus subtilis
In terms of molecular understanding of chromosome segregation, by far the best understood is that of sporulating cells of B. subtilis (Pogliano et al. 2003; Wu 2004). B. subtilis is a very well characterized Gram-positive bacterium. One of the key reasons for interest in this organism has been its ability to form spores. Sporulation is a developmental process involving the differentiation of two distinct cell types generated by a highly asymmetric cell division (Errington 2003; Hilbert & Piggot 2004; figure 2a,b). The positioning of the division septum close to one of the cell poles poses a special problem for the chromosome segregation systems of the cell because the chromosome destined to occupy the prespore cell needs to be moved to the extreme pole of the cell in order to be successfully segregated. A breakthrough in understanding this process emerged from the discovery that a gene called spoIIIE, identified by a classical screen for sporulation-defective phenotype, was found to have a specific defect in prespore chromosome segregation (Wu & Errington 1994; figure 3a,b). Furthermore, the phenotypic block in spoIIIE mutants was highly revealing about the mechanism of segregation. Most spoIIIE missense mutants have a terminal phenotype in which the polar division occurs but only a small portion of the prespore chromosome is successfully captured in the small polar compartment (Wu et al. 1995). Normally, the prespore and mother cell compartments both acquire a single completely replicated chromosome. Cytometric measurements of the DNA content of the mutant cells revealed that the chromosome destined for the prespore was partitioned between the two cells, such that about 30% of the DNA was correctly located in the prespore, whereas the remaining 70% of the chromosome was incorrectly located in the mother cell, which consequently had a total DNA content of about 1.7 chromosome equivalents of DNA, instead of the usual single chromosome (Wu & Errington 1994). Precise measurements on wild-type cells revealed that they transiently passed through a state very reminiscent of that of the SpoIIIE terminal phenotype during normal development (Wu et al. 1995). This surprising finding led to a model for prespore chromosome segregation in which the cell division septum forms in advance of the completion of segregation, thereby requiring most of the prespore chromosome to enter the prespore compartment by a DNA translocation process that is dependent on SpoIIIE protein (figure 3c–f). Support for this model came from localization studies, which showed that SpoIIIE is targeted to the leading edge of the septum (Wu & Errington 1997), and in vitro experiments demonstrating that SpoIIIE has the DNA tracking activity needed for it to play a direct role in transporting DNA (Bath et al. 2000).
Figure 2.

Asymmetric cell division during sporulation B. subtilis. Electron micrographs of thin sections through (a) a vegetative cell, exhibiting a partly formed, medial division septum, and (b) a sporulating cell with a complete sub-polar septum.
Figure 3.

Chromosome segregation during asymmetric cell division in sporulating cells of B. subtilis, and the role of the SpoIIIE protein. (a,b) Highly magnified fluorescence images (DAPI stain) of typical wild-type (a) and spoIIIE mutant (b) cells about 2 h after the onset of sporulation. The cartoons below show the outlines of the cells and the polar sporulation septa in each pair of sporulating cells: p, prespore; mc, mother cell. (c–f) Schematic summary of the key steps in chromosome segregation during sporulation.
Although at first sight SpoIIIE appears to be a very specialized protein, it is actually highly conserved throughout the eubacteria, including many diverse non-spore-forming bacteria. Proteins similar to SpoIIIE are associated with the transfer of DNA between cells during conjugative mating in Gram-positive bacteria (Wu et al. 1995; Possoz et al. 2001). Experiments with the E. coli homologue of SpoIIIE, FtsK, have confirmed its ability to translocate on DNA, and it has emerged that this protein family probably has several distinct activities associated with the final steps of cell division and chromosome segregation in bacteria (reviewed by Errington et al. 2001; Sherratt 2003).
5. Precise spatial arrangement of the BACILLUS subtilis chromosome
Discovery of the chromosome translocation function of SpoIIIE helped to resolve a puzzling feature of the spoIIIE mutant phenotype. The expression of certain sporulation-specific reporter genes is extremely position dependent (Sun et al. 1991). A transcription factor called σF plays a critical role in establishing the correct differentiated pattern of gene expression in the prespore compartment. Expression of several different σF dependent genes had been reported to be either blocked or unaffected by spoIIIE mutations, depending on the chromosomal location. In the light of the SpoIIIE DNA translocation function, a simple explanation for the chromosome position effect was that the segment of DNA trapped in the small compartment in the absence of spoIIIE function (about 30% of the chromosome) was highly specific (Wu & Errington 1994). Systematic analysis of this effect indeed showed that the segment of DNA was highly specific. Markers located within about 500 kbp of the unique site of bidirectional chromosome replication (oriC) were almost always trapped in the small compartment, whereas more oriC-distal sites were almost never trapped (Wu & Errington 1998). This provided strong evidence for a very precise orientation of the chromosome, at least at the onset of sporulation, but it is now clear that this applies to the chromosomes of most eubacteria (see above).
In vegetative cells of B. subtilis, nucleoids have a relatively diffuse ovoid or dumbbell appearance. Nucleoid-free spaces are discernible towards the cell poles and, just prior to division, at mid cell (figure 1b). Nucleoids of many other non-spore-forming bacteria have a similar appearance (e.g. E. coli; figure 1a). In contrast, soon after the onset of sporulation, the B. subtilis nucleoid becomes elongated (about 50% longer) and thinner, until it extends the full length of the cell, impinging into the normally nucleoid-free cell poles. This elongated nucleoid structure is called the axial filament and was first observed by electron microscopy (Ryter 1965), but it is most easily visualized by fluorescence microscopy of cells stained for DNA (Bylund et al. 1993; Hauser & Errington 1995; Pogliano et al. 2002; figure 1c,d). The formation of the axial filament represents the first key step in chromosome segregation in sporulating cells, and the studies with spoIIIE mutant cells suggested that the structure was formed by movement of oriC regions towards opposite poles of the sporulating cell. This pole-ward movement of oriC regions was confirmed when labelling methods for the oriC region were developed (Glaser et al. 1997; Webb et al. 1997; Graumann & Losick 2001), and the mechanism underlying this movement has been an important area for research on chromosome segregation in the last few years.
6. The soj–spo0J system
The chromosome position effect on gene expression generated by mutation of spoIIIE provided a powerful handle with which to analyse and dissect the cis- and trans-acting factors needed for chromosome arrangement. The first system found to affect chromosome arrangement was again identified by classical mutations affecting sporulation. The spo0J mutations block sporulation at an early stage of development, preventing the formation of the polar division septum. Ireton et al. (1994) showed that the spo0J block in sporulation could be suppressed by the deletion of an adjacent gene called soj (suppressor of spo0J). The predicted products of the two genes bore a striking resemblance to a family of gene pairs frequently found on low copy number plasmids in bacteria, and required for stable inheritance of the plasmids. Moreover, similar genes were found across a wide range of bacteria (though curiously not in E. coli and close relatives; Yamaichi & Niki 2000). The genes were usually located close to the oriC site, and Ireton et al. (1994) showed that mutation of spo0J (but not soj) led to a 100-fold defect in chromosome segregation in vegetative cells of B. subtilis. Sharpe & Errington (1996) then showed that deletion of soj and spo0J led to a partial relaxation of the chromosome orientation effect defined by reporter gene trapping in spoIIIE mutant cells. Thus, soj and/or spo0J are likely to contribute to the chromosome orientation effect. Unfortunately, probably because the Soj–Spo0J system is only one of the factors that contribute to accurate chromosome segregation in B. subtilis, its precise role in this process is not yet clear. It is even possible that its function is indirect because the system has been implicated in the regulation of chromosome replication (Lee et al. 2003; Ogura et al. 2003). Nevertheless, in experiments with plasmids, it has been clearly demonstrated that Spo0J and Soj can enhance segregational stability (Lin & Grossman 1998), and they can do this even when transplanted into E. coli, an organism that does not have homologues of these genes (Yamaichi & Niki 2000).
Given their interest as important segregation factors, the functions of Soj, Spo0J and related proteins from various bacteria and plasmids have been studied in considerable detail. Some features of the proteins appear to be widespread, but the situation is complicated by subtle differences in detailed function from system to system. For example, the B. subtilis proteins clearly have a role in the control of transcription at the onset of sporulation, for which there is no direct equivalent in the non-sporulating bacteria. Here we focus on current knowledge only of the B. subtilis system. Spo0J is a site-specific DNA-binding protein. It binds to at least eight sites located around the oriC region of the B. subtilis chromosome (Lin & Grossman 1998). Binding to the preferred sites is proposed to be followed by rapid lateral spreading that coats the oriC-proximal DNA (Lin & Grossman 1998; H. Ferreira et al., unpublished results). Examination of Spo0J protein localization by fusions to GFP or immunofluorescence revealed a striking pattern of discrete spots or foci. Analysis of the spot pattern during cell cycle progression and co-localization experiments showed that the foci were tightly associated with the oriC region of the chromosome. Furthermore, time-lapse experiments showed that the foci duplicated and separated in parallel with replication of the oriC region, providing some of the first evidence for abrupt and direct separation of oriC regions, probably indicating the existence of an active chromosome segregation system acting at or near oriC (Glaser et al. 1997; Lewis & Errington 1997; Lin et al. 1997; Lee et al. 2003).
Formation of compact Spo0J foci depends on functional Soj protein, though this does not seem to be critical for the segregation function of Spo0J. In the absence of Soj, multiple smaller, scattered Spo0J foci are evident, suggesting that organization or condensation of the oriC region is perturbed, and that Soj has an activity that can condense or organize the chromosome (Marston & Errington 1999a). Soj belongs to a family of small ATPases, many of which appear to have roles in cell cycle events or cell organization. A remarkable feature of Soj is its localization behaviour (Autret & Errington 2003; Marston & Errington 1999a; Quisel et al. 1999). Localization is highly asymmetric, and most of the protein in any given cell forms a dispersed ‘patch’ that covers part of the nucleoid mass. In cells with two nucleoids (e.g. late in the cell cycle), it is always associated with only one of the sisters. This cooperative localization is strongly suggestive of self-assembly into some kind of aggregate or mass of filaments. These patches are not static, and occasionally jump cooperatively from one nucleoid to the other. The jumping is very irregular but is influenced by at least two other proteins. In the absence of Spo0J, the dynamic behaviour is completely abolished, as is the asymmetry, and Soj associates statically and relatively uniformly with all of the DNA in the cell. In the absence of the cell division protein, MinD, jumping can still occur, but the movement is altered in a curious manner revealed only when cell length is perturbed. In elongated cells with multiple nucleoids, in which MinD is only localized at the extreme cell poles, Soj becomes trapped on one of the nucleoids located immediately adjacent to one of the poles, and accumulates only on that nucleoid in large quantities. In the absence of MinD, the tight association with the cell pole is lost and the patches can ‘wander’ onto non-polar nucleoids, though a single patch is usually still formed. The MinD protein is involved in regulation of the positioning of the cell division site, and is recruited to the cell poles by another pole associated protein, DivIVA, which will be discussed below (Edwards & Errington 1997; Marston et al. 1998). Although the molecular basis for Soj jumping behaviour and its regulation by the MinD/pole effect is not yet understood, the possibility that Soj associates with the cell pole, at least transiently, during jumping is probably important for its role in prespore chromosome segregation, as will be discussed below.
7. A role for DivIVA protein in prespore chromosome segregation
DivIVA is a small coiled-coil protein that was first identified through mutations that gave a strong cell division block, but also resulted in the formation of occasional minicells (tiny anucleate cells) by aberrant division close to an existing cell pole (Reeve et al. 1973; Cha & Stewart 1997; Edwards & Errington 1997). Detailed analysis of the divIVA gene revealed that its role in division is regulatory. The DivIVA protein is recruited to sites of cell division as a late step in the process. Unlike most other division proteins, DivIVA is retained at the cell poles after division (Edwards & Errington 1997; Marston et al. 1998). The basis of this targeting is not clear but it is extremely promiscuous and can occur in E. coli (phylogenetically a very distant bacterium from B. subtilis) and even in fission yeast (Edwards et al. 2000). DivIVA controls division by recruiting a bipartite division inhibitor, composed of MinC and MinD proteins, to the cell poles (Edwards & Errington 1997; Marston & Errington 1999b). The division inhibitor prevents division from occurring close to the cell poles, which would produce a minicell. In the absence of DivIVA, uncontrolled MinCD activity exerts a strong and dispersed block in cell division, giving rise to a filamentous phenotype. The division block of divIVA mutants can be suppressed by the mutation of the minC or minD genes, as expected. However, such double mutants have a strong sporulation defective phenotype, much stronger than minC or minD single mutants, suggesting that divIVA has an additional role in sporulation (Cha & Stewart 1997; Thomaides et al. 2001). Investigation of this additional divIVA effect led to the discovery that it was required for efficient prespore chromosome segregation. The divIVA mutants had a novel phenotype in which prespores failed to capture the normal 30% of oriC-proximal DNA and appeared almost devoid of DNA. Moreover, the oriC region of the chromosome, as defined by Spo0J foci, fails to move to its extreme polar position in divIVA mutants (Wu & Errington 2003).
8. An adapter protein RacA
Although it was possible that DivIVA might have a DNA-binding activity, it seemed more likely that one or more DNA-binding proteins associated with the oriC region of the chromosome might be recruited to DivIVA at the cell pole, in much the same way that MinCD is recruited to DivIVA in vegetative cells. The racA gene was found independently by two groups (Ben-Yehuda et al. 2003; Wu & Errington 2003). The mutant phenotype and predicted protein had a number of features consistent with an adapter protein. The racA (previously ywkC) gene lay within the segment of DNA trapped in the prespore compartment of the spoIIIE mutants (see above). Secondly, the expression of the gene was under the control of the σH transcription factor, and mutations in the sigH gene appeared to affect oriC migration during sporulation. Thirdly, the predicted protein contained a classical helix-turn-helix motif, characteristic of many sequence-specific DNA binding proteins in bacteria. Finally, mutations in racA gave rise to a weak sporulation phenotype in which prespore compartments deficient in DNA were produced.
Analysis of RacA function and localization showed that the N-terminal domain of the protein is indeed required for DNA binding, and that the protein localizes over the nucleoid but with prominent foci corresponding roughly to the oriC region. In the absence of DNA-binding activity, the protein co-localized with cell poles, and this localization was dependent on the presence of DivIVA protein. RacA therefore had the two key properties needed to bind the oriC region of the chromosome to the cell pole at the onset of sporulation. A surprising feature of the RacA phenotype, if it was indeed the putative adaptor protein, was that the effect of the null mutation on sporulation was much less prominent than that of divIVA mutants. Furthermore, the use of the spoIIIE/reporter gene trapping assay described above showed that in racA mutants, the orientation of the chromosome was almost normal, with markers close to oriC still being trapped in the prespore compartment much more frequently than oriC-distal markers. This suggested that at least one other protein was involved in the positioning of the chromosome close to the cell pole.
9. Synergistic action of the Soj and RacA proteins
The likely identity of a second protein involved in delivery of the oriC region to the cell pole was revealed by the effects of combining racA and soj mutations (Wu & Errington 2003). Although neither single mutant had a strong effect on sporulation frequency (both less than twofold) the double mutant had a 15-fold reduction in spore frequency, similar to that of the divIVA mutant. Furthermore, the double mutant also showed an almost complete loss of oriC-trapping activity in the spoIIIE/reporter trapping assay, indicating an almost complete loss of oriC region delivery to the cell pole, similar to the divIVA mutant. The simplest interpretation of this observation is that Soj and RacA have overlapping partially redundant roles in oriC region segregation, and that these two proteins cooperate in association of the oriC region with DivIVA at the cell pole.
An unexpected and still not understood feature of the phenotype of divIVA mutants is that the prespore compartments formed are frequently not completely anucleate, but often contain small segments of DNA (probably less than 10% of a chromosome equivalent). Moreover, the small segments of DNA are highly enriched for two small regions of chromosome located about 200–300 kbp either side of oriC (Wu & Errington 2003). One possible explanation is that in divIVA mutants, the chromosome folds in such a way that these regions come to lie closest to the cell poles and are thus most likely to be trapped in the small compartment. The central oriC region, as defined by Spo0J binding, seems to lie far from the cell poles in these mutants. It is therefore possible that the distribution of the chromosome around the actively positioned oriC/Spo0J domain leads to the regions flanking this core domain localizing closer to the cell poles. Whatever the explanation, a similar pattern of flanking region trapping was seen in cells with wild-type divIVA but bearing a double disruption of soj and racA (Wu & Errington 2003). This curious set of observations lends further support to the notion that Soj and RacA have overlapping, partly redundant functions in delivery of the oriC core region to DivIVA at the cell poles during sporulation.
10. A model for prespore chromosome segregation
Figure 4 summarizes our current understanding of the key players and their putative roles in the various steps of chromosome segregation during sporulation. In wild-type cells, during vegetative growth (figure 4a), the region flanking oriC (black circle) is condensed by the binding and lateral spreading of Spo0J protein (green circles) to various parS sites. The oriC–Spo0J complexes are positioned at subpolar sites by an as yet poorly understood, probably multifaceted, segregation system (represented by the black arrow). At the onset of sporulation (figure 4b), Soj protein drives the oriC–Spo0J complex pole-ward, perhaps involving its dynamic jumping behaviour and influenced by MinD protein (not shown), which is recruited to the cell poles by DivIVA protein (red bar). Meanwhile, the sporulation-specific RacA protein has been synthesized (blue circles), and it binds to multiple sites mainly clustered just to the left of oriC. The RacA protein also binds directly or indirectly to DivIVA, effectively tethering the oriC region to the cell pole. The effects of mutations in various components of this machinery are summarized in figure 4c–f. In the absence of DivIVA (figure 4c), the RacA protein complexes to DNA but it has no anchoring target at the cell pole. Soj protein also lacks its target at the cell pole (MinD, which requires DivIVA), so the pole-ward movement of the oriC–Spo0J complex is lacking and the complex remains positioned at its vegetative location. The ‘cloud’ of chromosomal DNA arranges itself around the oriC–Spo0J complex in such a way that the sequences flanking the region, to the left and right, are most likely to be trapped in the prespore compartment by the septum (dotted line). Double mutation of soj and racA (D) has a similar effect because neither of the factors capable of positioning the oriC–Spo0J region close to the cell pole are present. In a soj single mutant (figure 4e), the oriC–Spo0J region reaches the cell pole in most cells because random diffusion results in some RacA molecules reaching the cell pole, where they can be captured by DivIVA. Moreover, the binding would probably have an element of cooperativity because once one RacA molecule has bound, the tethering effect would increase the chances of further binding occurring. Similarly, in an racA mutant (figure 4f), although the tight binding to the cell pole has been lost, the putative pole-ward movement of the oriC–Spo0J complex, driven by Soj, would still facilitate capture of the region in the prespore compartment.
Figure 4.
Model for the mechanism underlying the first steps of chromosome segregation in sporulating cells of B. subtilis, and the effects of various mutations. See the text for a full description of the notation.
This model summarizes the overall functions of the most significant elements in prespore chromosome segregation that have been identified so far. In principle, it appears that these proteins are sufficient to explain (in outline) how the process works. The model can explain many aspects of the phenotypes associated with single and double mutants. However, it is clear that much remains to be discovered about the molecular details of the functions associated with each protein. It is worth noting that, with the exception of RacA, all of the proteins are associated with chromosome segregation or cell-cycle events in vegetative cells of B. subtilis, and are conserved outside of the spore forming bacteria. The SpoIIIE protein, similarly, is widely conserved throughout the bacteria. It seems likely that further work on chromosome segregation in sporulating cells will have broad significance for understanding of general cell cycle mechanisms in bacteria.
Acknowledgments
Work on chromosome segregation in the Errington lab was funded by grants from the Biotechnology and Biological Sciences Research Council. H.M. is supported by an EMBO Long-term Fellowship. We thank A. Hunt and H. Niki for providing illustrations.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Chromosome segregation’.
References
- Autret S, Errington J. A role for division-site-selection protein MinD in regulation of internucleoid jumping of Soj (ParA) protein in Bacillus subtilis. Mol. Microbiol. 2003;47:159–169. doi: 10.1046/j.1365-2958.2003.03264.x. [DOI] [PubMed] [Google Scholar]
- Bath J, Wu L.J, Errington J, Wang J.C. Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science. 2000;290:995–997. doi: 10.1126/science.290.5493.995. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda S, Rudner D.Z, Losick R. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science. 2003;299:532–536. doi: 10.1126/science.1079914. [DOI] [PubMed] [Google Scholar]
- Bylund J.E, Haines M.A, Piggot P.J, Higgins M.L. Axial filament formation in Bacillus subtilis: induction of nucleoids of increasing length after addition of chloramphenicol to exponential-phase cultures approaching stationary phase. J. Bacteriol. 1993;175:1886–1890. doi: 10.1128/jb.175.7.1886-1890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballido-López R, Errington J. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell. 2003;4:19–28. doi: 10.1016/s1534-5807(02)00403-3. [DOI] [PubMed] [Google Scholar]
- Cha J.-H, Stewart G.C. The divIVA minicell locus of Bacillus subtilis. J. Bacteriol. 1997;179:1671–1683. doi: 10.1128/jb.179.5.1671-1683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R.A, Errington J. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell. 2003;113:767–776. doi: 10.1016/s0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- Defeu Soufo H.J, Graumann P.L. Dynamic movement of actin-like proteins within bacterial cells. EMBO Rep. 2004;5:789–794. doi: 10.1038/sj.embor.7400209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc. Natl Acad. Sci. USA. 2002;99:14089–14094. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.H, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol. Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- Edwards D.H, Thomaides H.B, Errington J. Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J. 2000;19:2719–2727. doi: 10.1093/emboj/19.11.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Regulation of endospore formation in Bacillus subtilis. Nature Rev. Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- Errington J, Bath J, Wu L.J. Bacterial DNA transport. Nature Rev. Mol. Cell. Biol. 2001;2:538–545. doi: 10.1038/35080005. [DOI] [PubMed] [Google Scholar]
- Espeli O, Nurse P, Levine C, Lee C, Marians K.J. SetB: an integral membrane protein that affects chromosome segregation in Escherichia coli. Mol. Microbiol. 2003;50:495–509. doi: 10.1046/j.1365-2958.2003.03736.x. [DOI] [PubMed] [Google Scholar]
- Figge R.M, Divakaruni A.V, Gober J.W. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol. Microbiol. 2004;51:1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- Formstone A, Errington J. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol. Microbiol. 2005;15:1646–1657. doi: 10.1111/j.1365-2958.2005.04506.x. [DOI] [PubMed] [Google Scholar]
- Glaser P, Sharpe M.E, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behaviour of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- Graumann P.L, Losick R. Coupling of asymmetric division to polar placement of replication origin regions in Bacillus subtilis. J. Bacteriol. 2001;183:4052–4060. doi: 10.1128/JB.183.13.4052-4060.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P.M, Errington J. Characterization of cell cycle events during the onset of sporulation in Bacillus subtilis. J. Bacteriol. 1995;177:3923–3931. doi: 10.1128/jb.177.14.3923-3931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert D.W, Piggot P.J. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Gunther N.W.I, Grossman A.D. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Brenner S, Cuzin F. On the regulation of DNA replication in bacteria. Cold Spring Harb. Symp. Quant. Biol. 1963;28:329–348. [Google Scholar]
- Jensen R.B, Shapiro L. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc. Natl Acad. Sci. USA. 1999;96:10 661–10 666. doi: 10.1073/pnas.96.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.J.F, Carballido-López R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Kadoya R, Hassan A.K, Kasahara Y, Ogasawara N, Moriya S. Two separate DNA sequences within oriC participate in accurate chromosome segregation in Bacillus subtilis. Mol. Microbiol. 2002;45:73–87. doi: 10.1046/j.1365-2958.2002.03016.x. [DOI] [PubMed] [Google Scholar]
- Kruse T, Moller-Jensen J, Lobner-Olesen A, Gerdes K. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 2003;22:5283–5292. doi: 10.1093/emboj/cdg504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau I.F, Filipe S.R, Soballe B, Okstad O.A, Barre F.X, Sherratt D.J. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol. Microbiol. 2003;49:731–743. doi: 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- Lee P.S, Lin D.C.-H, Moriya S, Grossman A.D. Effects of the chromosome partitioning protein Spo0J (ParB) on oriC positioning and replication initiation in Bacillus subtilis. J. Bacteriol. 2003;185 doi: 10.1128/JB.185.4.1326-1337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon K.P, Grossman A.D. The extrusion–capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. doi: 10.1101/gad.913301. [DOI] [PubMed] [Google Scholar]
- Lewis P.J, Errington J. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the Spo0J partitioning protein. Mol. Microbiol. 1997;25:945–954. doi: 10.1111/j.1365-2958.1997.mmi530.x. [DOI] [PubMed] [Google Scholar]
- Lewis P.J, Thaker S.D, Errington J. Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J. 2000;19:710–718. doi: 10.1093/emboj/19.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sergueev K, Austin S. The segregation of the Escherichia coli origin and terminus of replication. Mol. Microbiol. 2002;46:985–996. doi: 10.1046/j.1365-2958.2002.03234.x. [DOI] [PubMed] [Google Scholar]
- Lin D.C.-H, Grossman A.D. Identification and characterization of a bacterial chromosome partitioning site. Cell. 1998;92:675–685. doi: 10.1016/s0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Lin D.C.-H, Levin P.A, Grossman A.D. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc. Natl Acad. Sci. USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A.L, Errington J. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol. Cell. 1999a;4:673–682. doi: 10.1016/s1097-2765(00)80378-0. [DOI] [PubMed] [Google Scholar]
- Marston A.L, Errington J. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol. Microbiol. 1999b;33:84–96. doi: 10.1046/j.1365-2958.1999.01450.x. [DOI] [PubMed] [Google Scholar]
- Marston A.L, Thomaides H.B, Edwards D.H, Sharpe M.E, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 1998;12:3419–3430. doi: 10.1101/gad.12.21.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H.L.T, Koch A.L, Doyle R.J, Streips U.N. Insertion and fate of the cell wall in Bacillus subtilis. J. Bacteriol. 1984;158:169–179. doi: 10.1128/jb.158.1.169-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller-Jensen J, Jensen R.B, Löwe J, Gerdes K. Prokaryotic DNA segregation by an actin-like filament. EMBO J. 2002;21:3119–3127. doi: 10.1093/emboj/cdf320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller-Jensen J, Borch J, Dam M, Jensen R.B, Roepstorff P, Gerdes K. Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Mol. Cell. 2003;12:1477–1487. doi: 10.1016/s1097-2765(03)00451-9. [DOI] [PubMed] [Google Scholar]
- Niki H, Hiraga S. Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev. 1998;12:1036–1045. doi: 10.1101/gad.12.7.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H, Yamaichi Y, Hiraga S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 2000;14:212–223. [PMC free article] [PubMed] [Google Scholar]
- Norris V. Hypothesis: transcriptional sensing and membrane-domain formation initiate chromosome replication in Escherichia coli. Mol. Microbiol. 1995;15:985–987. doi: 10.1111/j.1365-2958.1995.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Ogasawara N, Harry E.J, Moriya S. Increasing the ratio of Soj to Spo0J promotes replication initiation in Bacillus subtilis. J. Bacteriol. 2003;185:6316–6324. doi: 10.1128/JB.185.21.6316-6324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Sharp M.D, Pogliano K. Partitioning of chromosomal DNA during establishment of cellular asymmetry in Bacillus subtilis. J. Bacteriol. 2002;184:1743–1749. doi: 10.1128/JB.184.4.1743-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano K, Pogliano J, Becker E. Chromosome segregation in Eubacteria. Curr. Opin. Microbiol. 2003;6:586–593. doi: 10.1016/j.mib.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possoz C, Ribard C, Gagnat J, Pernodet J.L, Guerineau M. The integrative element pSAM2 from Streptomyces: kinetics and mode of conjugal transfer. Mol. Microbiol. 2001;42:159–166. doi: 10.1046/j.1365-2958.2001.02618.x. [DOI] [PubMed] [Google Scholar]
- Quisel J.D, Lin D.C.-H, Grossman A.D. Control of development by altered localization of a transcription factor in B. subtilis. Mol. Cell. 1999;4:665–672. doi: 10.1016/s1097-2765(00)80377-9. [DOI] [PubMed] [Google Scholar]
- Reeve J.N, Mendelson N.H, Coyne S.I, Hallock L.L, Cole R.M. Minicells of Bacillus subtilis. J. Bacteriol. 1973;114:860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos M, van Geel A.B, Aarsman M.E, Veuskens J.T, Woldringh C.L, Nanninga N. Cellular localization of oriC during the cell cycle of Escherichia coli as analyzed by fluorescent in situ hybridization. Biochimie. 1999;81:797–802. doi: 10.1016/s0300-9084(99)00218-7. [DOI] [PubMed] [Google Scholar]
- Roos M, van Geel A.B, Aarsman M.E, Veuskens J.T, Woldringh C.L, Nanninga N. The replicated ftsQAZ and minB chromosomal regions of Escherichia coli segregate on average in line with nucleoid movement. Mol. Microbiol. 2001;39:633–640. doi: 10.1046/j.1365-2958.2001.02263.x. [DOI] [PubMed] [Google Scholar]
- Ryter A. Etude morphologique de la sporulation de Bacillus subtilis. Ann. Inst. Pasteur. 1965;108:40–60. [PubMed] [Google Scholar]
- Sharpe M.E, Errington J. The Bacillus subtilis soj-spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol. Microbiol. 1996;21:501–509. doi: 10.1111/j.1365-2958.1996.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Sherratt D.J. Bacterial chromosome dynamics. Science. 2003;301:780–785. doi: 10.1126/science.1084780. [DOI] [PubMed] [Google Scholar]
- Shih Y.L, Le T, Rothfield L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc. Natl Acad. Sci. USA. 2003;100:7865–7870. doi: 10.1073/pnas.1232225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Fajardo-Cavazos P, Sussman M.D, Tovar-Rojo F, Cabrera-Martinez R.-M, Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by EσF: identification of features of good EσF-dependent promoters. J. Bacteriol. 1991;173:7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman A.A, Graumann P.L, Lin D.C.-H, Grossman A.D, Losick R. Chromosome arrangement within a bacterium. Curr. Biol. 1998;8:1102–1109. doi: 10.1016/s0960-9822(98)70464-6. [DOI] [PubMed] [Google Scholar]
- Thomaides H.B, Freeman M, El Karoui M, Errington J. Division-site-selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev. 2001;15:1662–1673. doi: 10.1101/gad.197501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollier P.H, Thanbichler M, McGrath P.T, West L, Meewan M, McAdams H.H, Shapiro L. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc. Natl Acad. Sci. USA. 2004;101:9257–9262. doi: 10.1073/pnas.0402606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C.D, Teleman A, Gordon S, Straight A, Belmont A, Lin D.C.-H, Grossman A.D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- Woldringh C.L. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol. Microbiol. 2002;45:17–29. doi: 10.1046/j.1365-2958.2002.02993.x. [DOI] [PubMed] [Google Scholar]
- Woldringh C.L, Huls P, Pas E, Brakenhoff G.J, Nanninga N. Topography of peptidoglycan synthesis during elongation and polar cap formation in a cell division mutant of Escherichia coli MC4100. J. Gen. Microbiol. 1987;133:575–586. [Google Scholar]
- Wu L.J. Structure and segregation of the bacterial nucleoid. Curr. Opin. Genet. Dev. 2004;14:126–132. doi: 10.1016/j.gde.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Errington J. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 1997;16:2161–2169. doi: 10.1093/emboj/16.8.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J, Errington J. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol. Microbiol. 1998;27:777–786. doi: 10.1046/j.1365-2958.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Errington J. RacA and the Soj–Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol. Microbiol. 2003;49:1463–1475. doi: 10.1046/j.1365-2958.2003.03643.x. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Lewis P.J, Allmansberger R, Hauser P.M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- Yamaichi Y, Niki H. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl Acad. Sci. USA. 2000;97:14 656–14 661. doi: 10.1073/pnas.97.26.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaichi Y, Niki H. migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J. 2004;23:221–233. doi: 10.1038/sj.emboj.7600028. [DOI] [PMC free article] [PubMed] [Google Scholar]



