Abstract
Bacteria ensure the fidelity of genetic inheritance by the coordinated control of chromosome segregation and cell division. Here, we review the molecules and mechanisms that govern the correct subcellular positioning and rapid separation of newly replicated chromosomes and plasmids towards the cell poles and, significantly, the emergence of mitotic-like machineries capable of segregating plasmid DNA. We further describe surprising similarities between proteins involved in DNA partitioning (ParA/ParB) and control of cell division (MinD/MinE), suggesting a mechanism for intracellular positioning common to the two processes. Finally, we discuss the role that the bacterial cytoskeleton plays in DNA partitioning and the missing link between prokaryotes and eukaryotes that is bacterial mechano-chemical motor proteins.
Keywords: Soj, Spo0J, ParAB, MinCDE, partitioning system, chromosome segregation
1. Introduction
In contrast to our understanding of eukaryotic mitosis, the principles that underlie the partitioning of DNA in prokaryotes are still largely unknown. Several models have been put forward. Forty years ago, Jacob et al. (1963) postulated the involvement of the elongating cellular envelope as a means of DNA segregation. However, recent experiments revealing that the origins of bacterial chromosomes rapidly separate, in a manner independent of cell elongation, have rendered this model invalid. The finding that DNA replication in Bacillus subtilis and Escherichia coli probably occurs at a stationary, centrally located replication factory has led to the proposal that bi-directional extrusion of newly replicated DNA from the replication factory followed by DNA condensation might constrain the motion of sister nucleoids to opposite sides of the division plane (Gordon & Wright 2000; Koppes et al. 1999; Lemon & Grossman 1998, 2000, 2001; Onogi et al. 2002; Sawitzke & Austin 2001). Transertion (coupled transcription, translation and insertion) of membrane proteins has been speculated to have a role in chromosome segregation (Norris 1995; Woldringh 2002) and more recently, Dworkin & Losick (2002) have proposed RNA polymerase as a novel candidate for driving the poleward motion of segregating bacterial chromosomes. The fifth, most radical model involves the existence of a prokaryotic mitotic-like machinery that actively transports plasmid and, possibly, chromosomal DNA into each daughter cell (Møller-Jensen et al. 2002).
2. DNA partitioning of the low copy number plasmids
The field of bacterial DNA partitioning has largely focused on studies of the low copy number plasmids P1, F factor and R1 of E. coli and the broad host range plasmid RP4. These model plasmids contain several different types of systems that prevent plasmid loss at cell division (Nordstrom & Austin 1989). One such type encodes a differentiation system that leads to the killing of plasmid-free cells and thereby confers plasmid stabilization (Gerdes et al. 1997; Jensen & Gerdes 1995). Conversely, true partitioning systems stabilize their replicons by actively distributing the plasmid molecules to the daughter cells, and this becomes essential for very low copy number plasmids.
3. E. coli plasmid P1 and F factor
In general, plasmid partitioning modules encode two trans-acting proteins and a cis-acting, centromere-like DNA sequence required for partitioning (table 1). The P1 par and F sop family of partitioning systems encode: (i) homologous ATPases, ParA and SopA, respectively, which are characterized by the presence of a conserved ‘deviant’ Walker A motif containing a signature lysine (Koonin 1993); and (ii) site-specific DNA-binding proteins containing helix-turn-helix (HTH) motifs, ParB and SopB, respectively. The centromere-like sites parS and sopC are located downstream of the genes encoding the partitioning proteins.
Table 1.
Gene and protein names. (MreB, bacterial actin homologue, forming intracellular helices; FtsZ, bacterial tubulin homologue, forming a ring at mid-cell during division; MinC, inhibitor of FtsZ polymerization, binds to MinD; MinD, ParA/NifH-like ATPase, binds to membrane and oscillates from pole to pole; MinE, MinD binding, enhances MinD ATP hydrolysis rate.)
| partitioning system | R1 par | P1 par | F1 sop | RP4 | chromosome | sporulation |
|---|---|---|---|---|---|---|
| cis-acting DNA | parC | parS | sopC | OB1-12 | parS | parS |
| DNA-binding protein | ParR | ParB | SopB | KorB | ParB | Spo0J |
| ATPase protein | ParM | ParA | SopA | IncC | ParA | Soj |
In the P1 system, ParB binds like a dimer to the parS site in conjunction with the host factor, IHF (Funnell 1988b) to form a higher‐order nucleoprotein complex called the partition complex (Davis & Austin 1988; Funnell 1988a, 1991). Additional ParB molecules can bind to the flanking sequences, leading to silencing of nearby genes (Bouet et al. 2000; Rodionov et al. 1999). Overexpression of both ParA and ParB interferes with partitioning. Overproduction of ParB was found to make all parS-carrying plasmids even more unstable than if they were randomly distributed at cell division (Lobocka & Yarmolinsky 1996). SopB of F plasmid has also been reported to disrupt partitioning when overexpressed (Mori et al. 1986) and, in a similar manner to P1 ParB, appears capable of silencing genes flanking the parS analogue sopC (Lynch & Wang 1995). ParB contains two multimerization domains including a primary dimerization domain located in the C-terminal 59, amino acids of the protein and a second self-association domain within the N-terminal half of ParB. These observations led to the proposal that the two domains play distinct roles in the formation of the partition complex (Surtees & Funnell 1999). It has long been assumed that the mechanism of partitioning would first involve pairing of the elements to be partitioned before they proceed to opposite poles of a dividing cell, a process not unlike the pairing of sister chromatids during the metaphase stage of eukaryotic mitosis. In vivo evidence that ParB can mediate intermolecular pairing of P1 plasmid centromeres (Edgar et al. 2001) has given weight to this hypothesis.
The postulated role of ParA is to supply energy for a subsequent step, the obvious one being plasmid movement (Davis et al. 1992). ParA has been shown to possess weak ATPase activity, which is stimulated in vitro by ParB and non-specific DNA (Davis et al. 1992). The determinant for ParB-mediated stimulation of ParA ATPase activity has been localized to the N-terminal 40 amino acids of ParB (Radnedge et al. 1998), whereas SopB has been shown to contain a species specific SopA interaction determinant within its N-terminal 45 amino acids (Ravin et al. 2003).
Autoregulation is common to the plasmid partitioning modules. Repression of P1 par transcription is exerted by the binding of ParA to a sequence that overlaps the parAB promoter (Davis et al. 1992). Binding of ParA to the operator site requires either ATP (Davis et al. 1992) or ADP (Davey & Funnell 1994), and repression is enhanced by ParB (Friedman & Austin 1988). However, studies of SopA of F plasmid have found that no nucleotide is required for its autoregulatory function (Mori et al. 1989), suggesting that the ATPase activity of SopA is directly involved in partitioning. Genetic data also show a requirement for ParA in partition, even when its regulatory role is bypassed (Davis et al. 1996; Friedman & Austin, 1988). ParA has been found to dimerize in the presence of ADP and ATP (Davey & Funnell 1994), suggesting that ParA binds DNA as a dimer. It has since been shown that ParA assembles onto the partition complex at parS and that this interaction is completely dependent on ATP, whereas the repressor activity of ParA prefers the ADP-bound form. These observations have led to the proposal that an ATP–ADP switch controls ParA entry into two separate pathways in which ParA plays separate roles (Bouet & Funnell 1999).
How is partitioning achieved in the cell? Time-lapse fluorescence microscopy studies have been used to investigate this question. P1 Plasmid copies have been visualized as a single focus at the cell centre or as two focuses at one-quarter and three-quarter positions in E. coli. After cell division, these quarter positions correspond to the new cell centres (Gordon et al. 1997). The observation that ParB forms focuses at the cell quarter positions in the presence of a P1 plasmid is consistent with a direct role in segregation (Erdmann et al. 1999). In the absence of ParA, focuses were formed that failed to localize to the quarter positions (Erdmann et al. 1999), and partition was defective. ParA and ParB have now been shown to actively mediate the segregation of P1 copies to the quarter positions in dividing cells. P1 plasmids expressing a dominant negative ParB, Δ30ParB form a plasmid focus at the cell centre, but never segregate to the cell quarter positions (Li & Austin 2002). In the absence of ParB, a plasmid focus is formed that does not localize to the cell centre, does not divide and is inherited by one daughter cell only. In the absence of ParA, focuses formed and frequently localized to the cell centre, but failed to segregate (Li et al. 2004). Thus, ParB appears to be required for plasmid localization to mid-cell, whereas ParA is required for focus division and ejection from the cell centre. Consistent with this, the ParA ATPase active site mutant, K122E, that abrogates nucleotide binding, blocks plasmid partitioning and the mutant M314I show a missegregation defect (Li et al. 2004) most probably attributable to a reduced affinity for nucleotide.
4. Structural insights from the broad-host-range plasmid RP4: IncC/KorB (ParA/ParB)
The self-transmissible plasmids of incompatibility group P (IncP) are maintained as stable, autonomously replicating elements in a wide variety of Gram-negative hosts (Sia et al. 1995). RP4 is a 60 kbp IncP-α plasmid that contains a partitioning module comprising the ParA-like ATPase IncC, a site-specific DNA-binding ParB-like protein, KorB, and DNA sequences to which KorB bind (Rosche et al. 2000; table 1). X-ray crystallographic studies of RP4 KorB have shed light on the dimerization of KorB and its mode of DNA binding. The C-terminal 62 amino acids of KorB (KorB-C) form a globular domain. KorB-C is a dimer with an extensive, predominantly hydrophobic subunit interface (Delbruck et al. 2002). Yeast two-hybrid studies have implicated the C terminus in dimerization of full length KorB (Lukaszewicz et al. 2002).
The crystal structure of the central, DNA-binding domain of KorB has been solved in complex with a 17 bp oligonucleotide containing the operator sequence (OB) (Khare et al. 2004). The structure shows two KorB-O (operator-binding domain of KorB) molecules symmetrically bound to two operator half-sites. KorB-O contains the predicted HTH motif commonly found in prokaryotic DNA-binding proteins. In the crystal structure, the operator DNA adopts a standard B-DNA conformation with a straight helix axis and two KorB-O molecules bound in successive major grooves of the DNA. Sequence recognition is not performed by the recognition helix of the HTH motif, but rather by two residues, Thr211 and Arg240, that form hydrogen bonds directly with bases in the operator site and when mutated to alanine, they abolish binding. There is only one protein–protein contact formed by the two KorB-O molecules in the structure between two glutamate residues (Khare et al. 2004). Whether this contact is important for dimerization in solution in the absence of DNA, remains to be determined, but KorB-O is monomeric in the absence of DNA.
The crystal structures of the two domains of KorB have led to a model in which KorB-C mediates dimerization of intact, full length KorB and owing to contacts between KorB-O subunits, the operator DNA is completely enclosed within the dimeric KorB. It is believed that KorB-C and KorB-O are connected by a flexible linker, which is required for the protein to undergo a conformational change upon binding that leads to engulfment of the DNA helix. The role of the N terminus remains to be elucidated (Khare et al. 2004).
5. The R1 par system: actin-like filaments involved in partitioning
Like the P1 par and F sop systems, the par locus of plasmid R1 encodes two genes, parM and parR, and a cis-acting site parC (table 1), which, in this case, is located upstream of parM. Plasmids containing par of R1 also move rapidly from mid-cell to positions near the cell poles in a symmetrical pattern (Jensen & Gerdes 1999). However, the components of the R1 par locus differ from the P1 and F systems. ParM of R1 belongs to a superfamily of ATPases that includes actin and its putative bacterial ancestor MreB, which has been confirmed by structural work (Bork et al. 1992; Jones et al. 2001; Møller-Jensen et al. 2002; van den Ent et al. 2001, 2002). Importantly, ParM forms actin-like, double helical filamentous structures in vivo extending along the longitudinal axis of the cell, and filament dynamics are required for active partitioning of the R1 plasmid. In vivo, filament formation and turnover is controlled by the ParR–parC complex and the intrinsic ParM ATPase activity. ATP binding is required for in vitro polymerization while hydrolysis is required for depolymerization (Møller-Jensen et al. 2002). ParR is a DNA-binding protein analogous, but not homologous, to ParB of P1 and SopB of F plasmids. ParR dimers bind cooperatively to two sets of five direct repeats (iterons) within the 160 bp parC site (Breuner et al. 1996; Jensen et al. 1994, 1998), thereby forming a complex that mediates specific pairing of plasmid molecules in vitro (Jensen et al. 1998). All 10 repeats in parC are required for optimal function in plasmid stabilization and autoregulation of the promoter by ParR (Breuner et al. 1996). ParR and ParM interact in vivo and in vitro (Jensen & Gerdes 1997). ParM filamentation is supported only by ParR–parC and not by ParR alone, even when overexpressed, lending support to the notion that ParR–parC acts as a nucleation point for the polymerization of ParM (Møller-Jensen et al. 2002, 2003).
The observations of ParR-mediated dimerization of R1 plasmids in vitro, dynamic filaments of ParM and the in vivo abrupt separation and segregation of plasmids from mid-cell to positions near to the cell poles, suggests a model for R1 partitioning that invokes the function of the ParM ATPase. The model assumes that plasmids are replicated at mid-cell by the replication factory. Following replication, the plasmids are paired rapidly by the ParR–parC complex (Jensen et al. 1998). ParR molecules in the paired complex then serve as nucleation points for ParM polymerization. Force is generated by inserting ParM subunits between the tip of the filament and ParR, and the plasmids are pushed towards the cell poles by a ‘polymerization motor’. Such a model would work even if the plasmids were not replicated at, or segregated from, mid-cell since the only requirement would be a minimal separation distance longer than half the dividing cell at cell division (Møller-Jensen et al. 2003).
Interestingly, database analyses have revealed that plasmids of both Gram-negative and Gram-positive bacteria encode actin-like proteins (putative ParM orthologues) more closely related to the chromosomal actin-like MreB family of proteins, than to each other. Thus, it is conceivable that plasmids recruited MreB at least twice during evolution to perform the task of DNA partitioning. This then raises the important question of whether the actin-like MreB protein plays a role in prokaryotic chromosome segregation.
6. Bacterial chromosome segregation
Over the past 40 years, various models have been proposed for the mechanism of chromosome segregation in bacteria, postulating the involvement of the elongating cell envelope, the DNA replication factory, transertion of membrane proteins, chromosome condensation and RNA polymerase. Crucial to recent progress has been the ability to determine precisely the subcellular localization of proteins and DNA within bacterial cells by fluorescence microscopy.
7. Orientation, positioning and movement of the bacterial chromosome
The first indications that the bacterial chromosome might have a distinct orientation within the cell came from studies on sporulation in B. subtilis. The formation of the prespore compartment is brought about by assembly of an asymmetric septum near one pole of the cell. At the completion of the asymmetric division that follows DNA replication, both the prespore compartment and the larger, adjacent mother cell each contain a complete chromosome. Differential gene expression pathways in each compartment direct the successful development of the spore. However, during invagination of the septum, only 30% of the prespore chromosome actually resides in the prespore compartment with the remaining 70% in the mother cell. Hence, the closing septum is set to bisect the prespore chromosome. How, then, is the remainder of the chromosome transported into the prespore compartment before septation is complete? The answer lies in the spoIIIE gene, mutations in which prevent the completion of DNA segregation into the prespore (Wu & Errington 1994). SpoIIIE is localized to the prespore septum and is proposed to translocate the chromosomal DNA into the prespore compartment as a DNA ‘pump’. Biochemical experiments have demonstrated that the conserved C-terminal domain of SpoIIIE possesses an ATPase activity that can be stimulated tenfold by the presence of double stranded DNA. Moreover, SpoIIIE affects the topology of plasmid molecules, consistent with tracking of the protein along the DNA (Bath et al. 2000). It has been determined that the DNA located in the prespore of a spoIIIE mutant always consists of the same region of chromosome, corresponding to a 500 kb region surrounding the origin of replication, oriC (Wu & Errington 1994; Wu et al. 1995). SpoIIIE homologues are widespread in non-sporulating bacteria, including the conserved cell division protein FtsK. FtsK probably functions like SpoIIIE to ensure the fidelity of chromosome segregation into the daughter cells prior to septation (Aussel et al. 2002), and has been shown in a recent single molecule experiment to act as a DNA translocase in vitro (Saleh et al. 2004).
Following the discovery that the prespore chromosome adopts a specific orientation in sporulating B. subtilis, specific orientation of the chromosome was discovered in E. coli and vegetative cells of B. subtilis (Gordon et al. 1997; Niki & Hiraga 1998; Sharpe & Errington 1998; Webb et al. 1997, 1998) by visualization of the origin and terminus regions using the LacI-GFP reporter system. High resolution data have demonstrated that in slow-growing E. coli, the origins move to one-quarter and three-quarter positions. Termini stay at mid-cell until very late in the cell cycle and are non-symmetrical with respect to the middle of the mother cell (Lau et al. 2003). The one-quarter and three-quarter positions represent predivisional sites in each of the nascent daughter cells. In fast‐growing E. coli cells, overlapping rounds of replication generate newborn cells in which replication has already been initiated. In these cells, the two copies of the origin are located at or near the one-quarter and three-quarter positions following DNA segregation. Upon completion of replication at mid-cell, the replication factories reposition themselves at the one-quarter and three-quarter positions where they reinitiate replication at the origins of the new chromosomes prior to the first round of septation. Multiple rounds of overlapping replication may proceed simultaneously with newly replicated origins moving to the one-eighth, three-eighth, five-eighth and seven-eighth positions shortly after replication has been initiated. Subsequent septation at all of these predivisional sites results in eight daughter cells. B. subtilis origin placement is similar to that observed in E. coli (Sharpe & Errington 1998; Webb et al. 1998).
The rapid, bi-directional migration of chromosomal origin regions is reminiscent of E. coli P1 and F plasmid partitioning described above (Gordon et al. 1997; Niki & Hiraga 1997) and points to the existence of a conserved, mitotic-like apparatus in bacteria. It appears that the origin-proximal region of the bacterial chromosome plays an analogous role to the eukaryotic centromere. However, although oriC itself is not sufficient for localization (Gordon et al. 2002), regions around oriC have been reported to facilitate correct orientation, as will be discussed below (Lin & Grossman 1998; Wu & Errington 2002; Yamaichi & Niki 2004).
8. Par proteins encoded by bacterial chromosomes—components of an active segregation mechanism?
Many bacterial chromosomes contain genes for homologues of the plasmid partitioning proteins ParA and ParB, immediately suggesting a role of these proteins in chromosome segregation. However, elucidation of that role has been complicated by the lack of a strong missegregation phenotype in deletion mutants. Chromosomal homologues of ParA and ParB (sometimes denoted as Soj and Spo0J, because of their involvement in sporulation) have been identified in a wide range of both Gram-negative and Gram-positive bacteria (Yamaichi & Niki 2000), covering most of the bacterial phylogenetic tree. The notable exceptions are the enteric bacteria, including E. coli and the gamma subdivision of proteobacteria (Yamaichi & Niki 2000). Although Soj/Spo0J were first discovered in B. subtilis, because of their role in chromosome segregation during sporulation, many chromosomal homologues of ParA and ParB are found in organisms that are not known to sporulate, suggesting a more fundamental role for these proteins.
Importantly, the chromosomal partitioning proteins can be engineered to function in the same way as plasmid par proteins to stabilize an otherwise unstable replicon and direct its correct positioning within the cell (Godfrin-Estevenon et al. 2002; Lin & Grossman 1998; Yamaichi & Niki 2000). Furthermore, null mutants of ParB are lethal in Caulobacter crescentus (Mohl et al. 2001), and mutations in spo0J in B. subtilis result in the formation of up to 3% anucleate cells (Ireton et al. 1994). Spo0J/ParB mutants of B. subtilis and Pseudomonas aeruginosa are viable but display perceptible defects in chromosome segregation in both vegetative and sporulating cells (Godfrin-Estevenon et al. 2002; Ireton et al. 1994; Lewis et al. 2002; Sharpe & Errington 1996). During vegetative growth, deletion of the parAB genes of Pseudomonas putida slightly increases the formation of anucleate cells, but during both the transition from exponential to stationary growth conditions and during overexpression of parAB, high levels of anucleate cells were formed (Godfrin-Estevenon et al. 2002). Deletion of parAB in Streptomyces coelicolor had no detectable effect in vegetatively growing cells, but also resulted in the production of significant numbers of anucleate spores (Kim et al. 2000).
The identification and characterization of a bacterial chromosome partitioning site has revealed 10 pseudo-palindromic 16 bp sequences found in the origin-proximal 20% of the B. subtilis chromosome (Lin & Grossman 1998). Research has shown that 8 of the 10 sequences are bound by Spo0J in vivo, and that the presence of one such site on an otherwise unstable plasmid stabilizes the plasmid in a Soj- and Spo0J-dependent manner, demonstrating that the site, parS, can function as a partitioning site (Lin & Grossman 1998). Moreover, it has since been shown that both the B. subtilis and P. aeruginosa partitioning machineries can function in E. coli (Godfrin-Estevenon et al. 2002; Yamaichi & Niki 2000).
The 16 bp parS sites identified in B. subtilis have since been identified in other bacterial species including P. aeruginosa and P. putida (Godfrin-Estevenon et al. 2002) and Thermus thermophilus (Nardmann & Messer 2000). More recently, a putative chromosomal partitioning site, migS, has been identified in E. coli (Yamaichi & Niki 2004). The migS site is located origin-proximally and is required for bipolar placement of oriC. When placed elsewhere on the chromosome or on a plasmid, migS mediates poleward localization of its new DNA context. The consensus sequence, 5′-tGTTTCACGTGAAACa-3′, shows a dyad symmetry typical of targets for dimeric DNA-binding proteins. The half-palindromes of these sequences (tGTTTCAC, GTGAAACa) are similar to ParB box A motifs of P1 plasmid centromeres (e.g. A1, ATTTCAC; A3, GTGAAAT; Bouet et al. 2000; Surtees & Funnell 2001).
Complexes of ParB proteins bound to nucleoids have been detected as discrete bipolar focuses that coincide with oriC in living cells (Glaser et al. 1997; Lin et al. 1997). Prespore-compartment-specific gene expression assays in B. subtilis show that spo0J mutants have defects in the orientation of the prespore chromosome (Sharpe & Errington 1996), suggesting that Spo0J is involved in the spatial organization of the origin proximal regions of the chromosome. However, Spo0J is not required for proper positioning or movement of the oriC region in B. subtilis during vegetative growth (Webb et al. 1998). This finding possibly reflects a level of redundancy in the system, which localizes the oriC region to the cell poles, at least during sporulation. Recent evidence has shown that the division site selection protein DivIVA has an additional role in positioning the origin region of the B. subtilis chromosome during sporulation (Thomaides et al. 2001). Together with the DNA-binding protein, RacA DivIVA plays a pivotal role in anchoring the chromosome at the cell pole during sporulation. RacA mutants often fail to trap prespore DNA prior to asymmetric septation, resulting in the production of anucleate cells. RacA interacts with DivIVA, localizing the origin region at the cell pole prior to spore formation (Ben-Yehuda et al. 2003). Furthermore, in the absence of Soj and RacA, a ΔdivIVA-like defect in prespore chromosome segregation is observed and deletion of RacA, Soj and Spo0J results in the elimination of the oriC-specificity of orientation of the prespore chromosome altogether (Wu & Errington 2002).
Deletion of soj (parA) alone does not result in a DNA segregation defect (Ireton et al. 1994). However, Soj is required for the stabilization of parS-containing plasmids (Yamaichi & Niki 2000). Soj and other ParA proteins are members of a large family of ATPases that include the bacterial cell division regulator MinD, nitrogenase iron protein involved in biological nitrogen fixation and the anion pump, ATPase ArsA (Koonin 1993). Soj has been shown to associate with the promoter regions to inhibit the transcription of several early sporulation genes in vivo, an effect that is antagonized by Spo0J (Ireton et al. 1994; Quisel & Grossman 2000; Quisel et al. 1999). It is also known to play a role in the formation of condensed Spo0J focuses on oriC/parS (Marston & Errington 1999).
Localization studies of Soj have shown that it oscillates within the nucleoid regions of the cell over a time period of approximately 20 min and to be statically nucleoid-associated in the absence of Spo0J (Marston & Errington 1999). Mutations in the nucleotide binding site of Soj disrupt localization and function, rendering it insensitive to regulation by Spo0J (Quisel & Grossman 2000; Quisel et al. 1999). These observations suggest that the non-oscillating form of Soj directs repression of early sporulation gene promoters and that the oscillating form does not. The switch between these two forms is probably linked to Soj ATPase activity, a hypothesis that is supported by MinE regulation of the Soj-related ATPase MinD. The MinD protein of E. coli regulates division site selection in conjunction with the division inhibitor, MinC, and the topological specificity factor, MinE, and exhibits an oscillatory pattern loosely related to that of Soj (Raskin & de Boer 1999). Whereas MinD oscillates all the way to the poles, Soj oscillation is restricted to the nucleoid. MinCD oscillation is dependent on MinE in a seemingly analogous fashion to Spo0J-dependent oscillation of Soj (Quisel et al. 1999). MinD undergoes ATP- and membrane-dependent self-association by virtue of a conserved C-terminal amphipathic helix which associates with the lipid bilayer (Lutkenhaus & Sundaramoorthy 2003). Members of the ParA family, including Soj, do not contain this helix and do not bind to the membrane, but instead associate dynamically with the nucleoid (Ebersbach & Gerdes 2001; Marston & Errington 1999; Quisel et al. 1999).
The par system has also been studied in C. crescentus. Like B. subtilis, the operon containing the parA and parB genes is near the origin of replication. ParB binds to several parS sequences adjacent to the par operon and the origin (Mohl & Gober 1997). Subcellular localization experiments have shown that a single focus of ParB is found at one pole of a cell containing a single chromosome (Mohl & Gober 1997; Mohl et al. 2001). Initiation of DNA replication results in the rapid formation of a second ParB focus at the opposite pole of the cell. This dynamic localization pattern coincides with the movement of the newly duplicated origin during the cell cycle (Jensen & Shapiro 1999). ParA localizes to the cell poles in C. crescentus but has not been assayed for polar oscillation in living cells (Mohl & Gober 1997). The case for the direct involvement of ParA and ParB in the movement of chromosomal DNA towards the cell poles in dividing cells is supported by the fact that both parA and parB are essential for viability in C. crescentus. Overexpression of either gene results in both cell division and chromosome partitioning defects. On the other hand, overexpression of both has only a mild effect on cell division but a relatively severe defect in partitioning, suggesting that the correct balance of the two proteins is essential for normal partitioning and division. In ParB depletion experiments, loss of the protein leads to cell filamentation and eventual death as a consequence of the inability to form FtsZ-rings; an identical phenotype is observed when ParA is overexpressed (Mohl et al. 2001).
It has been deduced that ParB of C. crescentus acts as a nucleotide exchange factor for ParA, stimulating the rapid exchange of ADP for ATP (Easter & Gober 2002). The N-terminal region of ParB of C. crescentus, like that of plasmid P1 ParB and F plasmid SopB, is the determinant for interaction with ParA (Figge et al. 2003).
The par proteins in both B. subtilis and C. crescentus are clearly required for efficient chromosome partitioning, but their exact roles in orienting the origin region, directing chromosome movement or regulating a cell cycle checkpoint coupling partitioning to cytokinesis remain unclear. The observation that increasing the expression of either protein in C. crescentus also causes a cell division defect could point to a role in coordinating cell division with chromosome movement. ParA and ParB may provide the cell with a mechanism to sense the arrival of the chromosomes at the cell poles, at which time division could proceed. This hypothesis is further strengthened by the homology between ParA/Soj and the cell division regulator MinD. The Min system discriminates between mid-cell and the cell poles through MinCD oscillation, which prevents septum formation at the poles but permits it at mid-cell. ParA/Soj could function to prevent septation in nucleoid occupied regions of the cell, or ‘sense’ the arrival of the chromosomes at the one-quarter positions of the cell or at the cell poles.
9. A common mechanism of ATP-dependent dimerization and ATPase activation shared by MinDE and parAB?
The ATPases of the two systems, Soj and MinD, are structurally homologous to the nitrogenase iron protein (Cordell & Löwe 2001; Hayashi et al. 2001; Leonard et al. 2004; Sakai et al. 2001), which, in conjunction with molybdenum iron protein, mediates electron transfer during biological nitrogen fixation (Schindelin et al. 1997). Nitrogenase, MinD, Soj and the anion pump ATPase ArsA are members of a family of ATPases characterized by the presence of a deviant Walker A motif containing a conserved ‘signature’ lysine at the start of the P-loop (Koonin 1993). Nitrogenase iron protein is a dimeric ATPase in which the constituent monomers are covalently linked by a 4Fe : 4S cluster. Each monomer binds a single adenine nucleotide. In the presence of , which mimics the transition state, the dimer switches from an ‘open’ to a more compact, ‘closed’ conformation, resulting in the rotation of the two subunits by approximately 13° towards the dimer interface. This transition state is then competent for interaction and complex formation with molybdenum iron protein (Schindelin et al. 1997). Until recently, all published structures of MinD-like proteins showed them to be monomeric (Cordell & Löwe 2001; Hayashi et al. 2001; Sakai et al. 2001), even in the presence of a non-hydrolysable ATP analogue, AMPPCP (Hayashi et al. 2001). Although the anion pump ArsA is also monomeric, it is twice the size of NifH and MinD and contains two similar domains connected by a short linker, such that each ArsA monomer is functionally a pseudodimer (Zhou et al. 2000). The crystal structure of T. thermophilus Soj D44A, which is deficient in nucleotide hydrolysis, shows conclusively for the first time that these MinD-like proteins do, indeed, form ATP-dependent nucleotide sandwich dimers (Leonard et al. 2004). The ‘signature’ lysine of each monomer is expected to stabilize the developing negative charge on the β-phosphate during the hydrolysis reaction.
The dynamic behaviours of MinD and Soj are regulated in vivo by the topological specificity factors MinE and Spo0J, respectively. MinE drives the pole-to-pole oscillation of the MinCD division inhibitor in many bacteria, restricting its activity to the vicinity of the cell poles (Fu et al. 2001; Hale et al. 2001; Hu & Lutkenhaus, 2001; Hu et al. 2002; Shih et al. 2003). The result is that the mid-cell is left free for formation of the division plane. In the Gram-positive bacteria, including B. subtilis, a different topological specificity factor, DivIVA, achieves a similar result by recruiting the inhibitor to the cell poles and retaining it there (Cha & Stewart 1997; Edwards & Errington 1997; Marston et al. 1998). In the presence of ATP, MinD binds to the division inhibitor MinC and goes to the membrane (Hu et al. 1999, 2003; Lackner et al. 2003). However, the ATPase is not stimulated and the complex is stable. MinD undergoes ATP-dependent dimerization in vitro and is expected to form a stable complex with a dimer of MinC (Hu & Lutkenhaus 2003). Subsequent interaction of this complex with MinE results in ATP hydrolysis and dissociation of both the complexes MinCD and MinD from the membrane. MinE mutants unable to stimulate MinD ATPase activity are unable to induce MinD oscillation and mutants with decreased ability to stimulate the ATPase have lower MinD oscillation frequencies (Hu & Lutkenhaus 2001), suggesting that the rate of MinE stimulation controls the oscillation period.
Likewise, oscillation of Soj within the nucleoid region, is abrogated both by mutations in the ATPase active site and by deletion of spo0J (Marston & Errington 1999; Quisel et al. 1999). It is not known how MinE stimulates MinD or how Spo0J stimulates Soj, but the interaction determinants of MinE and Spo0J have both been mapped to the N-terminal 22 and 20 amino acids, respectively (Leonard et al. 2004; Ma et al. 2003). Furthermore, Leonard et al. (2004) show that the activating regions of Spo0J and MinE contain significant sequence homology, including a conserved basic residue (see also Hu & Lutkenhaus 2001), mutation of which in Spo0J abrogates activation of Soj ATPase. Interestingly, the interaction determinants of both MinE and Spo0J also bear significant sequence homology to the conserved C terminus of FtsA, which presently has an unknown function, but may reasonably be expected to fulfil a similar role (Hu & Lutkenhaus 2001; Leonard et al. 2004). FtsA is part of the septum and interacts directly with FtsZ.
The emerging theme for members of the deviant Walker A class of ATPases is that binding of ATP drives the formation of an active dimer that has a high affinity for a dimeric binding partner. Thus, a NifH dimer binds the MoFe protein dimer and ArsA binds an ArsB dimer. Most likely, a MinD dimer binds to the dimeric MinC and we speculate that that a Soj dimer binds to dimeric Spo0J. The timing of ATP hydrolysis will determine the transient or persistent nature of such complexes since hydrolysis causes their dissociation. In the case of MinD, the MinCD complex exists for a relatively long period, as MinC does not stimulate the basal ATPase activity of MinD. Instead, MinCD dissociation is only brought about by the action of MinE, the rate of which controls the lifetime of the complex.
10. Speculation: Spo0J-assisted treadmilling of Soj
The similarities between MinDE and Soj/Spo0J are wide-ranging: (i) MinD and Soj oscillate (jump) between places in the cell; (ii) MinD oscillates by binding to the membrane, Soj, by binding to the nucleoid (DNA most probably); (iii) MinD and Soj have the same three-dimensional structure apart from the amphiphatic helix on MinD that is involved in membrane binding; (iv) MinD and Soj most probably from the same ATP-dependent dimer; (v) MinD and Soj ATPase activity is activated by a short, disordered peptide located on a dimeric binding partner (MinE N terminus, Spo0J N terminus, respectively); (vi) both MinD and Soj bind to extended surfaces (MinD: membrane, Soj: DNA) and binding is regulated by dimerization; (vii) both the MinDE and the Soj/Spo0J system are involved in accurate positioning (MinC and region of the nucleoid, respectively); (viii) copy numbers of MinD and Soj are high, copy numbers for MinE and Spo0J are low; and (ix) MinD and Soj oscillate by forming ‘helical tracks’ inside the cell.
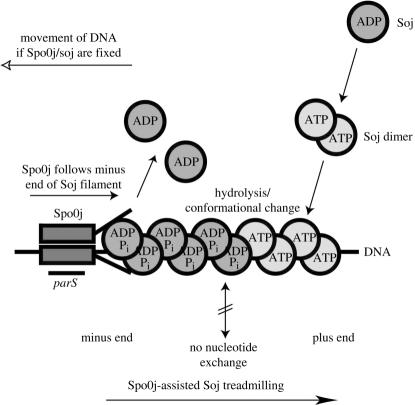
We believe that these parallels are strong and that it may be helpful to attempt to predict how Soj and Spo0J position regions of the nucleoid and help with DNA segregation. Central to Soj's function is its ability to polymerize in an ATP-dependent manner onto DNA (Leonard et al. 2004). This is analogous to MinD's ability to bind to the membrane once dimerized. By comparing the Soj dimer with nitrogenase iron protein, it is clear that a conformational change will occur upon nucleotide hydrolysis. The structure also shows that the nucleotide is occluded by the protein making nucleotide exchange improbable when dimeric. This leads to a model in which the polymer of Soj on the DNA has two different ends, a polymerizing plus end with an ATP cap and a minus end with the Soj subunit, which either depolymerize by themselves or bind to the DNA in an ADP-Pi state. The bulk ATPase activity of Soj is not enhanced by binding to the DNA, but by interaction with Spo0J, which is similar to MinD ATPase activation by MinE. Spo0J probably does not bind to the ATP dimer form of Soj but rather to the ADP-Pi form and functions as a nucleotide exchange factor for Soj. Therefore, Spo0J will bind preferentially to the minus end of the Soj filament and this will be enhanced by Spo0J's own DNA-binding activity. Spo0J might, thus, chase Soj off the DNA, just as MinE is thought to chase MinD off the membrane; we have termed this model ‘assisted treadmilling’ (figure 1). This model is somewhat related to the treadmilling of microtubules; however, the difference is that nucleotide hydrolysis is activated using an external factor (Spo0J). If polymerization and depolymerization are cooperative, then a pattern will form with waves of proteins oscillating and this can be modelled in silico (Howard et al. 2001; Hunding et al. 2003; Meinhardt & de Boer 2001). The time-averaged result is a concentration gradient that can be used to obtain positional information, as has been predicted for the MinCDE system. In the case of Soj/Spo0J, this is not as straightforward because oscillation does not extend along the whole length of the cell, but is restricted to the nucleoid, and positioning has to be with respect to the cell. What the system can do is mark the outer extremes of the nucleoid so that they can be recognized by other molecules to drag it towards the poles. We note that the marking could also be achieved by a stationary marker.
Figure 1.
Model of ‘assisted treadmilling’ of ParA-like proteins. Soj (ParA) binds to DNA in its ATP-dependent dimeric form. Because the nucleotide is almost completely occluded in the ‘sandwich dimer’ of Soj, there is probably very little nucleotide exchange in and out of the nucleoprotein filament—a pre-requisite for the two ends of the filament being different. Spo0J (ParB) enhances Soj ATP hydrolysis and, therefore, can chase Soj off the DNA. The result is treadmilling of Soj assisted by the N-terminal residues of Spo0J. The result is a relative movement of Soj/Spo0J against the DNA, and this movement could be used in various ways depending on what is more stationary in the cell. Note that a similar mechanism involving membrane-associated polymerization can be proposed for MinD (replacing Soj) and MinE (replacing Spo0J).
An alternative explanation involves using the assisted treadmilling for active DNA transport. The presence of parS sites near the chromosomal origin may impose directionality upon Soj polymerization, and detachment from the DNA may be initiated only near these sites. The model functions as follows: Soj polymerizes onto the DNA cooperatively. Spo0J has its highest affinity for the nucleoid at the 10 or so parS sites where it binds and starts depolymerizing Soj nucleoprotein filaments. Starting at parS means that depolymerization at the shrinking (−) end has a direction along the DNA away from the origins in both directions. Polymerization of Soj occurs at the other, growing (+) end of the filament. We speculate that if there is anything fixed in the cell binding to either Soj or Spo0J during this process of assisted treadmilling, then the DNA will be moved, just as with eukaryotic motor proteins. By extension, the many Min proteins found in some organisms may be used to move cargo, other than MinC, around in the cell in an analogous fashion.
None of these models explain the ‘tracks’ of MinD and Soj/ParA that can be seen by microscopy, and recent data indicates that there is no interaction with filamentous FtsZ or MreB (Shih et al. 2003; Thanedar & Margolin 2004). However, it seems theoretically possible that an interaction between Spo0J/Soj and MreB or FtsZ would explain the helical pattern of these proteins and could be included in the proposed model of assisted treadmilling of Soj/ParA/MinD.
11. Coordination of partitioning and division: the nucleoid occlusion model
Actively dividing cells must coordinate septation with accurate partitioning of the newly replicated nucleoids to ensure that each daughter cell receives an undamaged copy of the genetic material. Delays to DNA replication (e.g. for repair) or to chromosome segregation must, therefore, delay cell division until these processes are complete. In E. coli and vegetatively growing B. subtilis, the timing of division is regulated so that it follows the replication and segregation of sister chromosomes and division occurs at mid-cell in the DNA-free space between the partitioned nucleoids. The ‘nucleoid occlusion’ model, first proposed over a decade ago by Woldringh et al. (1991), in combination with the Min system could provide a dual mechanism for targeting the division machinery to mid-cell and simultaneously protecting the nucleoid from bisection by aberrantly forming septa. Wu & Errington (2004) have recently reported the identification of a novel nucleoid occlusion protein, Noc, which prevents the division machinery from assembling in the vicinity of the nucleoid. However, noc mutants do not exhibit any loss in specificity of division site selection in unperturbed cells, suggesting a level of redundancy in the systems controlling it (Wu & Errington 2004). It may be that the Min system can prevent division at significant distances from the cell pole or that a Noc-independent system biases division toward internucleoid spaces. Interestingly, Noc is 40% identical to the B. subtilis ParB homologue, Spo0J. Many bacteria do not contain a homologue of Noc whereas Spo0J is almost universally conserved (Yamaichi & Niki 2000), raising the question of how Noc has arisen. Additionally, many bacteria do not contain a complete Min system so it follows that bacteria may have evolved a range of mechanisms to control the correct timing and proper placement of their division septum.
12. A link between DNA segregation and the bacterial cytoskeleton?
In eukaryotic cells, the forces that drive chromosome segregation are well understood. Mitosis is a highly dynamic process involving a host of motor proteins which, together with cytoskeletal components, mediate the controlled movement of sister chromatids to opposite cell poles (Heald, 2000; Scholey et al. 2003). Microtubules of the spindle apparatus undergo alternating phases of polymerization and depolymerization, which, in combination with microtubule associated motor proteins that actively move microtubule filaments relative to one another in a ‘sliding filament’ mechanism, generates the force required for rapid poleward movement (Howard & Hyman 2003).
It was a long-held belief that bacteria did not possess a cytoskeleton and, therefore, that no such analogous means of segregating newly replicated chromosomes existed. However, the discovery of the bacterial cytoskeleton over the past decade has prompted a revision of our thinking regarding intracellular organization in bacteria. The bacterial tubulin homologue, FtsZ, forms rings at cell division sites (Bi & Lutkenhaus 1991; Löwe & Amos 1998; Mukherjee & Lutkenhaus 1994), whereas the actin homologues, MreB and Mbl, have established roles in cell shape determination (Jones et al. 2001; van den Ent et al. 2001).
The discovery of a plasmid partitioning determinant (ParM), also homologous to actin and capable of forming F-actin like filaments (van den Ent et al. 2002), has raised the question of whether MreB plays a role in chromosome segregation. MreB has recently been shown to be required for chromosome segregation in C. crescentus (Gitai et al. 2004), E. coli (Kruse et al. 2003) and B. subtilis (Soufo & Graumann 2003). In E. coli, depletion of MreB causes cells to segregate their chromosomes in pairs, consistent with their cohesion. Ectopic overexpression of wild-type MreB results in an impairment of cell division but does not affect chromosome segregation. Overexpression of active site mutants of MreB results in inhibition of cell division, abnormal MreB filament morphology and, importantly, induces severe defects in the localization of the origin and terminus regions of the chromosomes (Kruse et al. 2003). The striking observation that depletion or mutational inactivation of both actin homologues ParM and MreB result in a DNA segregation defect, raises the possibility that MreB filaments participate directly or indirectly in chromosome segregation. The discovery that MreB filaments in vivo are dynamic would satisfy a mechanism in which polymerization of MreB would actively move chromosomal DNA in opposite directions toward the cell poles (Carballido-Lopez & Errington 2003; Defeu Soufo & Graumann 2004). Alternatively, MreB filaments may serve as the tracks along which presently unidentified motor proteins move to drive chromosomal origin regions apart. Another possibility is that MreB filaments help to position the centrally located replication factory, such that, origin regions are extruded in opposite, poleward directions in a manner described by the ‘factory model’ of replication (Lemon & Grossman 1998). In B. subtilis, the bipolar localization of the origin regions during cell division is lost when MreB is depleted. Depletion of three other cell-shape determining proteins has no effect on chromosome segregation (Soufo & Graumann 2003). These data are consistent with any of the above mechanisms, although it remains a distinct possibility that the depletion of MreB or the overexpression of MreB mutants has an indirect effect on chromosome segregation owing to defects in cell shape that are not always detected given the resolution limits of microscopy. In addition to chromosomal origin regions, four independent cell polarity markers in Caulobacter, are mislocalized or become uniformly distributed when MreB is either depleted or overexpressed. This suggests that MreB plays a role in determining the polarity of the cell by delivering or localizing polar proteins to their correct positions within the cell (Gitai et al. 2004). Replenishment of MreB in depleted cells restores the polar localization of these proteins in a randomized fashion, suggesting that conservation of MreB filament polarity throughout generations is important. Deletion of the integral membrane protein SetB, which interacts with MreB, causes a delay in chromosome segregation, whereas its overproduction causes nucleoid stretching and disintegration (Espeli et al. 2003). This puzzling finding suggests that the chromosome might, indeed, be linked to the membrane as proposed initially, 40 years ago.
13. The search for bacterial motor proteins
Despite the discoveries of bacterial actin, tubulin and, most recently, an intermediate filament-forming protein in C. crescentus (Ausmees et al. 2003) that together make up the bacterial cytoskeleton, no motor proteins have been identified for any cytoskeletal element. Furthermore, no nucleation factors besides ParR of plasmid R1 (Møller-Jensen et al. 2003) have been identified and the only known bundling proteins are ZipA and ZapA, which both cross-link FtsZ filaments (Hale et al. 2000; Low et al. 2004). This is in stark contrast to the multitude of cytoskeleton-associated proteins that have been identified in eukaryotes, including the microtubule-associated directional motors of the dynein and kinesin families that mediate organelle movement along microtubules, as well as the actin motor myosin involved in force generation in skeletal muscle and a vast array of other functions. Do motor proteins related to myosin, kinesin or dynein not exist in bacteria? Are bacteria small enough that they do not require directed intracellular addressing or do they achieve it by mechanisms that do not require motor proteins, such as that proposed for the partitioning of the R1 plasmid in E. coli?
The discovery of the highly dynamic Min system initially suggested that the mystery of bacterial motors had been solved. The notion that MinD ATPase is a primitive motor protein is further supported by the observations that ParA and Soj (and its homologues involved in plasmid and chromosome partitioning) also display dynamic oscillatory behaviour in vivo (Ebersbach & Gerdes 2001; Marston & Errington 1999; Quisel et al. 1999). In addition, ParA is required for the bi-directional extrusion of replicated P1 plasmids from mid-cell towards the cell poles (Li et al. 2004). However, MinD and ParA share very little sequence or structural homology with myosin and kinesin that are distantly related in their motor domains. Furthermore, MinD and ParA have been reported not to colocalize with any cytoskeletal track (Shih et al. 2003; Thanedar & Margolin 2004). However, it is impossible to imagine the eukaryotic cell without the mechano-chemical motors myosin, kinesin and dynein, and despite the fact that no bacterial motors have been discovered to date, it is not unreasonable to expect that they exist in some form or another.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Chromosome segregation’.
References
- Ausmees N, Kuhn J.R, Jacobs-Wagner C. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell. 2003;115:705–713. doi: 10.1016/s0092-8674(03)00935-8. [DOI] [PubMed] [Google Scholar]
- Aussel L, Barre F.X, Aroyo M, Stasiak A, Stasiak A.Z, Sherratt D. FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell. 2002;108:195–205. doi: 10.1016/s0092-8674(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Bath J, Wu L.J, Errington J, Wang J.C. Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science. 2000;290:995–997. doi: 10.1126/science.290.5493.995. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda S, Rudner D.Z, Losick R. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science. 2003;299:532–536. doi: 10.1126/science.1079914. [DOI] [PubMed] [Google Scholar]
- Bi E.F, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc. Natl Acad. Sci. USA. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet J.Y, Funnell B.E. P1 ParA interacts with the P1 partition complex at parS and an ATP–ADP switch controls ParA activities. EMBO J. 1999;18:1415–1424. doi: 10.1093/emboj/18.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet J.Y, Surtees J.A, Funnell B.E. Stoichiometry of P1 plasmid partition complexes. J. Biol. Chem. 2000;275:8213–8219. doi: 10.1074/jbc.275.11.8213. [DOI] [PubMed] [Google Scholar]
- Breuner A, Jensen R.B, Dam M, Pedersen S, Gerdes K. The centromere-like parC locus of plasmid R1. Mol. Microbiol. 1996;20:581–592. doi: 10.1046/j.1365-2958.1996.5351063.x. [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R, Errington J. A dynamic bacterial cytoskeleton. Trends Cell Biol. 2003;13:577–583. doi: 10.1016/j.tcb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Cha J.H, Stewart G.C. The divIVA minicell locus of Bacillus subtilis. J. Bacteriol. 1997;179:1671–1683. doi: 10.1128/jb.179.5.1671-1683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell S.C, Löwe J. Crystal structure of the bacterial cell division regulator MinD. FEBS Lett. 2001;492:160–165. doi: 10.1016/s0014-5793(01)02216-5. [DOI] [PubMed] [Google Scholar]
- Davey M.J, Funnell B.E. The P1 plasmid partition protein ParA. A role for ATP in site-specific DNA binding. J. Biol. Chem. 1994;269:29 908–29 913. [PubMed] [Google Scholar]
- Davis M.A, Austin S.J. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988;7:1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.A, Martin K.A, Austin S.J. Biochemical activities of the parA partition protein of the P1 plasmid. Mol. Microbiol. 1992;6:1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Davis M.A, Radnedge L, Martin K.A, Hayes F, Youngren B, Austin S.J. The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol. Microbiol. 1996;21:1029–1036. doi: 10.1046/j.1365-2958.1996.721423.x. [DOI] [PubMed] [Google Scholar]
- Defeu Soufo H.J, Graumann P.L. Dynamic movement of actin-like proteins within bacterial cells. EMBO Rep. 2004;5:789–794. doi: 10.1038/sj.embor.7400209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbruck H, Ziegelin G, Lanka E, Heinemann U. An Src homology 3-like domain is responsible for dimerization of the repressor protein KorB encoded by the promiscuous IncP plasmid RP4. J. Biol. Chem. 2002;277:4191–4198. doi: 10.1074/jbc.M110103200. [DOI] [PubMed] [Google Scholar]
- Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc. Natl Acad. Sci. USA. 2002;99:14 089–14 094. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter J, Jr, Gober J.W. ParB-stimulated nucleotide exchange regulates a switch in functionally distinct ParA activities. Mol. Cell. 2002;10:427–434. doi: 10.1016/s1097-2765(02)00594-4. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Gerdes K. The double par locus of virulence factor pB171: DNA segregation is correlated with oscillation of ParA. Proc. Natl Acad. Sci. USA. 2001;98:15 078–15 083. doi: 10.1073/pnas.261569598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Chattoraj D.K, Yarmolinsky M. Pairing of P1 plasmid partition sites by ParB. Mol. Microbiol. 2001;42:1363–1370. doi: 10.1046/j.1365-2958.2001.02717.x. [DOI] [PubMed] [Google Scholar]
- Edwards D.H, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol. Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- Erdmann N, Petroff T, Funnell B.E. Intracellular localization of P1 ParB protein depends on ParA and parS. Proc. Natl Acad. Sci. USA. 1999;96:14 905–14 910. doi: 10.1073/pnas.96.26.14905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli O, Nurse P, Levine C, Lee C, Marians K.J. SetB: an integral membrane protein that affects chromosome segregation in Escherichia coli. Mol. Microbiol. 2003;50:495–509. doi: 10.1046/j.1365-2958.2003.03736.x. [DOI] [PubMed] [Google Scholar]
- Figge R.M, Easter J, Gober J.W. Productive interaction between the chromosome partitioning proteins, ParA and ParB, is required for the progression of the cell cycle in Caulobacter crescentus. Mol. Microbiol. 2003;47:1225–1237. doi: 10.1046/j.1365-2958.2003.03367.x. [DOI] [PubMed] [Google Scholar]
- Friedman S.A, Austin S.J. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid. 1988;19:103–112. doi: 10.1016/0147-619x(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Fu X, Shih Y.L, Zhang Y, Rothfield L.I. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc. Natl Acad. Sci. USA. 2001;98:980–985. doi: 10.1073/pnas.031549298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B.E. Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J. Bacteriol. 1988a;170:954–960. doi: 10.1128/jb.170.2.954-960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B.E. Participation of Escherichia coli integration host factor in the P1 plasmid partition system. Proc. Natl Acad. Sci. USA. 1988b;85:6657–6661. doi: 10.1073/pnas.85.18.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B.E. The P1 plasmid partition complex at parS. The influence of Escherichia coli integration host factor and of substrate topology. J. Biol. Chem. 1991;266:14 328–14 337. [PubMed] [Google Scholar]
- Gerdes K, Jacobsen J.S, Franch T. Plasmid stabilization by post-segregational killing. Genet. Eng. (NY) 1997;19:49–61. doi: 10.1007/978-1-4615-5925-2_3. [DOI] [PubMed] [Google Scholar]
- Gitai Z, Dye N, Shapiro L. An actin-like gene can determine cell polarity in bacteria. Proc. Natl Acad. Sci. USA. 2004;101:8643–8648. doi: 10.1073/pnas.0402638101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P, Sharpe M.E, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- Godfrin-Estevenon A.M, Pasta F, Lane D. The parAB gene products of Pseudomonas putida exhibit partition activity in both P. putida and Escherichia coli. Mol. Microbiol. 2002;43:39–49. doi: 10.1046/j.1365-2958.2002.02735.x. [DOI] [PubMed] [Google Scholar]
- Gordon G.S, Wright A. DNA segregation in bacteria. A. Rev. Microbiol. 2000;54:681–708. doi: 10.1146/annurev.micro.54.1.681. [DOI] [PubMed] [Google Scholar]
- Gordon G.S, Sitnikov D, Webb C.D, Teleman A, Straight A, Losick R, Murray A.W, Wright A. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- Gordon G.S, Shivers R.P, Wright A. Polar localization of the Escherichia coli oriC region is independent of the site of replication initiation. Mol. Microbiol. 2002;44:501–507. doi: 10.1046/j.1365-2958.2002.02901.x. [DOI] [PubMed] [Google Scholar]
- Hale C.A, Rhee A.C, de Boer P.A. ZipA-induced bundling of FtsZ polymers mediated by an interaction between C-terminal domains. J. Bacteriol. 2000;182:5153–5166. doi: 10.1128/jb.182.18.5153-5166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C.A, Meinhardt H, de Boer P.A. Dynamic localization cycle of the cell division regulator MinE in Escherichia coli. EMBO J. 2001;20:1563–1572. doi: 10.1093/emboj/20.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Oyama T, Morikawa K. Structural and functional studies of MinD ATPase: implications for the molecular recognition of the bacterial cell division apparatus. EMBO J. 2001;20:1819–1828. doi: 10.1093/emboj/20.8.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R. Motor function in the mitotic spindle. Cell. 2000;102:399–402. doi: 10.1016/s0092-8674(00)00044-1. [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman A.A. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422:753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- Howard M, Rutenberg A.D, de Vet S. Dynamic compartmentalization of bacteria: accurate division in E. coli. Phys. Rev. Lett. 2001;87:278 102. doi: 10.1103/PhysRevLett.87.278102. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Topological regulation of cell division in E. coli spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol. Cell. 2001;7:1337–1343. doi: 10.1016/s1097-2765(01)00273-8. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol. Microbiol. 2003;47:345–355. doi: 10.1046/j.1365-2958.2003.03321.x. [DOI] [PubMed] [Google Scholar]
- Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc. Natl Acad. Sci. USA. 1999;96:14 819–14 824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Gogol E.P, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc. Natl Acad. Sci. USA. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Saez C, Lutkenhaus J. Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J. Bacteriol. 2003;185:196–203. doi: 10.1128/JB.185.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunding A, Ebersbach G, Gerdes K. A mechanism for ParB-dependent waves of ParA, a protein related to DNA segregation during cell division in prokaryotes. J. Mol. Biol. 2003;329:35–43. doi: 10.1016/s0022-2836(03)00401-7. [DOI] [PubMed] [Google Scholar]
- Ireton K, Gunther N.W.t, Grossman A.D. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Brenner S, Cuzin F. On the regulation of DNA replication in bacteria. Cold Spring Harb. Symp. Quant. Biol. 1963;23:329–348. [Google Scholar]
- Jensen R.B, Gerdes K. Programmed cell death in bacteria: proteic plasmid stabilization systems. Mol. Microbiol. 1995;17:205–210. doi: 10.1111/j.1365-2958.1995.mmi_17020205.x. [DOI] [PubMed] [Google Scholar]
- Jensen R.B, Gerdes K. Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR–parC complex. J. Mol. Biol. 1997;269:505–513. doi: 10.1006/jmbi.1997.1061. [DOI] [PubMed] [Google Scholar]
- Jensen R.B, Gerdes K. Mechanism of DNA segregation in prokaryotes: ParM partitioning protein of plasmid R1 co-localizes with its replicon during the cell cycle. EMBO J. 1999;18:4076–4084. doi: 10.1093/emboj/18.14.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B, Shapiro L. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc. Natl Acad. Sci. USA. 1999;96:10 661–10 666. doi: 10.1073/pnas.96.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B, Dam M, Gerdes K. Partitioning of plasmid R1. The parA operon is autoregulated by ParR and its transcription is highly stimulated by a downstream activating element. J. Mol. Biol. 1994;236:1299–1309. doi: 10.1016/0022-2836(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Jensen R.B, Lurz R, Gerdes K. Mechanism of DNA segregation in prokaryotes: replicon pairing by parC of plasmid R1. Proc. Natl Acad. Sci. USA. 1998;95:8550–8555. doi: 10.1073/pnas.95.15.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.J, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Khare D, Ziegelin G, Lanka E, Heinemann U. Sequence-specific DNA binding determined by contacts outside the helix-turn-helix motif of the ParB homolog KorB. Nature. Struct. Mol. Biol. 2004;11:656–663. doi: 10.1038/nsmb773. [DOI] [PubMed] [Google Scholar]
- Kim H.J, Calcutt M.J, Schmidt F.J, Chater K.F. Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J. Bacteriol. 2000;182:1313–1320. doi: 10.1128/jb.182.5.1313-1320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E.V. A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J. Mol. Biol. 1993;229:1165–1174. doi: 10.1006/jmbi.1993.1115. [DOI] [PubMed] [Google Scholar]
- Koppes L.J, Woldringh C.L, Nanninga N. Escherichia coli contains a DNA replication compartment in the cell center. Biochimie. 1999;81:803–810. doi: 10.1016/s0300-9084(99)00217-5. [DOI] [PubMed] [Google Scholar]
- Kruse T, Møller-Jensen J, Lobner-Olesen A, Gerdes K. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 2003;22:5283–5292. doi: 10.1093/emboj/cdg504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner L.L, Raskin D.M, de Boer P.A. ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J. Bacteriol. 2003;185:735–749. doi: 10.1128/JB.185.3.735-749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau I.F, Filipe S.R, Soballe B, Okstad O.A, Barre F.X, Sherratt D.J. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol. Microbiol. 2003;49:731–743. doi: 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- Lemon K.P, Grossman A.D. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science. 1998;282:1516–1519. doi: 10.1126/science.282.5393.1516. [DOI] [PubMed] [Google Scholar]
- Lemon K.P, Grossman A.D. Movement of replicating DNA through a stationary replisome. Mol. Cell. 2000;6:1321–1330. doi: 10.1016/s1097-2765(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Lemon K.P, Grossman A.D. The extrusion-capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. doi: 10.1101/gad.913301. [DOI] [PubMed] [Google Scholar]
- Leonard T.A, Butler P.J.G, Löwe J. Bacterial chromosome segregation: structure and DNA binding of the Soj dimer—a conserved biological switch. EMBO J. Submitted doi: 10.1038/sj.emboj.7600530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R.A, Bignell C.R, Zeng W, Jones A.C, Thomas C.M. Chromosome loss from par mutants of Pseudomonas putida depends on growth medium and phase of growth. Microbiology. 2002;148:537–548. doi: 10.1099/00221287-148-2-537. [DOI] [PubMed] [Google Scholar]
- Li Y, Austin S. The P1 plasmid is segregated to daughter cells by a ‘capture and ejection’ mechanism coordinated with Escherichia coli cell division. Mol. Microbiol. 2002;46:63–74. doi: 10.1046/j.1365-2958.2002.03156.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Dabrazhynetskaya A, Youngren B, Austin S. The role of Par proteins in the active segregation of the P1 plasmid. Mol. Microbiol. 2004;53:93–102. doi: 10.1111/j.1365-2958.2004.04111.x. [DOI] [PubMed] [Google Scholar]
- Lin D.C, Grossman A.D. Identification and characterization of a bacterial chromosome partitioning site. Cell. 1998;92:675–685. doi: 10.1016/s0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Lin D.C, Levin P.A, Grossman A.D. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc. Natl Acad. Sci. USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobocka M, Yarmolinsky M. P1 plasmid partition: a mutational analysis of ParB. J. Mol. Biol. 1996;259:366–382. doi: 10.1006/jmbi.1996.0326. [DOI] [PubMed] [Google Scholar]
- Low H.H, Moncrieffe M.C, Löwe J. The crystal structure of ZapA and its modulation of FtsZ polymerisation. J. Mol. Biol. 2004;341:839–852. doi: 10.1016/j.jmb.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Löwe J, Amos L.A. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz M, Kostelidou K, Bartosik A.A, Cooke G.D, Thomas C.M, Jagura-Burdzy G. Functional dissection of the ParB homologue (KorB) from IncP-1 plasmid RK2. Nucleic Acids Res. 2002;30:1046–1055. doi: 10.1093/nar/30.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J, Sundaramoorthy M. MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation. Mol. Microbiol. 2003;48:295–303. doi: 10.1046/j.1365-2958.2003.03427.x. [DOI] [PubMed] [Google Scholar]
- Lynch A.S, Wang J.C. SopB protein-mediated silencing of genes linked to the sopC locus of Escherichia coli F plasmid. Proc. Natl Acad. Sci. USA. 1995;92:1896–1900. doi: 10.1073/pnas.92.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.Y, King G, Rothfield L. Mapping the MinE site involved in interaction with the MinD division site selection protein of Escherichia coli. J. Bacteriol. 2003;185:4948–4955. doi: 10.1128/JB.185.16.4948-4955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A.L, Errington J. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol. Cell. 1999;4:673–682. doi: 10.1016/s1097-2765(00)80378-0. [DOI] [PubMed] [Google Scholar]
- Marston A.L, Thomaides H.B, Edwards D.H, Sharpe M.E, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 1998;12:3419–3430. doi: 10.1101/gad.12.21.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H, de Boer P.A. Pattern formation in Escherichia coli: a model for the pole-to-pole oscillations of Min proteins and the localization of the division site. Proc. Natl Acad. Sci. USA. 2001;98:14 202–14 207. doi: 10.1073/pnas.251216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohl D.A, Gober J.W. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88:675–684. doi: 10.1016/s0092-8674(00)81910-8. [DOI] [PubMed] [Google Scholar]
- Mohl D.A, Easter J, Jr, Gober J.W. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol. Microbiol. 2001;42:741–755. doi: 10.1046/j.1365-2958.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- Møller-Jensen J, Jensen R.B, Löwe J, Gerdes K. Prokaryotic DNA segregation by an actin-like filament. EMBO J. 2002;21:3119–3127. doi: 10.1093/emboj/cdf320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller-Jensen J, Borch J, Dam M, Jensen R.B, Roepstorff P, Gerdes K. Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Mol. Cell. 2003;12:1477–1487. doi: 10.1016/s1097-2765(03)00451-9. [DOI] [PubMed] [Google Scholar]
- Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 1986;192:1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Mori H, Mori Y, Ichinose C, Niki H, Ogura T, Kato A, Hiraga S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J. Biol. Chem. 1989;264:15 535–15 541. [PubMed] [Google Scholar]
- Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J, Messer W. Identification and characterization of the dnaA upstream region of Thermus thermophilus. Gene. 2000;261:299–303. doi: 10.1016/s0378-1119(00)00507-2. [DOI] [PubMed] [Google Scholar]
- Niki H, Hiraga S. Subcellular distribution of actively partitioning F plasmid during the cell division cycle in E. coli. Cell. 1997;90:951–957. doi: 10.1016/s0092-8674(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Niki H, Hiraga S. Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev. 1998;12:1036–1045. doi: 10.1101/gad.12.7.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom K, Austin S.J. Mechanisms that contribute to the stable segregation of plasmids. Annu. Rev. Genet. 1989;23:37–69. doi: 10.1146/annurev.ge.23.120189.000345. [DOI] [PubMed] [Google Scholar]
- Norris V. Hypothesis: chromosome separation in Escherichia coli involves autocatalytic gene expression, transertion and membrane-domain formation. Mol. Microbiol. 1995;16:1051–1057. doi: 10.1111/j.1365-2958.1995.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Onogi T, Ohsumi K, Katayama T, Hiraga S. Replication-dependent recruitment of the beta-subunit of DNA polymerase III from cytosolic spaces to replication forks in Escherichia coli. J. Bacteriol. 2002;184:867–870. doi: 10.1128/JB.184.3.867-870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisel J.D, Grossman A.D. Control of sporulation gene expression in Bacillus subtilis by the chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) J. Bacteriol. 2000;182:3446–3451. doi: 10.1128/jb.182.12.3446-3451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisel J.D, Lin D.C, Grossman A.D. Control of development by altered localization of a transcription factor in B. subtilis. Mol. Cell. 1999;4:665–672. doi: 10.1016/s1097-2765(00)80377-9. [DOI] [PubMed] [Google Scholar]
- Radnedge L, Youngren B, Davis M, Austin S. Probing the structure of complex macromolecular >interactions by homolog specificity scanning: the P1 and P7 plasmid partition systems. EMBO J. 1998;17:6076–6085. doi: 10.1093/emboj/17.20.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin D.M, de Boer P.A. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc. Natl Acad. Sci. USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin N.V, Rech J, Lane D. Mapping of functional domains in F plasmid partition proteins reveals a bipartite SopB-recognition domain in SopA. J. Mol. Biol. 2003;329:875–889. doi: 10.1016/s0022-2836(03)00525-4. [DOI] [PubMed] [Google Scholar]
- Rodionov O, Lobocka M, Yarmolinsky M. Silencing of genes flanking the P1 plasmid centromere. Science. 1999;283:546–549. doi: 10.1126/science.283.5401.546. [DOI] [PubMed] [Google Scholar]
- Rosche T.M, Siddique A, Larsen M.H, Figurski D.H. Incompatibility protein IncC and global regulator KorB interact in active partition of promiscuous plasmid RK2. J. Bacteriol. 2000;182:6014–6026. doi: 10.1128/jb.182.21.6014-6026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Yao M, Itou H, Watanabe N, Yumoto F, Tanokura M, Tanaka I. The three-dimensional structure of septum site-determining protein MinD from Pyrococcus horikoshii OT3 in complex with Mg-ADP. Structure (Camb.) 2001;9:817–826. doi: 10.1016/s0969-2126(01)00638-4. [DOI] [PubMed] [Google Scholar]
- Saleh O.A, Perals C, Barre F.X, Allemand J.F. Fast, DNA-sequence independent translocation by FtsK in a single-molecule experiment. EMBO J. 2004;23:2430–2439. doi: 10.1038/sj.emboj.7600242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzke J, Austin S. An analysis of the factory model for chromosome replication and segregation in bacteria. Mol. Microbiol. 2001;40:786–794. doi: 10.1046/j.1365-2958.2001.02350.x. [DOI] [PubMed] [Google Scholar]
- Schindelin H, Kisker C, Schlessman J.L, Howard J.B, Rees D.C. Structure of ADP×AIF4(−)-stabilized nitrogenase complex and its implications for signal transduction. Nature. 1997;387:370–376. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
- Scholey J.M, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- Sharpe M.E, Errington J. The Bacillus subtilis soj–spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol. Microbiol. 1996;21:501–509. doi: 10.1111/j.1365-2958.1996.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Sharpe M.E, Errington J. A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol. Microbiol. 1998;28:981–990. doi: 10.1046/j.1365-2958.1998.00857.x. [DOI] [PubMed] [Google Scholar]
- Shih Y.L, Le T, Rothfield L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc. Natl Acad. Sci. USA. 2003;100:7865–7870. doi: 10.1073/pnas.1232225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia E.A, Roberts R.C, Easter C, Helinski D.R, Figurski D.H. Different relative importances of the par operons and the effect of conjugal transfer on the maintenance of intact promiscuous plasmid RK2. J. Bacteriol. 1995;177:2789–2797. doi: 10.1128/jb.177.10.2789-2797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufo H.J, Graumann P.L. Actin-like proteins MreB and Mbl from Bacillus subtilis are required for bipolar positioning of replication origins. Curr. Biol. 2003;13:1916–1920. doi: 10.1016/j.cub.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Surtees J.A, Funnell B.E. P1 ParB domain structure includes two independent multimerization domains. J. Bacteriol. 1999;181:5898–5908. doi: 10.1128/jb.181.19.5898-5908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees J.A, Funnell B.E. The DNA binding domains of P1 ParB and the architecture of the P1 plasmid partition complex. J. Biol. Chem. 2001;276:12 385–12 394. doi: 10.1074/jbc.M009370200. [DOI] [PubMed] [Google Scholar]
- Thanedar S, Margolin W. FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr. Biol. 2004;14:1167–1173. doi: 10.1016/j.cub.2004.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaides H.B, Freeman M, El Karoui M, Errington J. Division site selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev. 2001;15:1662–1673. doi: 10.1101/gad.197501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Amos L.A, Löwe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- van den Ent F, Møller-Jensen J, Amos L.A, Gerdes K, Löwe J. F-actin-like filaments formed by plasmid segregation protein ParM. EMBO J. 2002;21:6935–6943. doi: 10.1093/emboj/cdf672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C.D, Teleman A, Gordon S, Straight A, Belmont A, Lin D.C, Grossman A.D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- Webb C.D, Graumann P.L, Kahana J.A, Teleman A.A, Silver P.A, Losick R. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol. 1998;28:883–892. doi: 10.1046/j.1365-2958.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- Woldringh C.L. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol. Microbiol. 2002;45:17–29. doi: 10.1046/j.1365-2958.2002.02993.x. [DOI] [PubMed] [Google Scholar]
- Woldringh C.L, Mulder E, Huls P.G, Vischer N. Toporegulation of bacterial division according to the nucleoid occlusion model. Res. Microbiol. 1991;142:309–320. doi: 10.1016/0923-2508(91)90046-d. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Errington J. Bacillus subtilis spoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Errington J. A large dispersed chromosomal region required for chromosome segregation in sporulating cells of Bacillus subtilis. EMBO J. 2002;21:4001–4011. doi: 10.1093/emboj/cdf393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J, Errington J. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell. 2004;117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Wu L.J, Lewis P.J, Allmansberger R, Hauser P.M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- Yamaichi Y, Niki H. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl Acad. Sci. USA. 2000;97:14 656–14 661. doi: 10.1073/pnas.97.26.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaichi Y, Niki H. migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J. 2004;23:221–233. doi: 10.1038/sj.emboj.7600028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Radaev S, Rosen B.P, Gatti D.L. Structure of the ArsA ATPase: the catalytic subunit of a heavy metal resistance pump. EMBO J. 2000;19:4838–4845. doi: 10.1093/emboj/19.17.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]