Abstract
Susceptibility genes for complex diseases are characterized by reduced penetrance, caused by the influence of other genes, the environment or stochastic events. Recently, positional cloning efforts have yielded several candidate susceptibility genes in different complex disorders such as Crohn's disease and asthma. Within a genetic locus, however, the identification of the effector gene may pose further challenges and require functional studies. I review two examples of such challenges: the cloning of GPR154 (GPRA) and AAA1 on chromosome 7p14 at a susceptibility locus for atopy and asthma, and the study of HLA-Cw6, CCHCR1 (HCR) and CDSN on chromosome 6p21 at PSORS1, the major susceptibility locus for psoriasis. The susceptibility locus for atopy and asthma contains two genes and only one of them is protein coding. We studied its isoform-specific expression in bronchial biopsies and in a mouse model of ovalbumin-induced inflammation of bronchial epithelia. In the PSORS1 locus, strong linkage disequilibrium between genes has made it difficult to distinguish the effects of the three nearby genes. We engineered transgenic mice with either a HCR non-risk allele or the HCR*WWCC risk allele controlled by the cytokeratin-14 promoter. The results suggested that the overexpression of HCR in mouse skin was insufficient to induce a psoriasiform phenotype, but it appeared to induce allele-specific gene expression changes that were similar to those observed in psoriatic skin.
Keywords: complex disease, multifactorial inheritance, genetic susceptibility, genome scan, positional cloning, immune system
1. Introduction
Positional cloning of susceptibility genes in complex diseases has turned out to be much more challenging than appreciated a decade ago. Early failures to detect consistent linkage signals from genome scans and apparently contradictory results between studies have led to general disappointment. Examples are not difficult to find and asthma research to detect susceptibility genes is a good demonstration. Immunologists have suggested the existence of functionally important regulatory genes but genetic linkage and association studies on these candidate genes have often failed to support population-level differences between alleles. One such gene is Interleukin-4 (IL4), a key regulator of T-cell maturation. Repeated attempts to find atopy or asthma-associated genetic polymorphisms within it have led to the conclusion that it may have, at most, a small role in modifying genetic susceptibility between individuals (Lonjou et al. 2000). On the other hand, positional cloning may have implicated genes that are poorly characterized. Thus, these findings have suggested the occurrence of new pathways in disease processes with little previous information; evaluating and confirming such results has been a lengthy process. Again, the recently positionally cloned asthma susceptibility genes provide an example (Kere & Laitinen 2004). It is becoming increasingly clear that the identification of new susceptibility genes for complex diseases marks the beginning, rather than the end, of a vast amount of work. The biological processes are not especially easy to address experimentally because the genetic alterations associated with reduced penetrance and late-onset effect in life are often non-coding, affecting the gene function through more subtle mechanisms such as alternative splicing, altered transcript stability and transcription or suppressor factor binding (Pagani et al. 2003). Different haplotypes carry regulatory elements that affect transcript balance and, thus, individuals with different haplotype combinations will produce different levels of transcripts, which may contribute to subtle individual phenotypic or functional differences (Pastinen & Hudson 2004; Pastinen et al. 2004). This list is by no means exhaustive and new disease-associated effector mechanisms are likely to emerge for newly identified susceptibility genes. At the same time, the future of candidate gene studies may seem less bright than before because a large fraction of genes seem to be regulated in trans rather than in cis; therefore, it is highly likely that a good candidate gene may mechanistically have its genetic regulation elsewhere in the genome through a trans-acting factor (Cheung et al. 2003).
In this brief review, I shall present two case studies on common complex diseases that both involve immune mechanisms in their pathogenesis, namely, asthma and psoriasis. These examples highlight different problems in the research on susceptibility genes. For asthma, the main problem has turned out to be the relatively weak genetic effects that each susceptibility locus confers and, thus, genetic linkage studies, subsequent positional cloning efforts and even replication studies have been laborious. For psoriasis, a locus with a strong and indisputable genetic effect on susceptibility has been known for two decades but identifying the actual risk gene within the locus continues to be the subject of ongoing research.
2. Genetic linkage studies in asthma
Asthma has a modest but definite genetic component. Based on a number of twin and family studies, the risk of first-degree relatives of asthma patients developing asthma appears to be approximately fivefold of that of the average population (Laitinen et al. 1998). If the risk increase was attributable to just one or two susceptibility genes, it should be relatively easy to map them using genome-wide scans with some hundreds of families. Such considerations were probably in the minds of researchers who embarked on genome-wide scans to map asthma susceptibility genes in the early 1990s (table 1). Results from these studies, however, led to widespread disappointment (Altmuller et al. 2001). Nevertheless, some groups claimed successful studies with genome-wide significant results implicating susceptibility loci. As more genome-wide scans continued to emerge, some loci were repeatedly observed, suggesting that the loci are real (Illig & Wjst 2002). The perceived need for replication stimulated several consortium studies that were designed to evaluate individual loci (Lonjou et al. 1999, 2000).
Table 1.
Examples of genome-wide linkage studies in asthma.
| study | no. of subjects, populations | implicated chromosomes | phenotypes and remarks |
|---|---|---|---|
| Daniels et al. (1996) | 364, Western Australia; replication UK | 4, 6, 7, 11, 13, 16 | asthma, five related and subphenotypes |
| CSGA (1997) | 540, African–American, Caucasian, Hispanic | none for all populations. 2, 5, 11, 12, 13, 17, 19, 21 for one population each | asthma, bronchial hyperreactivity, atopy |
| Ober et al. (1998) | 653, Hutterites (USA) | 5q23–q31, 12q15–q24, 19q13, 21q21, 3p24–p22 | asthma and three related phenotypes |
| Wjst et al. (1999) | 415, mostly German | 2pter–p13, 6p21, 9q13–q32, 12q21 | asthma and six subphenotypes |
| Malerba et al. (1999) | 1083, North Italy | 12, 14 | asthma and three related phenotypes |
| Dizier et al. (2000) | 493, French (EGEA study) | 11p13, 12q24, 17q12–q21 | asthma and four related phenotypes |
| Laitinen et al. (2001) | Finnish, French Canadian | 7p | asthma, high IgE and their combination |
| Hakonarson et al. (2002) | 1134, Icelandic | 14q24 | asthma |
| Bouzigon et al. (2004) | 1355, French (EGEA study) | 6q14, 12p13, 17q22–q24, 21q21 | asthma and seven subphenotypes |
Genome scans on asthma susceptibility have used a few strongly correlated but distinct phenotypic components. Based on commonly accepted criteria, the presence or absence of asthma as a dichotomous trait has been considered in most studies. Two distinct features, broncial hyperreactivity as measured by lung function tests and serum total or specific immunoglobulin E (IgE) concentrations, have both been used in a number of studies, either as quantitative or dichotomous threshold traits. The inclusion of alternative phenotypes poses a problem for statistical testing but their strong correlations suggest that the correction factor need not be the same as the number of phenotypes tested. There is evidently some degree of uncertainty as to what should be considered the most relevant phenotype but the degree of uncertainty has not rendered mapping studies obsolete.
3. Why is genetic linkage so hard to find?
In retrospect, it is possible to assess the reasons for poor consistency between the genome scans. One obvious reason is that the assumptions made about the strength of genetic effects attributable to individual loci were optimistic. Recent findings on replicated susceptibility genes have shown that the relative risk effects of individual genes are far below two and power estimations for such effects suggest that much larger study materials than those involved in these studies would be needed to achieve even modest power. Thus, it is quite clear that genome scans have been underpowered to detect weak genetic effects. Another factor correlated with successful mapping efforts is the homogeneity of the study population. This effect may be caused in at least two alternative ways. It is possible to use more consistent phenotyping schemes in homogenous population groups, resulting in more accurate and reliable ascertainment of patients and, thus, stronger genetic correlations. Alternatively, the effect may depend on genetic homogeneity in the study population. Indeed, reduction of genetic heterogeneity has been suggested as a useful strategy for genetic studies in complex diseases (Lander & Schork 1994). Risk alleles may have different carrier frequencies in different populations and it is conceivable that a relatively high carrier frequency may translate to a higher power for genetic mapping in some populations.
4. Positional cloning of asthma susceptibility genes
The logical continuation of genetic mapping studies, positional cloning of risk genes, has been attempted by several groups, each focusing on different loci. So far, four genes have been directly implicated as asthma susceptibility genes on the basis of pure positional cloning efforts, without functional a priori evidence to suggest these genes are involved in the pathogenesis of asthma. Rather, each of these four genes has seemed to suggest the hypothesis of a new asthma-related novel pathway because no obvious links have been established between the genes and previously characterized pathways (table 2).
Table 2.
Positionally cloned asthma susceptibility genes for asthma.
| study | gene name(s), chromosome | predicted primary function | suggested function in asthma | effect of polymorphisms |
|---|---|---|---|---|
| Van Eerdewegh et al. (2002) | ADAM33, 20p13 | metalloproteinase | airway remodelling by fibroblasts and smooth muscle hyperreactivity | both amino acid substitutions and 3′ non-coding changes |
| Zhang et al. (2003) | PHF11, 13q14 | zinc finger transcription factor | immuno-regulation, particularly B lymphocytes | non-coding. Regulation of alternative splicing? |
| Allen et al. (2003) | DPP10 (DRPR3), 2q14 | dipeptidyl peptidase | cytokine processing, particularly T-lymphocytes | non-coding. Altered transcription factor binding to promoter, alternative splicing? |
| Laitinen et al. (2004) | GPRA (GPR154, PGR14, VRR1), 7p15 | G-protein coupled receptor | bronchial epithelial and smooth muscle receptor | non-coding. Regulation of alternative splicing? |
(a) ADAM33
The first positionally cloned asthma susceptibility gene to be localized to chromosome 20p was genetically mapped and positionally cloned in a single study (Van Eerdewegh et al. 2002). Remarkably, the locus on chromosome 20p was mapped with high confidence, even though it had not been observed in any previous genome scan that had included patients from similar population groups (table 1). The linked region was further fine mapped using single nucleotide polymorphisms (SNP) picked at especially high densities around predicted genes, but leaving relatively long intervals between genes sparsely covered. Most significant allelic associations were further followed up with additional markers. Results from this approach implicated the region that harbours the ADAM33 gene, a disintegrin and metalloproteinase homologue. The association results posed, however, a new surprise. The strongest genetic associations were observed to be very common but different in the US and UK populations. This result was counterintuitive, because the common history of these populations would suggest that the same alleles would act as susceptibility genes in both populations. Heterogeneity of susceptibility alleles between different populations has continued to be observed in replication studies of ADAM33 associations (table 3). The replication studies have either failed to observe allelic association between asthma and ADAM33 polymorphisms, or have found associations to different alleles in different populations. These results might be interpreted to suggest that ADAM33 does not confer a replicable risk gene for asthma susceptibility, even though further association studies are clearly warranted to more firmly assess the genetic risk.
Table 3.
Replication studies for positionally cloned candidate genes in asthma.
| study, target gene | number of cases, populations | associated SNPs (no. of SNPs studied) | notes |
|---|---|---|---|
| Raby et al. (2004), ADAM33 | 549, US White, African American, US Hispanic | T1, T+1 (17) | associations only in US Hispanics. KL+1, V2, ST+7 were excluded as unreliable markers |
| Lee et al. (2004), ADAM33 | 326, Korean | none (5) | T1 associated with metacholine provocation |
| Howard et al. (2003), ADAM33 | 644, US White, African American, Dutch, US Hispanic | ST+7, V4 (8) | associated only in the Dutch |
| Lind et al. (2003), ADAM33 | 373, Mexico, Puerto Rico | none (6) | — |
| Jongepier et al. (2004), ADAM33 | 152, Dutch | S2 (8) | FEV decline as phenotype |
| Werner et al. (2004), ADAM33 | 91, German | ST+5, ST+7 (15) | — |
| Shin et al. (2004), GPRA | 439, Korean | none (1) | only 1 SNP genotyped |
| Melén et al. (2005), GPRA | 441, Austria, Germany, Netherlands, Sweden, Switzerland | rs324384, rs324396 (7) | haplotype associations in addition to SNP associations. Patients and controls are children |
| Kormann et al. (2005), GPRA | 624, German (3 centres) | SNP546333, SNP585883 (7) | haplotype associations in addition to SNP associations. Patients and controls are children |
Replication studies that consider the asthma phenotype have been published for ADAM33 and GPRA.
Functional studies on ADAM33 have revealed that it might well have a role in airways. ADAM33 undergoes complex alternative splicing with several variant transcripts and their relative functional significance to each other is not clear (Umland et al. 2003; Powell et al. 2004).
(b) DPP10 and PHF11
Two following projects to complete positional cloning of candidate susceptibility genes for asthma were reported by the group led by Cookson in Oxford (Allen et al. 2003; Zhang et al. 2003). They used essentially the same approach for both projects that focused on genes on chromosomes 2q and 13q. Their asthma families were collected primarily from two geographically distinct populations, the first mapping series of families from Western Australia and the second, replication set from the UK. Both projects were based on previously repeatedly observed genetic linkage to the regions on chromosome 2q and 13q. Again, the genetically linked region was fine-mapped using both microsatellite and SNP markers, and genetic associations were sought for. Strongest allele and haplotype associations were observed for the segment that contained the PHF11 gene on chromosome 13q14 and for the segment containing the DPP10 gene on chromosome 2q14. Both genes have an apparently modest influence on the risk of asthma and high IgE values, or clearly reduced penetrance, although odds ratios have not been estimated for the risk alleles of these two genes yet. Both genes remain biologically highly interesting, but the exact mechanisms by which these genes might influence asthma or high IgE susceptibility remain uncharacterized. Recently, the functions of DPP10 were highlighted as a modulator of Kv4-mediated A-type potassium channels in the brain (Jerng et al. 2004; Zagha et al. 2005).
(c) GPRA
The fourth positionally cloned gene was identified by our group working on a mapping set of families collected from the Kainuu province in eastern central Finland; genetic associations were replicated by the study of a sample set from Northeastern Quebec, Canada, and an independently collected, smaller set from a geographically and historically distinct population from a region of Finland (Laitinen et al. 2004). We performed fine mapping with microsatellite as well as SNP markers and primarily analysed the data using a data mining application that searches for associated marker patterns by comparing haplotypes from affected and control individuals and performs a randomization test to assess the significance of suggested associations. The genetic fine mapping results implicated a segment on chromosome 7p14 that was embedded in two partially predicted genes. A thorough characterization of the genes revealed that only one of them had protein-coding properties and we named this gene GPRA (for G protein-coupled receptor in asthma, now renamed according to the standard GPR gene family nomenclature as GPR154). We found that it is alternatively spliced to encode two major isoforms, GPRA-A and GPRA-B, that result from the usage of different terminal exons for coding part of the intracellular carboxy tail. Besides genetic evidence, additional evidence for its role in asthma was presented. In all asthma patients studied, the GPRA-B isoform was found to be distinctly upregulated in bronchial smooth muscle cells that only expressed the GPRA-A isoform in healthy controls. In a mouse model of ovalbumin-induced bronchial inflammation, mouse Gpra mRNA was found to be significantly upregulated in airways. Taken together, these results suggested that the GPRA gene harbours genetic variation that may affect an individual's susceptibility to asthma-related traits and that, in addition, altered regulation of GPRA expression may be a general feature in the pathogenesis of asthma.
In parallel with our work, other investigators have characterized systematically novel GPR family genes and found the GPRA-A variant that they called PGR14 or VRR1 (Vassilatis et al. 2003; Gupte et al. 2004). Because GPR154 (alias GPRA or PGR14 or VRR1) belongs to the subfamily of vasopressin receptor-like receptors, Gupte et al. (2004) used it in a chimeric receptor model to study its signalling properties. To confirm the results of the chimeric receptor assay, they used the native GPR154 in experiments with a peptide ligand that had been identified by Mori and co-workers (see Gupte et al. 2004). We have recently shown that the corresponding mRNA is transcribed in bronchial epithelial cells, thus suggesting an autocrine or paracrine model of GPR154 activation (Vendelin et al. 2005). However, the corresponding locus on chromosome 10 has not been implicated in linkage studies, suggesting that it may not harbour variation with consequences on asthma risk. This suggestion will need to be verified by a targeted association study of the haplotypes around the locus because weak genetic effects may well have been overlooked by genetic linkage studies due to their low sensitivity for such weak effects.
Recently, both the ligand and the GPR154 receptor were also studied by Xu et al. (2004), who detected their expression in the brain and named the ligand as neuropetide S (NPS). They implicated the NPS–GPR154 pathway in the control of wakefulness, and possibly anxiety, through the study of mouse and rat models by directly injecting the NPS ligand into the brain of animals. Both NPS and GPR154 are widely expressed in the brain; however, they are expressed at variable levels in different parts of the brain.
The fortuitous parallel studies have thus rapidly revealed important characteristics for the new asthma-related pathway. An endogenous linear peptide ligand NPS, encoded by chromosome 10 and produced in large amounts in the brain (but also produced at lower levels by bronchial epithelial cells), binds to the GPRA154 receptor, leading to the activation of both the Gq and Gs signalling pathways (Gupte et al. 2004; Vendelin et al. 2005). GPR154 protein is produced in two different isoforms with different carboxyterminal cytoplasmic tails and may, thus, have partially different roles. The isoform balance appears to be cell type specific; for example, both isoforms are present in bronchial epithelial cells, whereas smooth muscle normally expresses only the A isoform (Laitinen et al. 2004).
5. Replication studies to verify risk effects
In monogenic disorders, genetic associations are usually readily and indisputably detected between specific coding variants of the gene and the occurrence of disease. However, in studies concerning cancer genes with age-dependent penetrance, such as BRCA1 and BRCA2 in breast cancer, it has been observed that it may be difficult to assess the role of family-specific single amino acid coding changes that do not truncate the proteins. In complex diseases, it is expected that the susceptibility alleles may be common in populations but have highly reduced penetrance, thus causing only a modest increase in disease susceptibility. Examples of such susceptibility alleles are the risk variants of the PPARG gene in diabetes and the NOD2 gene in Crohn's disease (Altshuler et al. 2000; Hugot et al. 2001; Ogura et al. 2001). Unreplicated, possibly false candidate gene association results have become commonplace in a number of complex diseases, including asthma. Primed by these considerations, it is obviously necessary to replicate the genetic associations that have been observed in the course of positional cloning studies in independent populations.
Several such studies have been rapidly completed for ADAM33 (for asthma-related phenotypes) and studies have been completed for PHF11 (in atopic dermatitis rather than asthma) and GPR154 (in asthma-related phenotypes). Results of the replication studies for asthma are summarized in table 3. At time of writing, replication studies for DPP10 are yet to appear. In addition, the SPINK5 gene that was earlier identified as causing a rare recessive skin disorder with dermatitis as a symptom, Netherton syndrome, has been associated with atopy and atopic dermatitis and, more recently, asthma susceptibility (Kabesch et al. 2004). Among the functional candidate genes, many associations that have been reported once have not been replicated (Hoffjan et al. 2003; Weiss & Raby 2004).
Results of replication studies for positionally cloned genes appear as equally unsatisfactory as results for functional candidate genes without any prior genetic evidence. One obvious problem is the lack of rigorous criteria for conducting, evaluating and publishing such genetic association studies. To achieve substantial power to detect genetic effects that have been initially observed in the positional cloning studies, with odds ratios around or below two, quite large case and control groups are needed. The smallest studies reported so far have certainly been underpowered. In a replication study, the investigators should always perform and report power calculations, especially because such calculations can be performed to test a known prior hypothesis about the genetic effect. In addition, in very small studies, false positive results due to chance variation commonly occur and reporting bias ensures that such results, but not necessarily the negative ones, are reported. Another critical factor of relevance to genetic replication studies is the set of SNPs used for genotyping. One SNP can barely be considered adequate, even if an original report may have identified one SNP as the most critical. With the HapMap data emerging, an early replication study should minimally assess the complete common (>1% population frequency) haplotype spectrum for the putative susceptibility gene.
6. Conclusions and prospects for asthma research
In a short period of time, positional cloning studies as well as successful candidate gene studies have equipped asthma researchers with a set of biochemically tractable targets and even suggested new pathways. It is expected that the identification of upstream and downstream molecules in these pathways will progress rapidly, as has already happened for GPR154. One goal of this research will be to integrate the presently distinct pathways, as well as pre-existing observations about the asthma process, into a coherent picture of pathogenetic events and its different actors. The physiological roles of the different genes will be studied using genetically modified mice as well as tissue culture models. Taken together, such results will suggest new targets for pharmaceutical development.
At the same time, the genetic research is not over. The identified risk effects, as such, do not yield information about their joint effects and yield even less information about the joint effects of the risk genes and environmental effectors. Many configurations are indeed possible. Different haplotypes may be associated with different risk effects, as has recently been suggested for GPR154 (Melén et al. 2005). In their study of about 4000 children from five European populations, Melén and co-workers determined that the most common haplotype H1 appeared to protect children against sensitization and asthma, whereas other haplotypes appeared rather neutral for risk or risk-increasing. Thus, one could envision that different combinations of protective, neutral and risk haplotypes may produce slightly different risk levels. It is expected that other identified risk genes modify these risks further, and it is indeed quite possible that the different risk haplotypes convey different risk levels depending on varying environmental exposure.
The picture that emerges from these realistic expectations is complex and involves almost individualized risk levels, but its distinct components are measurable for the first time. It is expected that possessing multiple risk haplotypes at different loci will increase an individual's risk of developing asthma compared with possessing just one or the other gene, but whether the risk increase is additive or multiplicative for different risk gene combinations remains to be determined. Of course, the protective haplotypes will complicate the picture further. Large, representative and epidemiologically well-characterized cohorts will be needed to assess the risk effects of different gene–gene and gene–environment combinations, preferably with longitudinal follow-up data to study the dynamics of disease development using life-table analyses. A few such cohorts already exist, such as, for example, the Northern Swedish longitudinal study (Lundbäck et al. 2001), but relatively rare combined effects will probably still require larger numbers of subjects and the studies will need to be repeated in multiple environments.
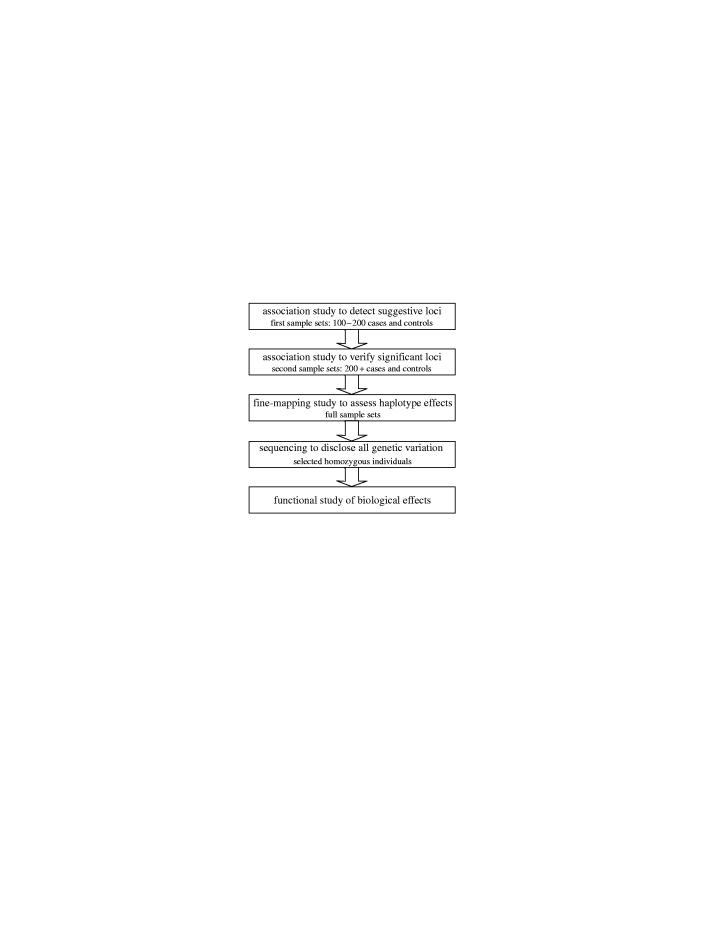
Finally, the lessons learned during these positional cloning efforts are currently being applied to similar projects that address distinct diseases. Genome-wide multiphased association studies (figure 1), as well as ongoing positional cloning efforts, are likely to suggest several new candidate susceptibility genes. These, in turn, will need similar approaches to genetic epidemiology, providing a strong motivation for large cohort studies, such as those already proposed, that will simultaneously record data for several important target diseases.
Figure 1.
A multiphase approach to identifying susceptibility genes in complex disorders. In the initial genome-wide association scanning phase, the number of SNPs used will determine the power to detect relevant loci. For the second phase, the study can be targeted to suggestively associated regions using higher local densities of markers. In the third phase, only the confirmed loci are studied at high enough density to distinguish neighbouring haplotype block effects. Genomic re-sequencing of implicated segments is used to reveal all potentially relevant genetic variants. Finally, the biological effects of genetic variation are considered and hypotheses tested experimentally.
7. Psoriasis genetics: a different kind of a problem
Psoriasis is the most common chronic skin disorder and is estimated to affect 2–3% of populations in Europe and North America. Several clinical subtypes of psoriasis have been recognized but the most consistent division of relevance for genetic studies involves the age of onset of psoriasis (Barker 1991). Two categories are distinguished: type I psoriasis that begins under the age of 30 and type II psoriasis that begins after the age of 40. Type I psoriasis is rather strongly associated with specific alleles of the HLA region on chromosome 6p and the association of psoriasis to its most specific risk allele HLA-Cw6 has been known for 25 years (Tiilikainen et al. 1980). The strong association has been corroborated with more recent linkage studies that have unequivocally determined that this locus that includes the HLA-C gene, now known as PSORS1, is by far the strongest susceptibility locus for psoriasis (International Psoriasis Genetics Consortium 2003). Furthermore, the strength of both the genetic linkage and association is among the strongest for any complex disease and far stronger than any genetic effect implicated for any asthma locus.
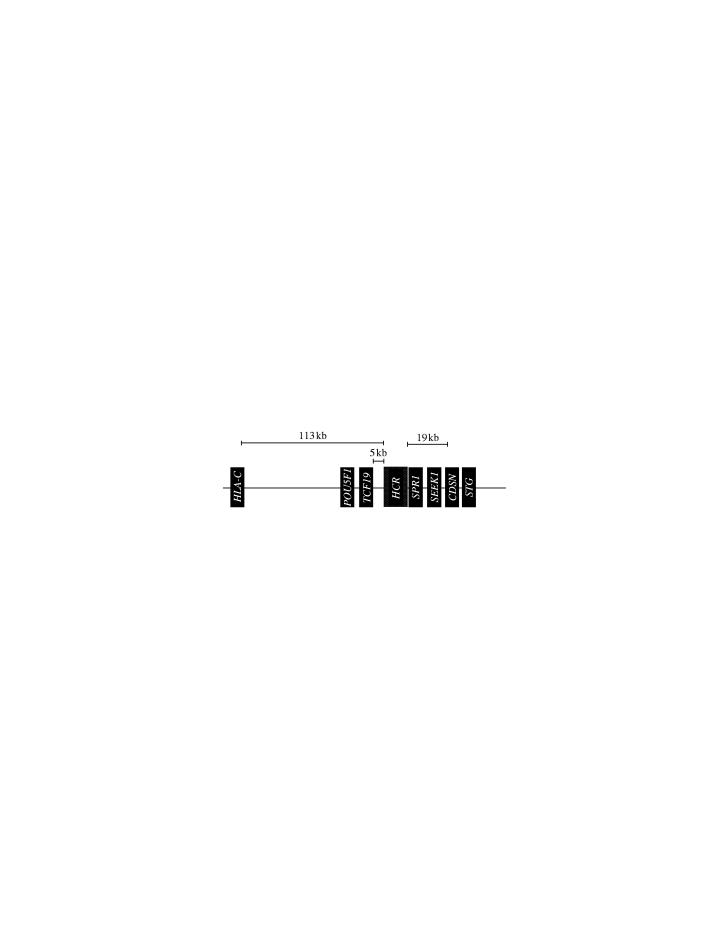
It may therefore be surprising that a current unsolved research problem still focuses on the identity of the actual susceptibility gene within PSORS1 (figure 2). Remarkably, decades of research on the exact immunological mechanism by which HLA-Cw6 might induce increased susceptibility to psoriasis have left the research community unconvinced (Bos & De Rie 1999). Several research groups have collected hundreds of families or case–control pairs and studied the PSORS1 region with dense microsatellite and SNP marker maps in genetic fine-mapping studies to localize the susceptibility gene within PSORS1 (table 4). These studies have suggested interesting new candidate genes that have already been assessed by functional studies.
Figure 2.
Genes in the PSORS1 locus on chromosome 6p21.
Table 4.
Examples of fine-mapping studies of PSORS1 to identify the psoriasis susceptibility gene.
| study | number of subjects, population | number and type of markers | suggested limits of gene region |
|---|---|---|---|
| Oka et al. (1999) | 208, Japanese | 11 microsatellites | 89–200 kb telomeric of HLA-C (incl. HCR, CDSN) |
| Nair et al. (2000) | 1337, North American | 62 microsatellites | 30–90 kb telomeric of HLA-C (‘RH1’, excl. HCR and CDSN) |
| Asumalahti et al. (2002) | 1049, six populations pooled | 12 SNPs (for HLA-C, HCR, CDSN genes) | HLA-C and HCR indistinguishable, CDSN excluded |
| Veal et al. (2002) | 667, UK European and Indian | 59 SNPs | 10 kb centromeric of HLA-C |
The first candidate gene to emerge was corneodesmosin, or the S gene (CDSN), that localizes 133 kb telomeric of HLA-C (Ishihara et al. 1996; Jenisch et al. 1999; Tazi Ahnini et al. 1999). Immediately upon finding its genetic association to psoriasis, it attracted wide interest because of its expression in the skin and its functional role in the cornified envelope of keratinocytes. As with HLA-C, however, convincing mechanistic explanations as to exactly how the risk allele of CDSN might increase the risk of developing psoriasis are nonexistent. In addition, the frequency of the suggested risk allele of CDSN in some populations appears inconsistent with its suggested role and, indeed, its risk effect has been measured as much lower that that of HLA–Cw6 in such populations (Asumalahti et al. 2002). Thus, the consistent genetic effect that one would expect from a true susceptibility gene appears problematic.
Its existence first suggested by ab initio gene prediction programmes when analysing the complete sequence of the HLA region (Guillaudeux et al. 1998), the alpha-helical coiled-coil rod protein gene (HCR, first named Pg8 for putative gene 8, now officially CCHCR1) has emerged as a surprising new candidate gene for PSORS1. The exon structure of HCR was first verified by Oka et al. (1999) and Asumalahti et al. (2000), with a new 5′ exon later identified (Asumalahti et al. 2002). We reported that the HCR gene is highly polymorphic with at least 12 coding variants, some of which are associated with psoriasis as strongly as HLA-Cw6 in our Finnish case–control study (Asumalahti et al. 2000). We followed up this finding with a larger multipopulation study that included samples from six different populations and determined that the same haplotypes, including four amino acid changes in the protein, are consistently associated with psoriasis in all six populations. The association of the HCR risk allele (named HCR*WWCC) was indistinguishable from the association of HLA-Cw6 with psoriasis in this study that included over 700 patients. Even though smaller studies that failed to replicate the strong association were soon published (Chia et al. 2001; O'Brien et al. 2001), it has later been confirmed by larger studies (Veal et al. 2002). Indeed, taken together, these studies have confirmed that in all populations studied so far, there appears to be a particularly long risk haplotype that spans the HLA-C and HCR genes, extending all the way to CDSN.
The PSORS1 region still includes other genes but, in spite of careful sequencing efforts by several laboratories, they have not been observed to carry genetic variants that would associate as strongly with psoriasis as the HLA-Cw6 or HCR*WWCC alleles. Thus, they remain less likely candidate genes, a notion also supported by their known functional roles.
Of interest is the mutational history of this PSORS1 risk haplotype. In their extensive study, Nair et al. (2000) suggested that the risk haplotype would consist of two segments with different risk profiles, named RH1 (starting from 60 kb telomeric of HLA-C) and RH2 (including HCR and CDSN). Their analysis and separation of these two segments was based on two microsatellite markers where specific alleles appeared to break the continuous haplotype. In a public discussion at the National Psoriasis Foundation International Genetic Committee Meeting in Rome, the present author suggested that the apparent break of continuous haplotypes was due to microsatellite mutation events. This suggestion was followed up by Dr Elder and his colleagues and was demonstrated as correct through careful sequencing and surrounding SNP analysis around the mutated microsatellite markers. Thus, all available data consistently suggests that the common risk haplotype for psoriasis spans three strong candidate genes. The genetic fine-mapping of the region has thus remained elusive.
8. Approaches to identify the PSORS1 risk gene
The abundance of very high resolution genotyping data, along with HapMap data, has made it possible to estimate whether the long continuous segment might be broken down by genetic analysis using a large enough sample set (Mark Daly, personal communication). Indeed, a project to attempt such analysis was recently approved for funding by the National Psoriasis Foundation and 11 European and U.S. laboratories are currently collaborating in a consortium project to dissect the individual gene associations.
Even though at least some functions of the HLA-C and CDSN genes are known, that of HCR remains much less understood. In situ hybridization and immunohistochemical analyses suggested that HCR is expressed in the skin with basal keratinocytes and that in psoriasis, HCR expression is reduced in areas where the most rapidly proliferating basal keratinocytes localize (Asumalahti et al. 2002). The lack of homologous proteins that would suggest specific roles for HCR and complete biochemical ignorance of its function prompted us to engineer transgenic mice to directly assess possible differences that the human risk and non-risk HCR alleles might induce in mouse skin. Because HCR*WWCC exerts an obviously dominant risk effect (a single copy of the risk allele suffices to increase one's risk for psoriasis), we chose to attempt overexpression of human HCR alleles in transgenic mice. As the promoter, we chose to use the cytokeratin 14 (K14) promoter that targets transgene expression to basal keratinocytes, the site of physiological expression of HCR in human skin.
The initial results showed that the mice were apparently unaffected by the overexpression of either transgene in skin (Elomaa et al. 2004). The mice bred normally and were indistinguishable from wild-type mice, even with microscopical analysis. Both southern, northern, western, cDNA sequencing and immunohistochemical analyses confirmed the presence and overexpression of the intended human alleles in our mouse strains. Because we considered it inconceivable that such intrusion to the cellular interior would go unnoted by the regulatory networks of the skin cells, we then assessed specific gene alterations using gene expression microchips to compare wild-type, non-risk allele and risk allele mice. The results of these analyses revealed that, in addition to gene expression changes that were induced by both transgenes in comparison to the wild-type mice, there were allele-specific differences between the transgenic strains overexpressing either risk or non-risk HCR alleles. The induced changes did not simply result from the level of HCR overexpression.
A more detailed analysis of the altered gene expression profile revealed two further notions of interest. First, a large fraction of the genes that were dysregulated by HCR*WWCC allele as compared with the non-risk allele were such that they had been either found dysregulated in human psoriatic plaques by gene expression profiling (Bowcock et al. 2001) or had been found altered in numerous direct gene-specific analyses performed on psoriatic skin. Examples of such genes included keratins 6, 5, 16 and 17, and small proline-rich proteins 1B, 2A and 2D. Second, two-dimensional clustering analyses considering different groups of genes showed that the alterations involved mostly cytoskeletal proteins, keratins and keratin-associated proteins. These results directly supported a relevant functional role for HCR in the skin and, furthermore, suggested that the four amino acids that were different between the risk and non-risk alleles have direct biochemical consequences on the function of the HCR protein in skin.
Why did the mice not develop psoriasis? This question cannot be fully answered at present. No natural model of a mouse psoriasis-like disease is known and, even though several mice models have been engineered that resemble human psoriasis, there are still important differences. Structurally, mouse skin differs considerably from human skin, for example, with respect to its thickness and number of keratinocyte layers. It is conceivable that, even though overexpression of the risk allele of HCR induced changes reminiscent of the changes in human psoriatic skin, these alterations were insufficient to trigger a psoriasiform phenotype. Clearly, further experiments and insights into the function of HCR protein are necessary to understand its effects on human and mouse skin.
These results on HCR complicate current psoriasis research because they challenge the community to try to understand the functional roles of the candidate genes in the PSORS1 locus. Because of the complexity and need for individual recognition inherent in the immune systems, the testing of HLA-Cw6 or other MHC alleles in transgene models is not as easy as it might be with other genes. Furthermore, indirect evidence of the roles of several other genes in the PSORS1 region does not invite researchers to look at their roles as potential psoriasis candidate susceptibility genes.
9. Conclusions and prospects for psoriasis research
Perhaps surprisingly, psoriasis as a model disease has highlighted some of the most difficult problems that may arise with the genetic approaches for identifying important disease-related genes. Similar to PSORS1, other risk genes for a number of diseases showing associations to the HLA region have remained elusive, even though numerous research groups have attempted to identify them. The problem is not unforeseen in other genomic regions either. A genomic segment near the Interleukin gene cluster on chromosome 5q31 has been implicated by genetic linkage and association studies as a locus in Crohn's disease but it has been difficult to identify the actual gene, even though the OCTN gene has been highlighted as a more likely candidate gene (Rioux et al. 2001; Peltekova et al. 2004).
With attention turning now to genome-wide association studies using high-density SNP maps, ultimately at the resolution of haplotype blocks, it is expected that candidate loci for susceptibility will be identified and then confirmed by repeated significant associations in several populations. In many cases these results will pinpoint individual genes, analogous to the case of GPR154 in asthma. But in a large number of cases, the disease-associated locus may still include several genes, many of which may even share common expression patterns and possibly common regulatory elements. Attributing the risk effect in such cases to one or another gene may turn out to be challenging. Possible solutions include functional studies in cell or animal models, such as that attempted for understanding the role of HCR in PSORS1. It is also conceivable that cumulative knowledge of the pathways through unique identification of disease-related genes at other loci may come to the rescue. For example, if one of the alternative risk genes at a multigene locus is identified as belonging to the same pathway as a definite risk gene at another locus, this suggests that that particular gene is the functionally relevant one in the extended locus. Global, systematic understanding of cellular networks will yield insights that have not been previously possible.
A particular problem for psoriasis research is the overdominant role of PSORS1 as a risk locus. All other suggested PSORS loci, at least nine so far, have turned out to have risk effects so low or population-specific that they have been impossible to replicate, even in large consortium studies. One possible solution is to condition high-density association studies in large case–control settings with the presence or absence of PSORS1 as a risk factor. Indeed, in one such attempt, we reported the mapping of a new psoriasis locus, PSORS10, on chromosome 18p in families that were selected to be PSORS1 negative (Asumalahti et al. 2003). This strategy may help to identify distinct groups of susceptibility genes, some of which work synergistically with PSORS1 to increase risk and others that may work independently of PSORS1 but perhaps interfere with other genes of this group to increase susceptibility.
10. Conclusions
The application of positional cloning with a better understanding of genetic models that guide detailed strategies has finally yielded results. Several interesting and credible susceptibility genes in complex diseases have been discovered in the past few years. The pessimism that followed early genome scans with seemingly inconsistent results was clearly unwarranted and patience has paid off. The rapid progress in developing genotyping methods, and the completion of the HapMap project, suggest that genetic association studies with high-density marker sets will accelerate the dissection of genetic influences and complexity even further. These results will then reveal not only the advantages but also the limits of genetic analyses. Linkage disequilibrium within haplotype blocks is helpful in studying genetic associations but, at the same time, haplotype blocks set the limit for resolution. Human history is too short to have resulted in recombinations between all neighbouring genes and understanding gene functions becomes essential for understanding the causalities of associations. Undoubtedly, molecular pathways and regulatory networks within cells and tissues will also be discovered at an accelerated pace. Perhaps genetic studies will then link together unexpected genes, new pathways and common, complex diseases.
Acknowledgments
I wish to thank Drs Tarja Laitinen, Lauri A. Laitinen and Tom Hudson for long-term collaboration on asthma genetics, Drs Kati Asumalahti, Outi Elomaa and Ulpu Saarialho-Kere for collaboration on the genetics of psoriasis and Dr Mark Daly for advice and collaboration on many projects. Significant support for our work was provided by the Academy of Finland, the Sigrid Jusélius Foundation and the Medical Research Council of Sweden.
Footnotes
One contribution of 12 to a Discussion Meeting Issue ‘Genetic variation and human health’.
References
- Allen M, et al. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nature Genet. 2003;35:258–263. doi: 10.1038/ng1256. [DOI] [PubMed] [Google Scholar]
- Altmuller J, Palmer L.J, Fischer G, Scherb H, Wjst M. Genomewide scans of complex human diseases: true linkage is hard to find. Am. J. Hum. Genet. 2001;69:936–950. doi: 10.1086/324069. [Erratum in: Am. J. Hum. Genet. 2001 69, 1413.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler D, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nature Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- Asumalahti K, et al. A candidate gene for psoriasis near HLA-C, HCR (Pg8), is highly polymorphic with a disease-associated susceptibility allele. Hum. Mol. Genet. 2000;9:1533–1542. doi: 10.1093/hmg/9.10.1533. [DOI] [PubMed] [Google Scholar]
- Asumalahti K, et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum. Mol. Genet. 2002;11:589–597. doi: 10.1093/hmg/11.5.589. [DOI] [PubMed] [Google Scholar]
- Asumalahti K, et al. Psoriasis susceptibility locus on 18p revealed by genome scan in Finnish families not associated with PSORS1. J. Invest. Dermatol. 2003;121:735–740. doi: 10.1046/j.1523-1747.2003.12483.x. [DOI] [PubMed] [Google Scholar]
- Barker J.N.W.N. The pathophysiology of psoriasis. Lancet. 1991;338:227–230. doi: 10.1016/0140-6736(91)90357-u. [DOI] [PubMed] [Google Scholar]
- Bos J.D, De Rie M.A. The pathogenesis of psoriasis: immunological facts and speculations. Immunol. Today. 1999;20:40–46. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- Bouzigon E, et al. Clustering patterns of LOD scores for asthma-related phenotypes revealed by a genome-wide screen in 295 French EGEA families. Hum. Mol. Genet. 2004;13:3103–3113. doi: 10.1093/hmg/ddh340. [DOI] [PubMed] [Google Scholar]
- Bowcock A.M, Shannon W, Du F, Duncan J, Cao K, Aftergut K, Catier J, Fernandez-Vina M.A, Menter A. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum. Mol. Genet. 2001;10:1793–1805. doi: 10.1093/hmg/10.17.1793. [DOI] [PubMed] [Google Scholar]
- Cheung V.G, Conlin L.K, Weber T.M, Arcaro M, Jen K.Y, Morley M, Spielman R.S. Natural variation in human gene expression assessed in lymphoblastoid cells. Nature Genet. 2003;33:422–425. doi: 10.1038/ng1094. [DOI] [PubMed] [Google Scholar]
- Chia N.V, et al. Variations in the HCR (Pg8) gene are unlikely to be causal for familial psoriasis. J. Invest. Dermatol. 2001;116:823–824. doi: 10.1046/j.1523-1747.2001.01245-2.x. [DOI] [PubMed] [Google Scholar]
- Collaborative Study on the Genetics of Asthma (CSGA) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nature Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- Daniels S.E, et al. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383:247–250. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- Dizier M.H, et al. Genome screen for asthma and related phenotypes in the French EGEA study. Am. J. Respir. Crit. Care Med. 2000;162:1812–1818. doi: 10.1164/ajrccm.162.5.2002113. [DOI] [PubMed] [Google Scholar]
- Elomaa O, et al. Transgenic mouse models support HCR as an effector gene in the PSORS1 locus. Hum. Mol. Genet. 2004;13:1551–1561. doi: 10.1093/hmg/ddh178. [DOI] [PubMed] [Google Scholar]
- Guillaudeux T, Janer M, Wong G.K, Spies T, Geraghty D.E. The complete genomic sequence of 424,015 bp at the centromeric end of the HLA class I region: gene content and polymorphism. Proc. Natl Acad. Sci. USA. 1998;95:9494–9499. doi: 10.1073/pnas.95.16.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte J, Cutler G, Chen J.L, Tian H. Elucidation of signaling properties of vasopressin receptor-related receptor 1 by using the chimeric receptor approach. Proc. Natl Acad. Sci. USA. 2004;101:1508–1513. doi: 10.1073/pnas.0308250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonarson H, et al. A major susceptibility gene for asthma maps to chromosome 14q24. Am. J. Hum. Genet. 2002;71:483–491. doi: 10.1086/342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffjan S, Nicolae D, Ober C. Association studies for asthma and atopic diseases: a comprehensive review of the literature. Respir. Res. 2003;4:14. doi: 10.1186/1465-9921-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T.D, Postma D.S, Jongepier H, Moore W.C, Koppelman G.H, Zheng S.L, Xu J, Bleecker E.R, Meyers D.A. Association of a disintegrin and metalloprotease 33 (ADAM33) gene with asthma in ethnically diverse populations. J. Allergy Clin. Immunol. 2003;112:717–722. doi: 10.1016/s0091-6749(03)01939-0. [DOI] [PubMed] [Google Scholar]
- Hugot J.P, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Illig T, Wjst M. Genetics of asthma and related phenotypes. Paediatr. Respir. Rev. 2002;3:47–51. doi: 10.1053/prrv.2002.0185. [DOI] [PubMed] [Google Scholar]
- International Psoriasis Genetics Consortium. The International Psoriasis Genetics Study: assessing linkage to 14 candidate susceptibility loci in a cohort of 942 affected sib pairs. Am. J. Hum. Genet. 2003;73:430–437. doi: 10.1086/377159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, et al. Genetic polymorphisms in the keratin-like S gene within the human major histocompatibility complex and association analysis on the susceptibility to psoriasis vulgaris. Tissue Antigens. 1996;48:182–186. doi: 10.1111/j.1399-0039.1996.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Jenisch S, et al. Corneodesmosin gene polymorphism demonstrates strong linkage disequilibrium with HLA and association with psoriasis vulgaris. Tissue Antigens. 1999;54:439–449. doi: 10.1034/j.1399-0039.1999.540501.x. [DOI] [PubMed] [Google Scholar]
- Jerng H.H, Qian Y, Pfaffinger P.J. Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10) Biophys. J. 2004;87:2380–2396. doi: 10.1529/biophysj.104.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongepier H, et al. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin. Exp. Allergy. 2004;34:757–760. doi: 10.1111/j.1365-2222.2004.1938.x. [DOI] [PubMed] [Google Scholar]
- Kabesch M, Carr D, Weiland S.K, von Mutius E. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypes in a large German population sample. Clin. Exp. Allergy. 2004;34:340–345. doi: 10.1111/j.1365-2222.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- Kere J, Laitinen T. Positionally-cloned susceptibility genes in allergy and asthma. Curr. Opin. Immunol. 2004;16:689–694. doi: 10.1016/j.coi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kormann M.S.D, Carr D, Klopp N, Illig T, Leupold W, Fritzsch C, Weiland S.K, von Mutius E, Kabesch M. G-protein-coupled receptor polymorphisms are associated with asthma in a large German population. Am. J. Respir. Crit. Care Med. 2005;171:1358–1362. doi: 10.1164/rccm.200410-1312OC. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Rasanen M, Kaprio J, Koskenvuo M, Laitinen L.A. Importance of genetic factors in adolescent asthma: a population-based twin-family study. Am. J. Respir. Crit. Care Med. 1998;157:1073–1078. doi: 10.1164/ajrccm.157.4.9704041. [DOI] [PubMed] [Google Scholar]
- Laitinen T, et al. A susceptibility locus for asthma-related traits on chromosome 7 revealed by genome-wide scan in a founder population. Nature Genet. 2001;28:87–91. doi: 10.1038/ng0501-87. [DOI] [PubMed] [Google Scholar]
- Laitinen T, et al. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- Lander E.S, Schork N.J. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Lee J.H, et al. ADAM33 polymorphism: association with bronchial hyper-responsiveness in Korean asthmatics. Clin. Exp. Allergy. 2004;34:860–865. doi: 10.1111/j.1365-2222.2004.01977.x. [DOI] [PubMed] [Google Scholar]
- Lind D.L, et al. ADAM33 is not associated with asthma in Puerto Rican or Mexican populations. Am. J. Respir. Crit. Care Med. 2003;168:1312–1316. doi: 10.1164/rccm.200306-877OC. [DOI] [PubMed] [Google Scholar]
- Lonjou C, Collins A, Ennis S, Tapper W, Morton N.E. Meta-analysis and retrospective collaboration: two methods to map oligogenes for atopy and asthma. Clin. Exp. Allergy. 1999;29(Suppl. 4):57–59. [PubMed] [Google Scholar]
- Lonjou C, et al. A first trial of retrospective collaboration for positional cloning in complex inheritance: assay of the cytokine region on chromosome 5 by the consortium on asthma genetics (COAG) Proc. Natl Acad. Sci. USA. 2000;97:10 942–10 947. doi: 10.1073/pnas.97.20.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbäck B, Ronmark E, Jonsson E, Larsson K, Sandström T. Incidence of physician-diagnosed asthma in adults—a real incidence or a result of increased awareness? Report from the obstructive lung disease in Northern Sweden studies. Respir. Med. 2001;95:685–692. doi: 10.1053/rmed.2001.1126. [DOI] [PubMed] [Google Scholar]
- Malerba G, Trabetti E, Patuzzo C, Lauciello M.C, Galavotti R, Pescollderungg L, Boner A.L, Pignatti P.F. Candidate genes and a genome-wide search in Italian families with atopic asthmatic children. Clin. Exp. Allergy. 1999;29(Suppl. 4):27–30. [PubMed] [Google Scholar]
- Melén E, et al. Haplotypes of G-protein-coupled receptor 154 are associated with childhood allergy and asthma. Am. J. Respir. Crit. Care Med. 2005;171:1089–1095. doi: 10.1164/rccm.200410-1317OC. [DOI] [PubMed] [Google Scholar]
- Nair R.P, Stuart P, Henseler T, Jenisch S, Chia N.V, Westphal E, Schork N.J. Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am. J. Hum. Genet. 2000;66:1833–1844. doi: 10.1086/302932. [Erratum in: Am. J. Hum. Genet. 2002 70, 1074.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, et al. Genome-wide search for asthma susceptibility loci in a founder population. The collaborative study on the genetics of asthma. Hum. Mol. Genet. 1998;7:1393–1398. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- O'Brien K.P, Holm S.J, Nilsson S, Carlén L, Rosenmüller T, Enerbäck C, Inerot A, Ståhle-Bäckdahl M. The HCR gene on 6p21 is unlikely to be a psoriasis susceptibility gene. J. Invest. Dermatol. 2001;116:750–754. doi: 10.1046/j.0022-202x.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Oka A, et al. Association analysis using refined microsatellite markers localizes a susceptibility locus for psoriasis vulgaris within a 111 kb segment telomeric to the HLA-C gene. Hum. Mol. Genet. 1999;8:2165–2170. doi: 10.1093/hmg/8.12.2165. [DOI] [PubMed] [Google Scholar]
- Pagani F, Buratti E, Stuani C, Baralle F.E. Missense, nonsense, and neutral mutations define juxtaposed regulatory elements of splicing in cystic fibrosis transmembrane regulator exon 9. J. Biol. Chem. 2003;278:26 580–26 588. doi: 10.1074/jbc.M212813200. [DOI] [PubMed] [Google Scholar]
- Pastinen T, Hudson T.J. Cis-acting regulatory variation in the human genome. Science. 2004;306:647–650. doi: 10.1126/science.1101659. [DOI] [PubMed] [Google Scholar]
- Pastinen T, et al. A survey of genetic and epigenetic variation affecting human gene expression. Physiol. Genomics. 2004;16:184–193. doi: 10.1152/physiolgenomics.00163.2003. [DOI] [PubMed] [Google Scholar]
- Peltekova V.D, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nature Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- Powell R.M, Wicks J, Holloway J.W, Holgate S.T, Davies D.E. The splicing and fate of ADAM33 transcripts in primary human airways fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004;31:13–21. doi: 10.1165/rcmb.2003-0330OC. [DOI] [PubMed] [Google Scholar]
- Raby B.A, Silverman E.K, Kwiatkowski D.J, Lange C, Lazarus R, Weiss S.T. ADAM33 polymorphisms and phenotype associations in childhood asthma. J. Allergy Clin. Immunol. 2004;113:1071–1078. doi: 10.1016/j.jaci.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Rioux J.D, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nature Genet. 2001;29:223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- Shin H.D, Park K.S, Park C.S. Lack of association of GPRA (G protein coupled receptor for asthma susceptibility) haplotypes with high serum IgE or asthma in a Korean population. J. Allergy Clin. Immunol. 2004;114:1226–1227. doi: 10.1016/j.jaci.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Tazi Ahnini R, Camp N.J, Cork M.J, Mee J.B, Keohane S.G, Duff G.W, di Giovine F.S. Novel genetic association between the corneodesmosin (MHC S) gene and susceptibility to psoriasis. Hum. Mol. Genet. 1999;8:1135–1140. doi: 10.1093/hmg/8.6.1135. [DOI] [PubMed] [Google Scholar]
- Tiilikainen A, Lassus A, Karvonen J, Vartiainen P, Julin M. Psoriasis and HLA-Cw6. Br. J. Dermatol. 1980;102:179–184. doi: 10.1111/j.1365-2133.1980.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Umland S.P, Garlisi C.G, Shah H, Wan Y, Zou J, Devito K.E, Huang W.M, Gustafson E.L, Ralston R. Human ADAM33 messenger RNA expression profile and post-transcriptional regulation. Am. J. Respir. Cell Mol. Biol. 2003;29:571–582. doi: 10.1165/rcmb.2003-0028OC. [DOI] [PubMed] [Google Scholar]
- Van Eerdewegh P, Little R.D, Dupuis J, Del Mastro R.G, Falls K, Simon J, Torrey D, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- Vassilatis D.K, et al. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl Acad. Sci. USA. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal C.D, et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am. J. Hum. Genet. 2002;71:554–564. doi: 10.1086/342289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelin J, Pulkkinen V, Rehn M, Pirskanen A, Räisänen-Sokolowski A, Laitinen A, Laitinen L.A, Kere J, Laitinen T. Characterization of GPRA, a novel G protein-coupled receptor related to asthma. Am. J. Respir. Cell Mol. Biol. 2005 doi: 10.1165/rcmb.2004-0405OC. Published online 9 June 2005. ( doi:10.1165/rcmb.2004-0405OC.) [DOI] [PubMed] [Google Scholar]
- Weiss S.T, Raby B.A. Asthma genetics 2003. Hum. Mol. Genet. 2004;13(Spec No. 1):R83–R89. doi: 10.1093/hmg/ddh080. [DOI] [PubMed] [Google Scholar]
- Werner M, Herbon N, Gohlke H, Altmuller J, Knapp M, Heinrich J, Wjst M. Asthma is associated with single-nucleotide polymorphisms in ADAM33. Clin. Exp. Allergy. 2004;34:26–31. doi: 10.1111/j.1365-2222.2004.01846.x. [DOI] [PubMed] [Google Scholar]
- Wjst M, et al. A genome-wide search for linkage to asthma. German Asthma Genetics Group. Genomics. 1999;58:1–8. doi: 10.1006/geno.1999.5806. [DOI] [PubMed] [Google Scholar]
- Xu Y.L, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Zagha E, et al. Dipeptidyl peptidase 10 modulates Kv4-mediated A-type potassium channels. J. Biol. Chem. 2005;280:18 853–18 861. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Leaves N.I, Anderson G.G, Ponting C.P, Broxholme J, Holt R, Edser P, et al. Positional cloning of a quantitative trait locus on chromosome 13q14 that influences immunoglobulin E levels and asthma. Nature Genet. 2003;34:181–186. doi: 10.1038/ng1166. [DOI] [PubMed] [Google Scholar]