Abstract
Peach harvest workers were evaluated for exposure to azinphosmethyl residues by measuring foliar residues, urinary alkylphosphate metabolites, butyrylcholinesterase (BChE), acetylcholinesterase (AChE), and dermal residues using clothing and skin washes. Workers entered orchards 51 days after application and worked in treated fields for 10 of the next 17 days. Dislodgeable foliar residues ranged from 0.82 to 1.72 microg/cm2 and did not change significantly over the study period. Combined mean dermal exposure for the 3 consecutive monitoring days was 32 mg and ranged from 17.9 to 60.5 mg. Overall mean excretion levels for the 5 monitoring days were 1.7 mg dimethylphosphate and 1.9 mg dimethlythiophosphate. There was no significant difference in BChE between the exposed harvesters and minimally exposed sorters. The exposed group had significantly lower AChE values than the sorters for 2 post-exposure blood draws by three testing methods, while no significant difference was found for the pre-exposure blood draw. The AChE values for the post-exposure blood samples for the exposed workers decreased significantly about 10-20% over the 3-week exposure period but increased or remained constant for the sorters. Urinary metabolite excretion increased with continuous exposure and was inversely correlated with both AChE and BChE but was not correlated with dermal exposure measurements. High correlations were generally observed between AChE measurements taken in the field using a new spectrophotometric kit and laboratory AChE measurements.
Full text
PDF
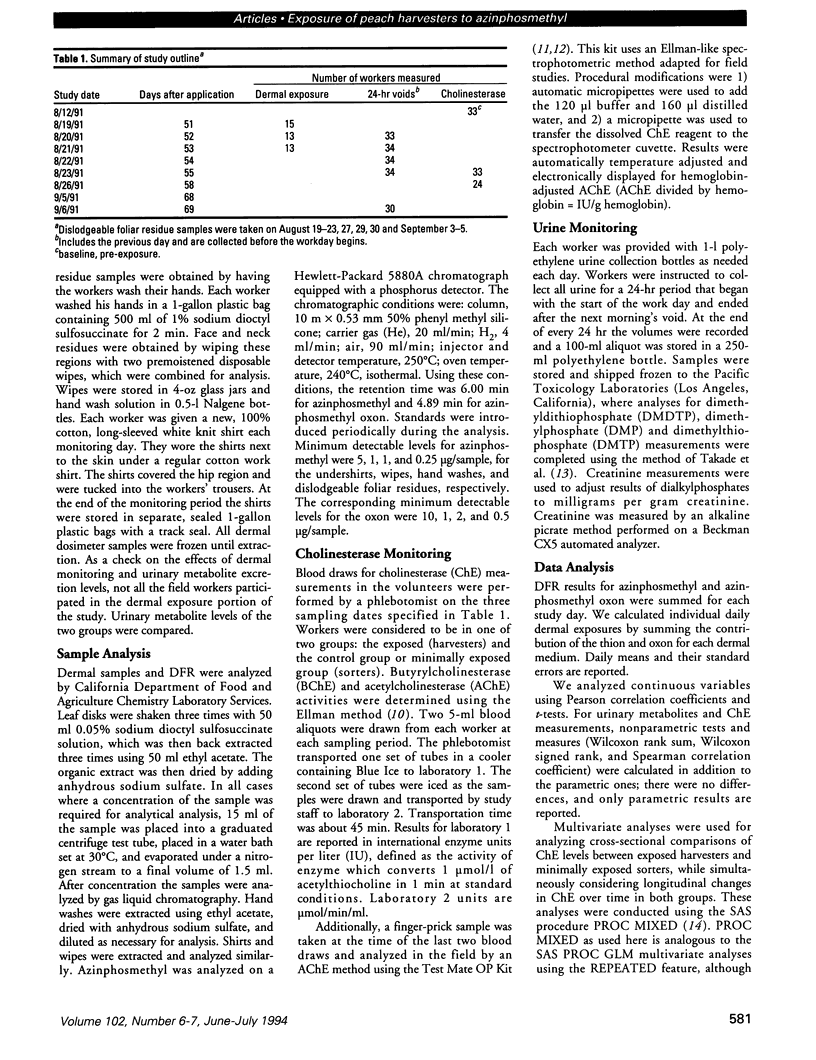


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Feldmann R. J., Maibach H. I. Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol. 1974 Apr;28(1):126–132. doi: 10.1016/0041-008x(74)90137-9. [DOI] [PubMed] [Google Scholar]
- Franklin C. A., Fenske R. A., Greenhalgh R., Mathieu L., Denley H. V., Leffingwell J. T., Spear R. C. Correlation of urinary pesticide metabolite excretion with estimated dermal contact in the course of occupational exposure to Guthion. J Toxicol Environ Health. 1981 May;7(5):715–731. doi: 10.1080/15287398109530014. [DOI] [PubMed] [Google Scholar]
- Franklin C. A., Muir N. I., Moody R. P. The use of biological monitoring in the estimation of exposure during the application of pesticides. Toxicol Lett. 1986 Oct;33(1-3):127–136. doi: 10.1016/0378-4274(86)90077-9. [DOI] [PubMed] [Google Scholar]
- Gunther F. A., Westlake W. E., Barkley J. H., Winterlin W., Langbehn L. Establishing dislodgeable pesticide residues on leaf surfaces. Bull Environ Contam Toxicol. 1973 Apr;9(4):243–249. doi: 10.1007/BF01684832. [DOI] [PubMed] [Google Scholar]
- Kraus J. F., Richards D. M., Borhani N. O., Mull R., Kilgore W. W., Winterlin W. Physiological response to organophosphate residues in field workers. Arch Environ Contam Toxicol. 1977;5(4):471–485. doi: 10.1007/BF02220926. [DOI] [PubMed] [Google Scholar]
- Magnotti R. A., Jr, Dowling K., Eberly J. P., McConnell R. S. Field measurement of plasma and erythrocyte cholinesterases. Clin Chim Acta. 1988 Sep 15;176(3):315–332. doi: 10.1016/0009-8981(88)90190-8. [DOI] [PubMed] [Google Scholar]
- Magnotti R. A., Jr, Eberly J. P., Quarm D. E., McConnell R. S. Measurement of acetylcholinesterase in erythrocytes in the field. Clin Chem. 1987 Oct;33(10):1731–1735. [PubMed] [Google Scholar]
- Maibach H. I., Feldman R. J., Milby T. H., Serat W. F. Regional variation in percutaneous penetration in man. Pesticides. Arch Environ Health. 1971 Sep;23(3):208–211. doi: 10.1080/00039896.1971.10665987. [DOI] [PubMed] [Google Scholar]
- McConnell R., Cedillo L., Keifer M., Palomo M. R. Monitoring organophosphate insecticide-exposed workers for cholinesterase depression. New technology for office or field use. J Occup Med. 1992 Jan;34(1):34–37. [PubMed] [Google Scholar]
- Richards D. M., Kraus J. F., Kurtz P., Borhani N. O., Mull R., Winterlin W., Kilgore W. W. A controlled field trial of physiological responses to organophosphate residues in farm workers. J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):493–512. [PubMed] [Google Scholar]
- Takade D. Y., Reynolds J. M., Nelson J. H. 1-(4-Nitrobenzyl)-3-(4-tolyl)triazene as a derivatizing reagent for the analysis of urinary dialkyl phosphate metabolites of organophosphorus pesticides by gas chromatography. J Agric Food Chem. 1979 Jul-Aug;27(4):746–753. doi: 10.1021/jf60224a003. [DOI] [PubMed] [Google Scholar]