Abstract
In a prospective cross-sectional ultrasound study the size of the fetal lumbar spinal canal was evaluated to determine reference values for the lumbar part of the vertebral canal. One hundred and sixty-seven pregnant women undergoing routine obstetric ultrasound were studied between 16 and 41 weeks of gestation. Exclusion criteria consisted of structural fetal anomalies or growth restriction. Area and volume of the vertebral canal at L1, L3 and L5 were calculated by three-dimensional (3D) ultrasound. Length of the lumbar spine was also determined. The size of the spinal canal and spinal length correlated well with gestational age. No gestational-age-dependent differences in area and volume measurements between upper and lower lumbar spine were found. The results provide an in vivo assessment of the spinal canal by 3D ultrasound over the entire gestation period.
Keywords: development, fetal spine, three-dimensional sonography, vertebral canal
Introduction
The human fetal spine reaches maturity early in life (Gepstein et al. 1991; Larsen & Smith, 1995). Importantly, the cross-sectional area of the mid-lumbar canal has already reached its adult size by the age of 1 year (O'Rahilly et al. 1980; Papp et al. 1994). Defective development at this stage is of clinical significance as a small lumbar vertebral canal is considered to be a significant risk factor for back pain syndromes in adulthood (Roth et al. 1976; Mooney, 1983; Papp & Porter, 1994).
Anatomical (Ursu et al. 1996) and histological (Noback & Robertson, 1951) in vitro studies have described the pattern of intrauterine growth of the fetal spine. While radiographic methods were used in the past (Flecker, 1942), nowadays in vivo evaluation of spinal development can be performed by ultrasound (Raghavendra, 1985; Budorick et al. 1995). An accurate knowledge of the normal spinal appearance is a prerequisite for diagnosing neural tube defects.
The fetal spine becomes visible by ultrasound at around 14 weeks of gestation (Raghavendra, 1985). There are several planes to examine the fetal spine sonographically. In the transverse plane, the three ossification centres can be detected as echogenic foci. In the longitudinal plane, the spine has a ‘rail-road track’ appearance (Raghavendra, 1985; Budorick et al. 1991). With the recent advent of three-dimensional (3D) sonography, the size and development of the fetal spine can be reliably assessed from early in the second trimester (Budorick et al. 1995).
Materials and methods
For our prospective cross-sectional study, 167 women with a singleton pregnancy between 16 and 41 weeks of gestation were recruited during their routine anomaly scan. All subjects were included only once. Inclusion criteria were an appropriate-for-gestational (AGA) age fetus (between the 10th and 90th centile for our reference population) and adequate scanning conditions. For the purpose of our study, only fetuses in a dorsoposterior position, facilitating data acquisition, were included. Exclusion criteria were known or suspected fetal anomalies and chromosomal abnormalities. All ultrasound examinations were performed by R.L.S. on a 530D VOLUSON ultrasound machine (Kretztechnik, Zipf, Austria) with a 5.0-MHz annular array transducer (lateral resolution: 1.38 mm, axial: 0.41 mm). Area and volume of the vertebral canal at L1, L3 and L5 were calculated by 3D ultrasound. Length of the lumbar spine was also determined. The technique for measuring the fetal spine has been described in detail before (Budorick et al. 1995; Riccabona et al. 1995, 1996; Wallny et al. 1999).
Statistical analysis
An SPSS program was used to test the relationship between ultrasound results and gestational age. Regression slopes for mean values and normal ranges (5th and 95th centiles) were calculated according to a method proposed by Royston & Wrigh (1998). A P-value of < 0.05 was considered statistically significant.
Results
The mean gestational age was 26.7 weeks. In the first 30 subjects to be studied, the area and volume of the spinal canal were measured at the level of each lumbar vertebral body. When measurements restricted to three levels (L1, L3 and L5) were compared, no statistically significant difference was apparent (P > 0.05).
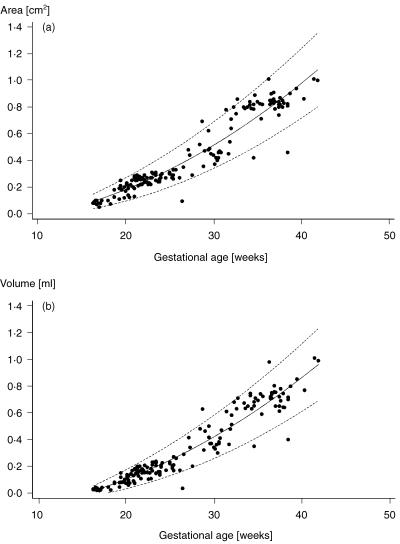
The area and volume of the vertebral canal showed close correlation with gestational age. Advancing gestational age was characterized by a statistically significant increase in all volumetric and conventional two-dimensional (2D) measurements. The relationship between area, volume and gestational age with 95% confidence intervals is plotted in Figs 1 and 2. A close correlation could also be found between lumbar spine length, head circumference, abdominal circumference and femur length (r = 0.91–0.97). Correlation coefficients between conventional biometric data and spinal measurements, gestational age and spinal measurements, respectively, are shown in Table 1.
Fig. 1.
(a) Cross-sectional area and (b) volume of the spinal canal at the first lumbar vertebra are plotted against gestational age with 95% confidence intervals, respectively.
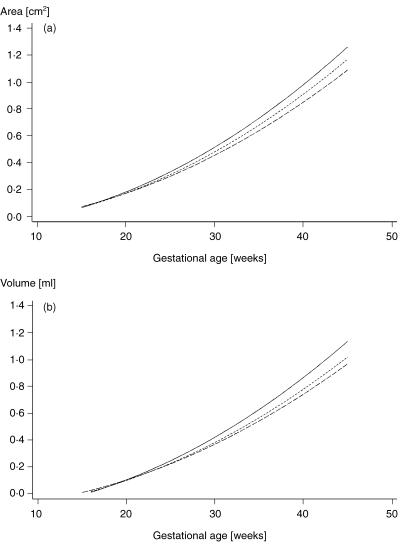
Fig. 2.
(a) Cross-sectional area and (b) volume of the spinal canal at the first (—), third (– –) and fifth (----) lumbar vertebra are plotted against gestational age, respectively.
Table 1.
Correlation (Pearson's correlation coefficient) between gestational age and spinal/conventional biometric measurements, as well as between standard biometry and spinal values. HC, head circumference; AC, abdominal circumference; FL, femur length; L a, area of the spinal canal; L v, volume of the spinal canal; LL, lumbar length; GA, gestational age
| HC | AC | FL | L1 a | L1 v | L3 a | L3 v | L5 a | L5 v | LL | |
|---|---|---|---|---|---|---|---|---|---|---|
| GA | 0.973 | 0.978 | 0.981 | 0.961 | 0.958 | 0.961 | 0.961 | 0.959 | 0.957 | 0.979 |
| HC | 0.926 | 0.924 | 0.923 | 0.887 | 0.911 | 0.879 | 0.970 | |||
| AC | 0.941 | 0.943 | 0.937 | 0.885 | 0.925 | 0.881 | 0.979 | |||
| FL | 0.931 | 0.929 | 0.927 | 0.893 | 0.914 | 0.878 | 0.975 |
The regression equations for area and volume of the spinal canal for the first, third and fifth lumbar vertebrae relative to gestational age are presented in Table 2.
Table 2.
Regression equations for area and volume of the spinal canal relative to gestational age. GA, gestational age; L, lumbar vertebra; SE, standard error; r, multiple regression coefficient; P, significance of regression
| Regression equation | SE | r | P | |
|---|---|---|---|---|
| Area L1 | −0.09575 + 0.000689 * ga2 | 0.34853 | 0.9502 | 0.0001 |
| Volume L1 | −0.1687 + 0.000670 * ga2 | 0.33066 | 0.9513 | 0.0001 |
| Area L3 | −0.08553 + 0.000644 * ga2 | 0.30997 | 0.9483 | 0.0001 |
| Volume L3 | −0.1533 + 0.000593 * ga2 | 0.24307 | 0.9126 | 0.0001 |
| Area L5 | −0.07620 + 0.000614 * ga2 | 0.30157 | 0.9329 | 0.0001 |
| Volume L5 | −0.1455 ± 0.000567 * ga2 | 0.27505 | 0.9046 | 0.0001 |
No gestational-age-dependent differences regarding area and volume measurements between upper and lower lumbar spine were apparent (see Fig. 2).
Discussion
Sonographic evaluation of the fetal spine is an important part of obstetric anomaly scans (Budorick et al. 1995). Prenatal diagnosis may also lead to interesting aspects concerning back pain in adults as maturity of the fetal spinal canal is reached surprisingly early (Clark et al. 1986). In a previous report we were able to demonstrate that 3D ultrasound is a valuable tool in the assessment of the fetal spinal canal (Wallny et al. 1999). The purpose of the current study was to provide additional data throughout pregnancy. Unlike Papp et al. (1994), we could not find significant gestational-age-dependent differences in the size of upper and lower lumbar spine. It should, however, be emphasized that in vivo data cannot be compared with the findings of an archaeological study.
Further data will show if our reference values may facilitate early detection of disorders of the vertebral canal. In an archaeological study, Papp & Porter (1994) found a significantly larger cross-sectional area at L5 proximal to spina bifida occulta lesions compared with unaffected spines. As early detection of these lesions is difficult, our reference values may help to improve accuracy of prenatal ultrasound.
The development of the lumbar vertebral is of clinical significance. A small vertebral canal is considered to be one of the causes for back pain in later life (Roth et al. 1976; O'Rahilly et al. 1980; Porter et al. 1980), but these syndromes are multifactorial. In a small spinal canal, disc lesions, osteophytes or segmental instability cause earlier compression of neural structures with a concomitant increase in the risk of symptomatic disk lesion (Mooney, 1983; Porter et al. 1980). Since interpedicular diameter of the spinal canal at the level of L1–L4 at birth is approximately 70% of the adult size, any kind of intra-uterine growth impairment will affect further development (Ursu et al. 1996). Porter et al. (1987) described a correlation between health status and spinal canal size in that fetal malnutritution may have an adverse effect on its growth. These authors suggested the vertebral canal as a morphometric indicator of neural development. Our results confirm the close correlation between fetal head circumference and spinal canal development.
With 3D ultrasound we may be able to predict occurrence of a narrow lumbar spinal canal. Further investigations on patients with spinal abnormalities are under way.
References
- Budorick NE, Pretorius DH, Grafe MR, Lou KV. Ossification of the fetal spine. Radiology. 1991;181:561–565. doi: 10.1148/radiology.181.2.1924805. [DOI] [PubMed] [Google Scholar]
- Budorick NE, Pretorius DH, Nelson TR. Sonography of the fetal spine. Technique, Imaging findings, and clinical Implications. Am. J. Roentgenol. 1995;164:421–428. doi: 10.2214/ajr.164.2.7839982. [DOI] [PubMed] [Google Scholar]
- Clark GA, Hall NR, Armelagos GJ, Borkan GA, Panjabi MM, Wetzel FT. Poor growth prior to early childhood: Decreased health and life-span in the adult. Am. J. Phys. Anthropol. 1986;70:145–160. doi: 10.1002/ajpa.1330700202. [DOI] [PubMed] [Google Scholar]
- Flecker H. Time of appearance and fusion of ossification centers as observed by roentgenographic methods. Am. J. Roentgenol. 1942;47:97–159. [Google Scholar]
- Gepstein R, Folman Y, Sagiv P, David Ben Y, Hallel T. Does the anteroposterior diameter of the bony canal reflect ist size? An anatomical study. Surg. Radiol. Anat. 1991;3:289–291. doi: 10.1007/BF01627760. [DOI] [PubMed] [Google Scholar]
- Larsen JL, Smith D. Size of the subarachnoid space in stenosis of the lumbar canal. Acta Radiol. 1995;21:627–632. doi: 10.1177/028418518002100509. [DOI] [PubMed] [Google Scholar]
- Mooney V. The syndromes of low back pain. Orthopedic Clinics North Am. 1983;14:401–408. [PubMed] [Google Scholar]
- Noback CR, Robertson GG. Sequences of appearance of ossification centers in the human skeleton during the first five prenatal months. Am. J. Anat. 1951;89:1–28. doi: 10.1002/aja.1000890102. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Muller F, Meyer DB. The human vertebral column at the end of the embryonic period. I. The whole column. J. Anat. 1980;131:565–575. [PMC free article] [PubMed] [Google Scholar]
- Papp T, Porter RW. Changes of the lumbar spinal canal proximal to spina bifida occulta. Spine. 1994;19:1508–1511. doi: 10.1097/00007632-199407000-00017. [DOI] [PubMed] [Google Scholar]
- Papp T, Porter RW, Aspden RM. The growth of the lumbar vertebral canal. Spine. 1994;19:2770–2773. doi: 10.1097/00007632-199412150-00006. [DOI] [PubMed] [Google Scholar]
- Porter RW, Hibbert C, Wellman P. Backache and the lumbar spinal canal. Spine. 1980;5:99–15. doi: 10.1097/00007632-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Porter RW, Drinkall JN, Porter DE, Thorp L. The vertebral canal. II. Health and academic status, a clinical study. Spine. 1987;12:907–911. [PubMed] [Google Scholar]
- Raghavendra BN. Ultrasonography of the spine and the spinal cord. In: Sanders RC, Hill MC, editors. Ultrasound Annual. New York: Raven Press; 1985. pp. 227–249. [Google Scholar]
- Riccabona M, Pretorius DH, Nelson TR, Davidson TE. Distance and Volume measurements using three dimensional ultrasound. Ultrasound Med. Biol. 1995;14:881–886. doi: 10.7863/jum.1995.14.12.881. [DOI] [PubMed] [Google Scholar]
- Riccabona M, Johnson D, Pretorius DH, Nelson TR. Three dimensional ultrasound: display modalities in the fetal spine and the thorax. Eur. J. Radiol. 1996;22:141–145. doi: 10.1016/0720-048x(95)00720-b. [DOI] [PubMed] [Google Scholar]
- Roth M, Krkoskaka J, Toman I. Morphogenesis of the spinal canal, normal and stenotic. Neuroradiology. 1976;10:227–286. doi: 10.1007/BF00327577. [DOI] [PubMed] [Google Scholar]
- Royston P, Wright EM. How to construct normal ranges for fetal variables. Ultrasound Obstetr. Gynecol. 1998;11:30–38. doi: 10.1046/j.1469-0705.1998.11010030.x. [DOI] [PubMed] [Google Scholar]
- Ursu TRS, Porter RW, Navaratnam V. Development of the lumbar and sacral vertebral canal in utero. Spine. 1996;21:2705–2708. doi: 10.1097/00007632-199612010-00001. [DOI] [PubMed] [Google Scholar]
- Wallny T, Schild RL, Fimmers R, Wagner UA, Hansmann ME, Schmitt O. The fetal lumbar spinal canal – a three dimensional study. Ultrasound Med. Biol. 1999;25:1329–1333. doi: 10.1016/s0301-5629(99)00106-4. [DOI] [PubMed] [Google Scholar]