Abstract
Myelin-associated glycoprotein (MAG) has been implicated in inhibition of nerve regeneration in the CNS. This results from interactions between MAG and the Nogo receptor and gangliosides on the apposing axon, which generates intracellular inhibitory signals in the neuron. However, because myelin–axon signaling is bidirectional, we undertook an analysis of potential MAG-activated signaling in oligodendrocytes (OLs). In this study, we show that antibody cross-linking of MAG on the surface of OLs (to mimic axonal binding) leads to the redistribution of MAG into detergent (TX-100)-insoluble complexes, hyperphosphorylation of Fyn, dephosphorylation of serine and threonine residues in specific proteins, including lactate dehydrogenase and the β subunit of the trimeric G-protein-complex, and cleavage of α-fodrin followed by a transient depolymerization of actin. We propose that these changes are part of a signaling cascade in OLs associated with MAG function as a mediator of axon–glial communication which might have implications for the mutual regulation of the formation and stability of axons and myelin.
Keywords: Antibody cross-linking, axo–glia interactions, fyn, fodrin
INTRODUCTION
CNS myelin is a unique, lipid-rich biological membrane that is produced by oligodendrocytes (OLs) (Pfeiffer et al., 1993; Madison et al., 1999). In addition to its important physiological role in facilitating nerve conduction, myelin also inhibits axonal regeneration (Schwab et al., 1993; Woolf and Bloechlinger, 2002). Although this might be important in the regulation of unwanted nerve sprouting in the mature nervous system, it severely limits neuron recovery after injury. Myelin associated glycoprotein (MAG), a sialic acid-binding protein on the periaxonal myelin membrane, is implicated in the inhibition of nerve regeneration (Vyas and Schnaar, 2001; Weiss et al., 2001; Spencer et al., 2003) through its interaction with molecules on axonal plasma membranes, such as microtubule-associated protein 1B (Franzen et al., 2001), gangliosides GD1a and GT1b (Kelm et al., 1994; Crocker et al., 1996; Vinson et al., 2001; Vyas et al., 2002), and the recently discovered glycosylphosphatidylinositol (GPI)-linked Nogo receptor (neuronal receptor for Nogo, another myelin inhibitor of axonal regeneration) (Fournier et al., 2001; Domeniconi et al., 2002; Liu et al., 2002). Binding of an extracellular domain of MAG to apposing molecules on the axonal surface generates an inhibitory signal in the neuron that involves p75, RhoA and Rac1 signaling (Niederost et al., 2002; Wang et al., 2002). In addition, intracellular domains of MAG bind to microtubules (Kursula et al., 2001) and Fyn tyrosine kinase (Umemori et al., 1994; Umemori et al., 1999) in OLs.
Myelin–axolemmal interactions regulate many cellular and molecular events (Menon et al., 2003). Axons elicit signals that modify oligodendrocyte gene expression, signal transduction and survival, and provide metabolic precursors (Friedrich and Mugnaini, 1983; Chakraborty et al., 1999; Chakraborty et al., 2001; LoPresti et al., 2001). Conversely, OLs and Schwann cells regulate axon caliber, microtubular properties and ion-channel clustering at nodes of Ranvier (Aguayo et al., 1979; Sanchez et al., 1996; Brady et al., 1999; Kirkpatrick et al., 2001; Rasband and Trimmer, 2001; Dashiell et al., 2002). Although some of the cellular and molecular mechanisms that control these processes have been described, myelin–axon signaling mechanisms are still poorly understood.
Glycosphingolipids and cholesterol form microdomains in the plasma membrane of cells (termed rafts) into which some proteins can partition and others are excluded (Simons and Ikonen, 1997; Brown and London, 1998; Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998; Taylor et al., 2002; Taylor et al., 2004). Lipid rafts have an important role as platforms for the initiation of signal transduction by favoring specific protein–protein interactions (Simons and Toomre, 2000). Using biochemical criteria to identify proteins in rafts, it has been shown that in myelin the GPI-linked proteins NCAM-120 and contactin, the doubly acylated proteins Fyn and Lyn kinases, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) and myelin oligodendrocyte glycoprotein (MOG) partition into rafts (Kim et al., 1995; Kramer et al., 1997; Kim and Pfeiffer, 1999; Kramer et al., 1999; Simons et al., 2000; Taylor et al., 2002), whereas MAG does not. Cross-linking of some proteins to either ligand or antibody can result in their enhanced partitioning into rafts and participation in early signal-transduction events (Simons and Toomre, 2000). Previous studies have validated the use of antibodies to mimic ligand binding (Atashi et al., 1992; Simons and Toomre, 2000; Filatov et al., 2003). For example, we have shown that whereas ∼40% of MOG in myelin is associated with detergent-insoluble complexes that are characteristic of rafts, MOG in OLs in culture is nearly entirely excluded from rafts (Marta et al., 2003). However, antibody cross-linking of MOG in OLs in culture results in its increased association with lipid rafts, and leads to rapid, novel signal-transduction events and pronounced morphological changes in OLs (Marta et al., 2003).
In the present study, we sought to identify OL signaling molecules involved in axon–glial interaction through MAG. We show that antibody cross-linking of MAG on the surface of OLs (to potentially mimic axonal binding) leads to a significant redistribution of MAG into TX-100 insoluble fractions (which are associated with lipid rafts and the cytoskeleton), increased phosphorylation of Fyn, dephosphorylation of serine and threonine residues in specific proteins, such as lactate dehydrogenase (LDH), and Gβ, cleavage of α-fodrin, and a transient depolymerization of actin. We propose that these changes are part of a MAG-mediated bidirectional signaling cascade between the axolemma and myelin that is associated with the control of myelin formation and stability on the one hand, and axon physiology, including inhibition of axonal regeneration, on the other.
OBJECTIVES
Bidirectional signaling between the myelin sheath and the axon influences crucial aspects of OL and neuron physiology. These include events that are regulated by MAG, a protein apposed to the axolemmal membrane on the inner lamella of the myelin sheath. Based on our recent observations of MOG, another myelin protein, we hypothesized that MAG signaling is initiated in glycosphingolipid–cholesterol microdomains (‘lipid rafts’). To test this hypothesis, we antibody cross-linked MAG on the OL cell surface to potentially mimic the axonal binding of ligand, analyzed the repartitioning of MAG into detergent-insoluble fractions that are characteristic of lipid rafts or cytoskeletal association, and evaluated by proteomic analysis of phosphoproteins and cytoskeletal proteins the signal-transduction cascade that was generated.
METHODS
Materials
Anti-MAG 513mAb, which recognizes an external, conformation-dependent epitope of MAG (Poltorak et al., 1987), was purified from its hybridoma supernatant in Dr. R. Quarles's laboratory. Rabbit polyclonal MAG (used for Western blotting) was obtained from Dr. J. Roder. O4 mAb (Sommer and Schachner, 1981) was prepared in our laboratory (Bansal and Pfeiffer, 1989). Other antibodies were obtained as follows: anti-phosphoserine and anti-phosphothreonine (Zymed); anti-phosphotyrosine (4G10), anti-Fyn and goat anti-mouse IgG (Transduction Laboratories); anti-Gβ1-4 (Santa Cruz Biotechnology Inc); anti-α-fodrin, and horseradish peroxidase-conjugated goat anti-mouse IgG (Chemicon); anti-β-tubulin (Sigma).
Vanadate, GT1b, nocodazol, taxol, cytochalasin D and Hoechst 33342 were obtained from Sigma. ASB-14 was obtained from Calbiochem. Texas Red-X phalloidin was obtained from Molecular Probes. Protein G Sepharose was obtained from Amersham Biosciences.
All solutions were prepared with MilliQ H2O. Protein concentrations were determined using the DC protein assay kit (Bio-Rad).
Cell culture
Mixed primary cultures and highly enriched populations of maturing OLs were prepared and maintained as described previously (Pfeiffer et al., 1993; Bansal et al., 1996). OL populations were grown in defined medium [modified N2 (mN2)] (Bottenstein and Sato, 1979; Gard and Pfeiffer, 1989) for 5–6 days to obtain OLs that expressed MAG.
MAG cross-linking
OLs grown in mN2 medium were washed with 1% bovine serum albumin (BSA) in Dulbecco's modified Eagle's medium (DMEM) and incubated for 15 minutes at 37°C with monoclonal anti-MAG antibody (513mAb, 1:100; directed against an extracellular epitope) diluted in freshly prepared mN2. The antibody was washed out by two changes of DMEM. Goat anti-mouse IgG (1:500, diluted in DMEM) was added for 15 minutes at 37°C. In some cases, the antibody-containing medium was removed and cells incubated with fresh medium for an additional 14 hours. In other cases, cells were incubated with 10 μM nocodazol for 3 hours at 37°C, 20 μM cytochalasin D for 3 hours at 37°C, and 20 μM taxol for 3 hours at 37°C before adding antibodies. Other conditions included incubation with GT1b [2–10 μg/mL (Vinson et al., 2001)] for 30, 60 or 120 minutes, and incubation with GT1b for either 15 or 120 minutes followed by further incubation with anti-MAG antibody and anti-mouse IgG to cross-link MAG–anti-MAG complexes. Controls were subjected to the same schedule of washes and incubations.
Immunofluorescence microscopy
After cross-linking, OL wells were put on ice and the antibody washed out by two changes of HEPES-buffered Earle's balanced salt solution (EBSS-HEPES).
Cells were incubated with EBSS-HEPES containing 3% normal goat serum (NGS) (also used for diluting antibodies) to block nonspecific absorption, and live cells were stained for 20 minutes at 4°C with either O4 mAb (1:25) or anti-MAG (513mAb, 1:100). Cells were then incubated with the appropriate secondary antibody for 20 minutes at 4°C: FITC-conjugated goat anti-mouse IgM (1:50, μ-chain specific, Chemicon) for O4; and goat anti-mouse IgG (1:500, γ-chain specific, Jackson ImmunoResearch) for MAG. In some cases, cells were fixed with 4% paraformaldehyde, permeabilized with 0.05% saponin for 3 minutes at room temperature and either stained with anti-MAG (1:100) or permeabilized with 0.3% TX-100 for 5 minutes at room temperature and stained with anti-β-tubulin (1:50), followed by the appropriate secondary antibodies for 20 minutes, or with Texas Red-X phalloidin (which binds to polymerized actin) for 20 minutes. A nuclear counterstain (1 μg ml−1 Hoechst dye 33342) was included with the secondary antibodies. Washing between steps was performed with three 5-minute-changes of 1% NGS and EBSS-HEPES. Cells were mounted in 50% glycerol, pH 8.6 and 2.5% diazobicyclo-(2,2,2) octane to suppress fading and examined by epifluorescence microscopy.
Preparation of cell lysates
After cross-linking, plates were put on an ice-tray and washed twice with ice-cold phosphate-buffered saline (PBS). Cells were scraped into 0.5 ml of 150 mM NaCl, 5 mM EDTA, 25 mM Tris-Cl buffer containing 1 mM PMSF, 10 μg ml−1 leupeptin/aprotinin, 50 mM NaF, 10 mM NaP2O7 and 1 mM Na o-Vanadate (scraping buffer) and passed 10 times (on ice) through a 30G needle.
Detergent extraction and sucrose-gradient ultracentrifugation
Triton X-100 (1% final concentration) was added to cell lysates and incubated for 30 minutes at either 4°C or 37°C (Kim and Pfeiffer, 1999; Taylor et al., 2002). The TX-100 extracts were centrifuged (13 000×g, 4°C for 10 minutes) to separate detergent-insoluble pellet and detergent-soluble supernatant fractions.
The supernatant fraction was precipitated with 2 volumes of ethanol at −20°C overnight, centrifuged (13 000×g, 4°C for 10 minutes) and the new supernatant discarded. Finally, both the original detergent-insoluble pellet and the ethanol-precipitated soluble-fraction pellets were solubilized in equal volumes of either sample-loading buffer or RIPA buffer for SDS-PAGE or immunoprecipitation, respectively, to compare the relative amount of each protein in the two fractions.
For sucrose-gradient ultracentrifugation, TX-100-insoluble pellet fractions were resuspended in 0.5 ml of scraping buffer plus 1% TX-100, mixed with 2 M sucrose (1 ml), overlaid with 1 M (2 ml) and 0.2 M (1.5 ml) sucrose, and centrifuged for 16 hours at 45 000 rpm (SW 55 Ti,∼200 000×g, Beckman) at 4°C. After centrifugation, 0.5 ml fractions were collected at 4°C, from the top to the bottom of the gradient.
Cholesterol extraction
To disrupt cholesterol in OL membranes before TX-100 extraction, cell lysates were treated with saponin (final concentration 0.2%) on ice for 30 minutes and centrifuged (13 000×g, 10 minutes) (Kim and Pfeiffer, 1999). The supernatants (S1) were collected. The pellets were extracted with 0.5 ml of 1% TX-100 in scraping buffer for 30 minutes (above), centrifuged (13 000×g, 10 minutes), and the TX-100 soluble fractions (S2) and pellets separated. Supernatants S1 and S2 were precipitated with ethanol at −20°C overnight and centrifuged (13 000×g, 10 minutes). Pellets and ethanol-precipitated supernatant fractions were resuspended in equal volumes (to compare the relative amount of each protein in the two fractions) of sample buffer for analysis by SDS-PAGE.
Immunoprecipitation
Equal volumes of the soluble and insoluble fractions (see above) of the various TX-100 extracts were solubilized in 500 μl of RIPA buffer (10 mM Tris pH 7.6, 150 mM NaCl, 0.1% SDS, 1% deoxycholate, 1% NP-40 and 1% TX-100) containing 1 mM PMSF, 10 μg ml−1 leupeptin/aprotinin and 1 mM Na o-Vanadate. Samples were incubated overnight with anti-phosphotyrosine (4G10, 1:100) at 4°C. Protein G Sepharose beads (0.01 μg ml−1) were added for 2 hours at 4°C. Immunocomplexes were separated by centrifugation (13 000×g, 4°C, 10 minutes) and washed four times with RIPA buffer. Supernatants were ethanol precipitated (see above). Immunocomplexes and supernatants were solubilized in equal volume of 50 mM Tris-HCl pH 6.8, 2.5% glycerol, 5% SDS, 4 M urea, 0.01% bromophenol blue and 10 mM DTT, for analysis by SDS-PAGE.
SDS-PAGE and Western-blotting
Equal volumes of the soluble and insoluble fractions (see above) of the various TX-100 extracts were solubilized in sample buffer (50 mM Tris-HCl pH 6.8, 2.5% glycerol, 5% SDS, 4 M urea, 0.01% bromophenol blue and 10 mM DTT), loaded onto acrylamide gels (Protean II mini-cell apparatus, Bio-Rad), and run at constant voltage (120 V for 1–2 hours). The proteins were transferred to PVDF membranes (Hybond-P, Amersham Biosciences) at 4°C with a constant voltage (100 V, 1 hour). The blots were blocked with either 5% non-fat milk or 5% BSA (Sigma) for 1 hour at room temperature, depending on the antibody before immunostaining and detection using enhanced chemiluminescence (ECL Plus, Amersham Biosciences).
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)
Mature OL cells were scraped into 25 mM Tris-Cl buffer (pH 7.5) containing 2% ASB-14, 1 mM PMSF, 10 μg ml−1 leupeptin/aprotinin, 50 mM NaF, 10 mM NaP2O7, and 1 mM Na o-Vanadate. Proteins were precipitated overnight with 2 volumes of ethanol at −20°C. Cell extracts (300 μg of protein) were solubilized in rehydration buffer [7 M urea, 2 M thiourea, 2% ASB-14, 0.5% IPG buffer 3-10 (Amersham Biosciences), 100 mM DTT, 0.001% bromophenol blue]. All samples were left in rehydration buffer for 1 hour at room temperature with occasional mixing before centrifugation (10 000×g, 10 minutes) to clear particulate matter. Sample supernatant was added to IPGphor coffins (Amersham Biosciences) and an Immobiline™ Dry Strip pH 3-10 isoelectricfocusing gel (Amersham Biosciences) was placed over the solution. The IPG strips were allowed to rehydrate overnight. Proteins were separated in the first dimension (200 V, 1 hour; 500 V, 1 hour; 1000 V, 1 hour; ramped to 6000 V, 30 minutes; held at 6000 V for 20 000 Vh) at 20°C using an IPGPhor electrophoresis unit (Amersham Biosciences). After isoelectricfocusing, the gel was equilibrated, first for 15 minutes with 130 mM DTT in an equilibration buffer containing 6 M urea, 50 mM Tris pH 8.8, 30% glycerol, 2% SDS, and, second, for 15 minutes with 135 mM iodoacetamide in the same equilibration buffer. SDS-PAGE was performed in 10% acrylamide running gels at a constant current (15 mA gel−1, 14 hours), using a Hoefer DALT vertical system (Amersham Biosciences). The proteins were transferred to PVDF membranes (Hybond-P, Amersham Biosciences) at constant current (400 mA, 14 hours). In some cases, gels were stained with either ammoniacal silver nitrate or Colloidal Blue (Invitrogen).
RESULTS
Antibody-induced cross-linking of MAG induces its partitioning into a detergent-insoluble fraction
Biochemical studies of glycosphingolipid-cholesterol microdomains often take advantage of their insolubility in non-ionic detergents at low temperatures. In agreement with previous results using purified myelin (Kim and Pfeiffer, 1999; Taylor et al., 2002), when cultures enriched for OLs were extracted with 1% TX-100 at 4°C, nearly all MAG was detected in the soluble fraction (Fig. 1A). However, when we treated MAG-positive OLs at 37°C with anti-MAG antibody (1:100) for 15 minutes before detergent extraction (TX-100, 4°C), ∼50% of MAG was recovered in the detergent-insoluble fraction. When OLs were treated at 37°C for 15 minutes with anti-MAG (primary antibody) followed by an additional 15 minutes with cross-linking secondary antibody (1:500, anti-IgG) nearly all MAG was found in the insoluble fraction (Fig. 1A). In subsequent experiments we used the latter secondary-cross-linking approach. As a control, secondary antibody alone had no effect on MAG partitioning (Fig. 1A). Additional control studies for cross-linking experiments are shown in (Marta et al. 2003). We conclude that cross-linking MAG on the surface of differentiated OLs in culture results in a major change in its detergent solubility properties. We therefore initiated a series of experiments to assess the biochemical characteristics and functional significance of this crosslinking-mediated detergent insolubility.
Fig. 1.
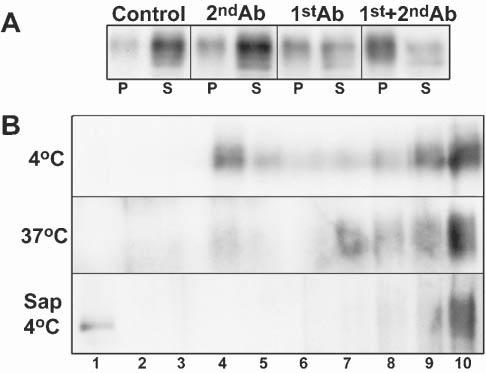
Antibody cross-linking leads to MAG partitioning into detergent-insoluble microdomains. (A) Western blot analyses of MAG from untreated OLs (control) or following incubation for 15 minutes with anti-mouse IgG (1:500, 2ndAb), anti-MAG IgG mAb (1:100, 1stAb) or anti-MAG mAb (1:100) followed by anti-mouse IgG (1:500, 15min; 1st+2ndAb). Cell lysate (10μg of protein) was extracted with TX-100 at 4°C, separated by centrifugation into insoluble (P) and soluble (S) fractions, and the entire yield in each fraction loaded on the gel. (B) OLs were extracted as above, with TX-100 at 4°C, at 37°C and at 4°C after pretreatment of the cell lysate with saponin. The insoluble fractions from MAG-cross-linked cells (anti-MAG + anti-mouse IgG, 50μg protein) were then further fractionated by centrifugation on sucrose gradients (see Methods). Fraction 1, is the top of gradient (i.e. lowest density). The figure represents a typical result of three independent experiments.
Detergent-insoluble MAG has low-density and high-density components
Insolubility of a protein in TX-100 at 4°C can be caused by association with lipid rafts and/or through protein–protein interactions that often involve cytoskeletal elements (Pfeiffer et al., 1993; De Angelis and Braun, 1996; Kim and Pfeiffer, 1999; Taylor et al., 2002; Marta et al., 2003). Therefore, we applied three, additional, well-established biochemical criteria to analyze the properties of detergent-resistant membrane complexes. Following TX-100 extraction at 4°C, the detergent-insoluble, raft-associated proteins float to a characteristic, low density in density (e.g. sucrose) gradients (Brown and Rose, 1992; Simons and Ikonen, 1997). In general, raft-associated proteins that are insoluble in TX-100 at 4°C become solubilized when the extraction is carried out at either 37°C (Brown and Rose, 1992) or 4°C following treatment with the cholesterol-binding agent saponin (Rothberg et al., 1990; Cerneus et al., 1993; Hanada et al., 1995; Stulnig et al., 1997; Ledesma et al., 1998). It has been reported (Marta et al., 2003) that markers of lipid rafts, such as GM1 ganglioside and caveolin (a marker for caveolae, a subgroup of lipid rafts) (Abrami et al., 2001), are enriched in low-density, detergent-insoluble fractions derived from OLs on TX-100 extraction at 4°C, and that they are completely solubilized following either extraction at 37°C or 4°C after pretreatment with saponin. Applying these three additional criteria, we found that detergent-insoluble MAG (TX-100, 4°C) from antibody-cross-linked OLs (above) was distributed between low and heavy-density fractions following flotation on sucrose gradients (Fig. 1B). The material in the low-density fraction was solubilized efficiently by extraction with either TX-100 at 37°C or by pretreatment of the cell lysate with saponin and extraction with TX-100 at 4°C (Fig. 1B). We conclude that a significant fraction of MAG that is detergent-insoluble upon antibody cross-linking is present in lipid rafts.
MAG/anti-MAG complexes are internalized upon antibody cross-linking of MAG
Several studies have linked raft-association of membrane proteins and endocytic uptake (Ikonen, 2001). In mature OLs in culture, MAG was distributed uniformly on the surface of OLs, as seen by staining of native MAG in live cells at 4°C (Fig. 2A). However, after cross-linking MAG at 37°C (treatment with anti-MAG for 15 minutes followed by unlabeled anti-mouse IgG for additional 15 minutes; see Methods), staining of MAG on the surface of live cells at 4°C was significantly reduced (Fig. 2B). In this case, detection of MAG could be achieved only by first permeabilizing cells to allow visualization of internalized antigens (Fig. 2C). Consistent with this, internalized MAG/anti-MAG complexes could also be visualized in unpermeabilized, live cells when a Cy3-labeled secondary antibody was used to cross-link MAG/anti-MAG at 37°C (Fig. 2D). We conclude that MAG/anti-MAG complexes are internalized following cross-linking of MAG.
Fig. 2.
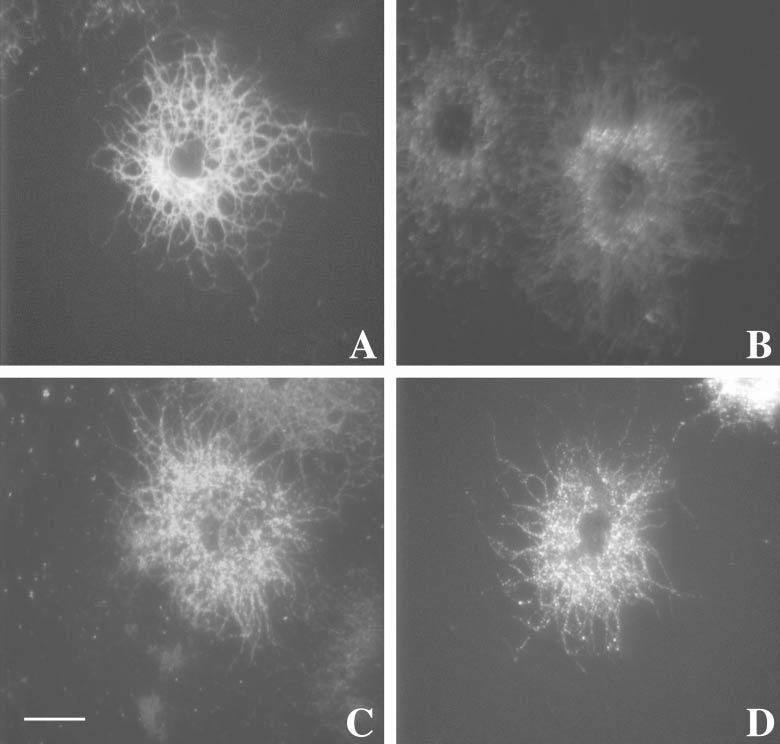
MAG–anti-MAG complexes are internalized upon antibody cross-linking. OLs in culture were either left untreated as controls (A), or (B,C) treated with anti-MAG mAb (1:100 for 15 minutes at 37°C) followed by anti-mouse IgG (1:500 for 15 minutes at 37°C). (A,B) Cells were immunostained live at 4°C with anti-MAG antibodies or (C) fixed (see Methods) and permeabilized prior to immunostaining with anti-MAG. (D) Cells were antibody-treated with anti-MAG mAb (1:100 for 15 minutes at 37°C) followed by a fluorescent-tagged anti-mouse IgG (IgG-Cy3, 1:500 for 15 minutes at 37°C). Cells were then fixed but not permeabilized or further stained before visualization by fluorescence microscopy. Scale bar: 5 μm.
Protein phosphorylation following antibody cross-linking of MAG
In a number of systems, either ligand- or antibody-mediated partitioning of proteins into lipid rafts results in the initiation of signal transduction cascades (Simons and Toomre, 2000). Because phosphorylation of proteins is an integral part of signal-transduction mechanisms, we used immunoprecipitation and 1D- and 2D-SDS-PAGE/Western blotting to examine the phosphorylation state of residues in proteins from untreated and MAG cross-linked OLs (Fig. 3).
Fig. 3.
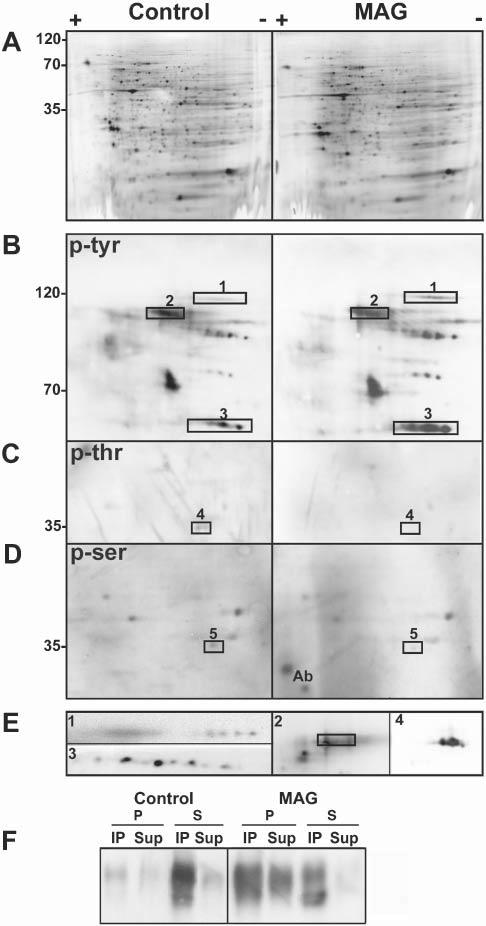
Specific proteins change their phosphorylation status upon antibody cross-linking of MAG. (A–E) Two-dimensional electrophoresis of cell lysates (300 μg total cell protein) from untreated, control OLs in culture and OLs treated with anti-MAG mAb (anti-MAG mAb, 1:100, 15 minutes; anti-mouse IgG, 1:500, 15 minutes) (MAG). Western blots are silver stained (A), and stained for anti-phosphotyrosine (p-tyr) (B), anti-phosphothreonine (p-thr) (C) and anti-phosphoserine (p-ser) (D). (E) Proteins that change their phosphorylation status upon MAG cross-linking identified by immunoblotting with specific antibodies (rectangles indicate position of these proteins in the respective phospho-immunoblot). 1, α-fodrin; 2, MAG, the rectangle encloses the pan-phosphotyrosine signal (2 in B). MAG overlaps with only the lower part of the phosphotyrosine signal because of the presence of other phosphoproteins in that region; 3, fyn; 4, Gβ; and 5, LDH. Ab: reactivity of some remaining antibody light chain. Images are enlarged in terms of pI and molecular weight in B–E compared to A. (F) Control and antibody-treated cells (MAG) were subjected to immunoprecipitation with anti-phosphotyrosine antibody followed by Western blot analysis of MAG. Cell lysates (20 μg) were extracted with TX-100 at 4°C and separated into pellets (P) and supernatants (S) by centrifugation. The entire yield in each fraction was used for immunoprecipitation (see Methods). The immunoprecipitated (IP) and non-immunoprecipitated (Sup) proteins were analyzed by Western blot using anti-MAG. Typical results of three independent experiments are shown.
A similar pattern of proteins in the total OL lysates (silver-stained gels) was observed for control and cross-linked cells (Fig. 3A), indicating that the overall protein content and profile was unchanged following MAG cross-linking. Furthermore, tyrosine phosphorylation of MAG, a known substrate of tyrosine kinases (Jaramillo et al., 1994), was similar in control and cross-linked cells (∼105 kDa, Fig. 3B,E, spot 2; identified by Western blotting). Consistent with this, immunoprecipitation with anti-phosphotyrosine antibody demonstrated that MAG is tyrosine phosphorylated in the detergent-soluble fraction before cross-linking, and in both the soluble and insoluble fractions after cross-linking (Fig. 3F).
By contrast, an increase in tyrosine phosphorylation of Fyn (∼60 kDa) was detected after cross-linking using a pan-tyrosine-phosphate antibody (Fig. 3B,E, spot 3; identified by Western blotting). Additional studies were carried out with antibodies that recognize specific phosphorylated tyrosines in Fyn. Anti-phosphotyrosine 418 (Y418) was unchanged, and anti-Y529 showed no or only minor enhancement in levels of phosphorylation upon cross-linking (data not shown). These data indicate that other tyrosine sites in Fyn must be phosphorylated on antibody cross-linking (see Discussion).
Similar analyses with anti-phosphothreonine and anti-phosphoserine antibodies identified proteins that were dephosphorylated on cross-linking (Fig. 3C,D, spots 4 and 5). These were identified as the 35 kDa β(1-2) subunit of the heterotrimeric G-protein complex (Fig. 3C,E, spot 4; identified by mass spectrometry and Western blotting) and LDH (Fig. 3D, spot 5; identified by mass spectrometry). We conclude that antibody cross-linking of MAG leads to increased phosphorylation of fyn and dephosphorylation of Gβ and LDH.
α-Fodrin cleavage and actin depolymerization following antibody cross-linking of MAG
Analysis with the anti-phosphotyrosine antibody upon cross-linking of MAG also detected the appearance of a ∼120 kDa phosphotyrosine-containing protein. This was identified by mass spectrometry and 2D-PAGE Western blot as a cleavage product of α-fodrin (Fig. 3B,E, spot 1). Further analysis by 1D-Western blot with anti-fodrin showed the presence of α-fodrin fragments of ∼120 kDa, ∼150 kDa and ∼180 kDa in the detergent-soluble fraction after MAG cross-linking (Fig. 4), but not in untreated control cells (intact α-fodrin of 250 kDa is not detected in 1D- and 2D-Western blots because of poor resolution of high molecular weight proteins). These results indicate that cleavage of α-fodrin increases on MAG cross-linking.
Fig. 4.
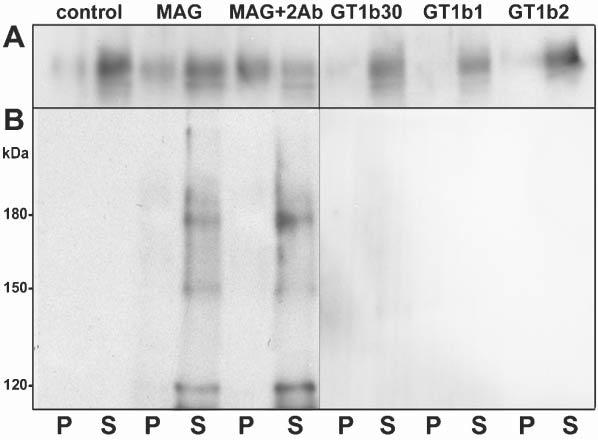
Antibody cross-linking of MAG leads to cleavage of α-fodrin. (A,B) Western blot analyses of MAG (A) and α-fodrin (B) in lysates of untreated OLs (control), and OLs incubated for 15 minutes with anti-MAG mAb (1:100, MAG), with anti-MAG mAb (1:100) followed by anti-mouse IgG (1:500, 15 minutes, MAG+2Ab), and with GT1b (2 μg ml−1) for 30 minutes (GT1b30), 1 hour (GT1b1) and 2 hours (GT1b2). Cell lysates (10 μg protein) were extracted with TX-100 at 4°C and separated into pellets (P) and supernatants (S) by centrifugation, and the entire yield in each fraction loaded on the gel. The figure represents a typical result of three independent experiments. Data shown are for 2 μg ml−1GT1b, the concentration used by (Vinson et al., 2001); concentrations up to 10 μg ml−1 gave similar results.
Cleavage of α-fodrin results in rearrangements of the actin cytoskeleton (Harris and Morrow, 1990). Consistent with this, polymerized actin (estimated by phalloidin staining) became disorganized and significantly reduced on treatment with anti-MAG antibody followed by anti-mouse IgG (Fig. 5B). This process was reversible; actin polymerization returned to control conditions within 14 hours of removing the antibodies (Fig. 5C).
Fig. 5.
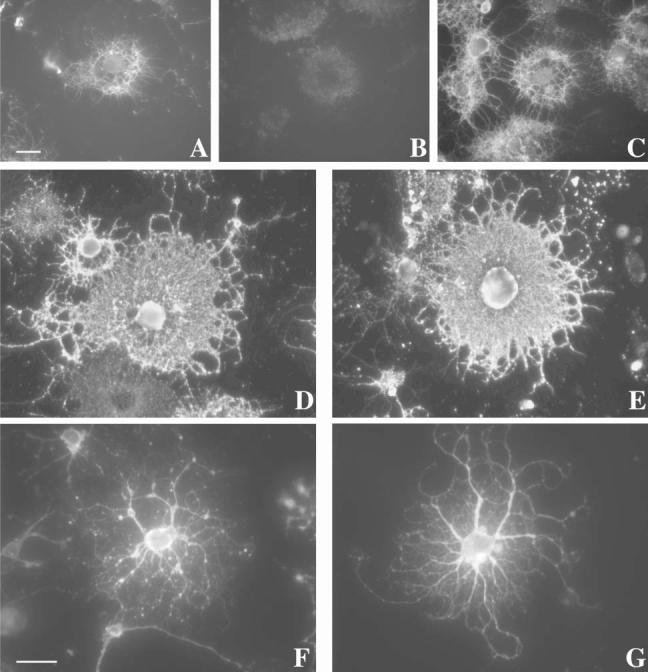
MAG cross-linking leads to a transient depolymerization of actin without major morphological alterations in OLs. OLs in culture were either left untreated (A,D,F) as controls or treated with anti-MAG mAb (1:100, 15 minutes at 37°C) followed by anti-mouse IgG (1:500, 15 minutes at 37°C) (B,C,E,G). In some cases (C), the antibody-containing medium was washed and cells were incubated with fresh medium for an additional 14 hours. Cells were then immunostained (see Methods) with phalloidin (A–C), O4 (D,E) and anti-tubulin (F,G) antibodies for epifluorescence microscopy. Scale bars: 5 μm.
In contrast to actin microfilaments, the morphology of OLs (determined by immunostaining with O4) and microtubule integrity (estimated by anti-tubulin staining after detergent solubilization) was unaltered (Fig. 5D-G). Nevertheless, when OLs were treated first with either nocodazol or cytochalasin D, to depolymerize microtubules and microfilaments, respectively, or taxol, to stabilize microtubules, repartitioning of MAG to the TX-100-insoluble fraction (4°C) was reduced (Fig. 6). This is consistent with previous obsevations that an intact cytoskeleton is necessary for the integrity of rafts in OLs (Holowka et al., 2000; Fassett et al., 2001; Nebl et al., 2002; Marta et al., 2003).
Fig. 6.
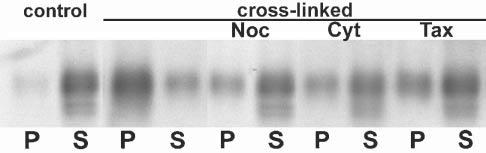
Repartitioning of MAG into detergent-insoluble fractions depends on cytoskeletal integrity. Western blot analysis of MAG from OLs incubated with anti-MAG mAb (1:100, 15 minutes) followed by anti-mouse IgG (1:500, 15 minutes) (cross-linked), and from untreated OLs (control), after 3-hour pretreatment with 10 μM nocodazol (noc), 20 μM cytochalasin D (cyt) and 20 μM taxol (tax). After scraping, cell lysates (10 μg protein) were extracted with TX-100 at 4°C and separated into pellets (P) and supernatants (S) by centrifugation. The entire yield in each fraction was loaded for each condition. The figure represents a typical result of three independent experiments.
GT1b, a ligand for MAG, does not mimic antibody-mediated effects
The ganglioside GT1b binds to MAG and has been proposed as a neuronal receptor for MAG (Vinson et al., 2001). Treatment of OLs with GT1b (2–10 μg ml−1) for 30–120 minutes neither induced the repartitioning of MAG into a detergent-insoluble fraction nor increased the cleavage of α-fodrin (Fig. 4). Moreover, pre-incubation of OLs with GT1b for 15–120 minutes did not prevent MAG repartitioning into the TX-100-insoluble fraction after cross-linking with anti-MAG/anti-mouse IgG (data not shown).
CONCLUSIONS
Antibody cross-linking of MAG on the surface of differentiated OLs in culture results in repartitioning of MAG into detergent-insoluble fractions with biochemical characteristics of lipid rafts; this repartitioning depends on an intact OL cytoskeleton.
MAG/anti-MAG complexes are internalized on antibody cross-linking.
Antibody cross-linking of MAG leads to increased phosphorylation of fyn and dephosphorylation of Gβ and LDH.
Antibody cross-linking of MAG leads to a cleavage of α-fodrin and to a transient depolymerization of actin microfilaments; microtubule integrity and morphology of OLs remain unaltered.
GT1b, a binding ligand for MAG, does not induce repartitioning of MAG or cleavage of α-fodrin.
DISCUSSION
Several lines of evidence indicate that MAG has an important role in the control of axon–glial interactions in the CNS. MAG is localized in the periaxonal myelin membrane, is apposed to the axollema and inhibits axonal regeneration after injury. MAG-null mice exhibit a significant delay of myelination, structural anormalities of myelin sheaths and OL dystrophy with aging (Quarles, 2002). These findings emphasize that MAG-mediated signaling from axons to OLs is needed for efficient myelination and maintenance of healthy, mature oligodendroglia. Although several studies have identified axonal molecules involved in MAG-mediated signaling, little is known about the molecular interactions within the OL.
Cross-linking of membrane proteins is a physiological phenomenon that can lead to the repartitioning of these proteins into lipid rafts, which results in novel protein interactions and the initiation of cell signaling (Simons and Toomre, 2000; Ikonen, 2001). Although this occurs naturally in response to multivalent ligands, similar responses have been observed using antibodies. In this report we show that, although MAG is largely detergent soluble (TX-100, 4°C) in OLs in culture, it becomes repartitioned into a detergent-insoluble fraction after antibody-induced cross-linking (anti-MAG plus secondary antibody). When the detergent-insoluble fraction is floated on sucrose gradients, MAG is distributed between low- and heavy-density fractions. The low-density fraction has characteristics of lipid rafts (low density, sensitive to pretreatment with cholesterol-perturbing agents before detergent extraction at 4°C and sensitive to detergent extraction at 37°C, which renders MAG soluble). The heavy-density fraction is enriched in proteins such as β-tubulin and β-actin, indicating that protein–protein interactions with cytoskeletal proteins are involved (Marta et al., 2003). Moreover, our results indicate that MAG/anti-MAG complexes are internalized upon cross-linking, thus, either endosomes or caveosomes might mediate the signaling that is triggered by antibody cross-linking (Conner and Schmid, 2003; Nabi and Le, 2003).
Concomitant with the cross-linking-dependent redistribution of MAG into the detergent-insoluble fraction, there are changes in the phosphorylation of specific proteins, including Fyn and Gβ1-2, which have been shown previously to reside in lipid rafts (Kramer et al., 1997; Kramer et al., 1999; Melkonian et al., 1999; Marta et al., 2003). Fyn is a member of the Src family of kinases and is associated with cell adhesion, migration, proliferation, differentiation, apoptosis and cytoskeletal rearrangements (Osterhout et al., 1999). Increased phosphorylation of Fyn following MAG cross-linking in double-transfected COS cells has been reported previously (Umemori et al., 1994; Umemori et al., 1999). The main site of Fyn autophosphorylation is Tyr418, which leads to activation of kinase activity. Phosphorylation of additional tyrosines (Tyr28, Tyr138 and, particularly, Tyr213) also leads to kinase activation (Hansen et al., 1997). By contrast, phosphorylation of the C-terminal Tyr529 results in the inhibition of Fyn kinase activity (Resh, 1998). Considering that our data show a significant increase in Fyn phosphorylation in tyrosine residues other than Tyr418 or Tyr529, we speculate that this is due primarily to phosphorylation of Tyr213, Tyr138 and/or Tyr28. Clearly, additional studies are needed to clarify this challenging aspect of signal transduction.
The G-protein βγ complex (Gβγ) regulates the activity of a diverse set of effectors, including phospholipases, adenyl cyclases and ion channels (Clapham and Neer, 1997). Phosphorylation of the β subunit activates the dissociated Gβγ complex (Sternweis, 1994; Nurnberg et al., 1996). Dephosphorylation of the Gβγ complex could, therefore, affect a number of downstream signaling pathways. Previously, we have reported the dephosphorylation of Gβ1-2 subunit after cross-linking of MOG (Marta et al., 2003).
Additional consequences of cross-linking MAG are an increase in the cleavage of α-fodrin and a concomitant depolymerization of actin filaments. α-Fodrin is involved in the organization of receptor domains by linking integral membrane proteins to cortical actin filaments. This process is regulated by the binding of calmodulin to α-fodrin and the subsequent cleavage by Ca2+-dependent proteases (Harris and Morrow, 1990). Fragments of fodrin (120–150 kDa) are generated by caspase and calpain activation during apoptotic cell death (Moore et al., 2002). Ca2+/calmodulin-dependent protein phosphatases, such as calcineurin (Asai et al., 1999), also regulate the phosphorylation of LDH, which is dephosphorylated upon MAG cross-linking. Changes in the phosphorylation of LDH have important implications for the control of cell metabolism (Yasykova et al., 2000). These findings indicate that MAG might have a role in the regulation of Ca2+/calmodulin. Taken together, these observations are consistent with a model in which contact between MAG and a receptor on the axolemma activates specific signal-transduction pathways, which results in alterations in OL metabolism and cytoskeletal rearrangements.
Treatment of mature OLs with GT1b ganglioside, which interacts with MAG, neither reproduced nor prevented the changes observed after MAG cross-linking. This might be because of a low binding affinity of GT1b compared to anti-MAG antibody. Alternatively, MAG–neuron interactions might require a multimeric protein–lipid complex, which includes not only gangliosides, but also proteins such as the Nogo receptor and its co-receptor p75 (Domeniconi et al., 2002; Liu et al., 2002; Wang et al., 2002). It was reported recently that MAG is associated with a lubrol-insoluble fraction (Vinson et al., 2003). Because the Nogo receptor is a GPI-linked protein, these authors suggested that the interaction between MAG and its receptors might involve the association between lipid rafts on opposing neuron and OL membranes. The regulated partitioning of MAG into lipid rafts that we report here is consistent with this concept.
Recently, we used a similar cross-linking approach to study MOG signaling in OLs. MOG is clearly implicated in the demyelinating disease multiple sclerosis (Marta et al., 2003). Unlike MAG, which is localized in the inner myelin lamella, MOG is concentrated in the outer lamella of the myelin sheath where it is exposed to the environment. Interestingly, although both anti-MOG and anti-MAG antibodies can be detected amongst the many autoantibody responses in multiple sclerosis, only anti-MOG antibodies appear to be pathogenic in this disease (Genain et al., 1999; Lassmann et al., 2001; Robinson et al., 2003). This indicates that in pathological situations MOG-mediated signaling occurs upstream of that mediated by MAG cross-linking. In agreement with these observations, the signaling and morphological modifications of OLs following MOG or MAG cross-linking are distinct. Specifically, antibody cross-linking of MOG results in the rapid repartitioning of MOG into lipid rafts, changes in the phosphorylation of a different set of proteins that respond to MAG (with the exemption of Gβ), and a rapid retraction of OL processes and myelin-like membranes (MAG cross-linking does not induce morphological alterations).
We propose that the antibody-induced association of MAG with lipid rafts leads to novel interactions with other proteins (e.g. kinases and phosphatases) that are also selectively recruited into lipid rafts. This triggers specific, MAG-mediated signaling that is involved in the cross-communication between OLs and axons. This process might require the reorganization of protein domains in the plasma membrane and, as a consequence, rearrangements of the actin cytoskeleton.
ACKNOWLEDGEMENTS
We would like to acknowledge A. Taylor and M. Montano for excellent technical support. Supported by grants from NIH NS10861, NS45440 and NS41078. This investigation was supported in part by a Postdoctoral Fellowship from the National Multiple Sclerosis Society FG1423A.
REFERENCES
- Abrami L, Fivaz M, Kobayashi T, Kinoshita T, Parton RG, van der Goot FG. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. Journal of Biological Chemistry. 2001;276:30729–30736. doi: 10.1074/jbc.M102039200. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, Bray GM, Perkins SC. Axon-Schwann cell relationships in neuropathies of mutant mice. Annals New York Academy of Science. 1979;317:512–531. [PubMed] [Google Scholar]
- Asai A, Qiu J, Narita Y, Chi S, Saito N, Shinoura N, et al. High level calcineurin activity predisposes neuronal cells to apoptosis. Journal of Biological Chemistry. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- Atashi JR, Klinz SG, Ingraham CA, Matten WT, Schachner M, Maness PF. Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron. 1992;8:831–842. doi: 10.1016/0896-6273(92)90197-l. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proceedings of the National Academy of Sciences of the U.S.A. 1989;86:6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Kumar M, Murray K, Pfeiffer SE. Developmental and FGF-2-mediated regulation of syndecans (1–4) and glypican in oligodendrocytes. Molecular and Cellular Neuroscience. 1996;7:276–288. doi: 10.1006/mcne.1996.0021. [DOI] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proceedings of the National Academy of Sciences of the U.S.A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST, Witt AS, Kirkpatrick LL, de Waegh SM, Readhead C, Tu PH, Lee VM. Formation of compact myelin is required for maturation of the axonal cytoskeleton. Journal of Neuroscience. 1999;19:7278–7288. doi: 10.1523/JNEUROSCI.19-17-07278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annual Reviews in Cell Developmental Biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Cerneus DP, Ueffing E, Posthuma G, Strous GJ, van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. Journal of Biological Chemistry. 1993;268:3150–3155. [PubMed] [Google Scholar]
- Chakraborty G, Drivas A, Ledeen R. The phosphoinositide signaling cycle in myelin requires cooperative interaction with the axon. Neurochemistry Research. 1999;24:249–254. doi: 10.1023/a:1022562021059. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. Journal of Neurochemistry. 2001;78:736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. G protein beta gamma subunits. Annual Review of Pharmacology and Toxicology. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Kelm S, Hartnell A, Freeman S, Nath D, Vinson M, et al. Sialoadhesin and related cellular recognition molecules of the immunoglobulin superfamily. Biochemistry Society Transactions. 1996;24:150–156. doi: 10.1042/bst0240150. [DOI] [PubMed] [Google Scholar]
- Dashiell SM, Tanner SL, Pant HC, Quarles RH. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. Journal of Neurochemistry. 2002;81:1263–1272. doi: 10.1046/j.1471-4159.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- De Angelis DA, Braun PE. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase binds to actin-based cytoskeletal elements in an isoprenylation-independent manner. Journal of Neurochemistry. 1996;67:943–951. doi: 10.1046/j.1471-4159.1996.67030943.x. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Fassett MS, Davis DM, Valter MM, Cohen GB, Strominger JL. Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proceedings of the National Academy of Sciences of the U.S.A. 2001;98:14547–14552. doi: 10.1073/pnas.211563598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov AV, Shmigol IB, Sharonov GV, Feofanov AV, Volkov Y. Direct and indirect antibody-induced TX-100 resistance of cell surface antigens. Immunology Letters. 2003;85:287–295. doi: 10.1016/s0165-2478(02)00244-4. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Franzen R, Tanner SL, Dashiell SM, Rottkamp CA, Hammer JA, Quarles RH. Microtubule-associated protein 1B: a neuronal binding partner for myelin-associated glycoprotein. Journal of Cell Biology. 2001;155:893–898. doi: 10.1083/jcb.200108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich VL, Jr, Mugnaini E. Myelin sheath thickness in the CNS is regulated near the axon. Brain Research. 1983;274:329–331. doi: 10.1016/0006-8993(83)90712-6. [DOI] [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106:119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nature Medicine. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. Journal of Biological Chemistry. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Hansen K, Alonso G, Courtneidge SA, Ronnstrand L, Heldin CH. PDGF-induced phosphorylation of Tyr28 in the N-terminus of Fyn affects Fyn activation. Biochemistry Biophysics Research Communications. 1997;241:355–362. doi: 10.1006/bbrc.1997.7743. [DOI] [PubMed] [Google Scholar]
- Harris AS, Morrow JS. Calmodulin and calcium-dependent protease I coordinately regulate the interaction of fodrin with actin. Proceedings of the National Academy of Sciences of the U.S.A. 1990;87:3009–3013. doi: 10.1073/pnas.87.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D, Sheets ED, Baird B. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. Journal of Cell Science. 2000;113:1009–1019. doi: 10.1242/jcs.113.6.1009. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Roles of lipid rafts in membrane transport. Current Opinions Cell Biology. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Jaramillo ML, Afar DE, Almazan G, Bell JC. Identification of tyrosine 620 as the major phosphorylation site of myelin-associated glycoprotein and its implication in interacting with signaling molecules. Journal of Biological Chemistry. 1994;269:27240–27245. [PubMed] [Google Scholar]
- Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Current Biology. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Kim T, Fiedler K, Madison DL, Krueger WH, Pfeiffer SE. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. Journal of Neuroscience Research. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- Kim T, Pfeiffer SE. Myelin glycosphingolipid/cholesterol-enriched microdomains selectively sequester the non-compact myelin proteins CNP and MOG. Journal of Neurocytology. 1999;28:281–293. doi: 10.1023/a:1007001427597. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Witt AS, Payne HR, Shine HD, Brady ST. Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. Journal of Neuroscience. 2001;21:2288–2297. doi: 10.1523/JNEUROSCI.21-07-02288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Koch T, Niehaus A, Trotter J. Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. Journal of Biological Chemistry. 1997;272:8937–8945. doi: 10.1074/jbc.272.14.8937. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. Journal of Biological Chemistry. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Kursula P, Lehto VP, Heape AM. The small myelin-associated glycoprotein binds to tubulin and microtubules. Brain Research. Molecular Brain Research. 2001;87:22–30. doi: 10.1016/s0169-328x(00)00270-9. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends in Molecular Medicine. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Simons K, Dotti CG. Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proceedings of the National Academy of Sciences of the U.S.A. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- LoPresti P, Muma NA, De Vries GH. Neu differentiation factor regulates tau protein and mRNA in cultured neonatal oligodendrocytes. Glia. 2001;35:147–155. doi: 10.1002/glia.1079. [DOI] [PubMed] [Google Scholar]
- Madison DL, Krueger WH, Cheng D, Trapp BD, Pfeiffer SE. SNARE complex proteins, including the cognate pair VAMP-2 and syntaxin-4, are expressed in cultured oligodendrocytes. Journal of Neurochemistry. 1999;72:988–998. doi: 10.1046/j.1471-4159.1999.0720988.x. [DOI] [PubMed] [Google Scholar]
- Marta CB, Taylor CM, Coetzee T, Kim T, Winkler S, Bansal R, et al. Antibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphology. Journal of Neuroscience. 2003;23:5461–5471. doi: 10.1523/JNEUROSCI.23-13-05461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. Journal of Biological Chemistry. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Menon K, Rasband MN, Taylor CM, Brophy P, Bansal R, Pfeiffer SE. The myelin-axolemmal complex: biochemical dissection and the role of galactosphingolipids. Journal of Neurochemistry. 2003;87:995–1009. doi: 10.1046/j.1471-4159.2003.02075.x. [DOI] [PubMed] [Google Scholar]
- Moore JD, Rothwell NJ, Gibson RM. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. British Journal of Pharmacology. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. Journal of Cell Biology. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. Journal of Biological Chemistry. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. Journal of Neuroscience. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberg B, Harhammer R, Exner T, Schulze RA, Wieland T. pecies- and tissue-dependent diversity of G-protein beta subunit phosphorylation: evidence for a cofactor. Biochemistry Journal. 1996;318:717–722. doi: 10.1042/bj3180717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. Journal of Cell Biology. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends in Cell Biology. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Poltorak M, Sadoul R, Keilhauer G, Landa C, Fahrig T, Schachner M. Myelin-associated glycoprotein, a member of the L2/HNK-1 family of neural cell adhesion molecules, is involved in neuron-oligodendrocyte and oligodendrocyte-oligodendrocyte interaction. Journal of Cell Biology. 1987;105:1893–1899. doi: 10.1083/jcb.105.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cellular and Molecular Life Sciences. 2002;59:1851–1871. doi: 10.1007/PL00012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Subunit composition and novel localization of K+ channels in spinal cord. Journal of Comparative Neurology. 2001;429:166–176. doi: 10.1002/1096-9861(20000101)429:1<166::aid-cne13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fyn, a Src family tyrosine kinase. International Journal of Biochemistry and Cell Biology. 1998;30:1159–1162. doi: 10.1016/s1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- Robinson WH, Fontoura P, Lee BJ, de Vegvar HE, Tom J, Pedotti R, et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nature Biotechnology. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. holesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. Journal of Cell Biology. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. Journal of Neuroscience. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annual Reviews Neuroscience. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid Rafts and Signal Transduction. Nature Reviews Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons M, Kramer EM, Thiele C, Stoffel W, Trotter J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. Journal of Cell Biology. 2000;151:143–154. doi: 10.1083/jcb.151.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Developmental Biology. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Spencer T, Domeniconi M, Cao Z, Filbin MT. New roles for old proteins in adult CNS axonal regeneration. Current Opinion in Neurobiology. 2003;13:133–139. doi: 10.1016/s0959-4388(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Sternweis PC. The active role of beta gamma in signal transduction. Current Opinion in Cell Biology. 1994;6:198–203. doi: 10.1016/0955-0674(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Stockinger H, Horejsi V, Waldhausl W. Signal transduction via glycosyl phosphatidylinositol-anchored proteins in T cells is inhibited by lowering cellular cholesterol. Journal of Biological Chemistry. 1997;272:19242–19247. doi: 10.1074/jbc.272.31.19242. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Coetzee T, Pfeiffer SE. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. Journal of Neurochemistry. 2002;81:993–1004. doi: 10.1046/j.1471-4159.2002.00884.x. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Marta CB, Bansal R, Pfeiffer SE. The Transport, Assembly, and Function of Myelin Lipids. In: Lazzarini RA, editor. Myelin Biology and Disorders. Vol. 1. Academic Press; 2004. pp. 57–88. [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Umemori H, Kadowaki Y, Hirosawa K, Yoshida Y, Hironaka K, Okano H, et al. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. Journal of Neuroscience. 1999;19:1393–1397. doi: 10.1523/JNEUROSCI.19-04-01393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, et al. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. Journal of Biological Chemistry. 2001;276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- Vinson M, Rausch O, Maycox PR, Prinjha RK, Chapman D, Morrow R, et al. Lipid rafts mediate the interaction between myelin-associated glycoprotein (MAG) on myelin and MAG-receptors on neurons. Molecular and Cellular Neurosciences. 2003;22:344–352. doi: 10.1016/s1044-7431(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Schnaar RL. Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration. Biochimie. 2001;83:677–682. doi: 10.1016/s0300-9084(01)01308-6. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Patel HV, Fromholt SE, Heffer-Lauc M, Vyas KA, Dang J, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proceedings of the National Academy of Sciences of the U.S.A. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of nogo-a and nogo-66 receptor proteins at sites of axon- myelin and synaptic contact. Journal of Neuroscience. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MD, Luciano CA, Quarles RH. Nerve conduction abnormalities in aging mice deficient for myelin-associated glycoprotein. Muscle and Nerve. 2001;24:1380–1387. doi: 10.1002/mus.1159. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Bloechlinger S. Neuroscience. It takes more than two to Nogo. Science. 2002;297:1132–1134. doi: 10.1126/science.1076247. [DOI] [PubMed] [Google Scholar]
- Yasykova MY, Petukhov SP, Muronetz VI. Phosphorylation of lactate dehydrogenase by protein kinases. Biochemistry (Moscow) 2000;65:1192–1196. [PubMed] [Google Scholar]


