Abstract
The neurotrophins are a family of polypeptide growth factors that are essential for the development and maintenance of the vertebrate nervous system. In recent years, data have emerged indicating that neurotrophins could have a broader role than their name might suggest. In particular, the putative role of NGF and its receptor TrkA in immune system homeostasis has become a much studied topic, whereas information on the other neurotrophins is scarce in this regard. This paper reviews what is known about the expression and possible functions of neurotrophins and their receptors in different immune tissues and cells, as well as recent data obtained from studies of transgenic mice in our laboratory. Results from studies to date support the idea that neurotrophins may regulate some immune functions. They also play an important role in the development of the thymus and in the survival of thymocytes.
Keywords: immunocompetent cells, lymphoid organs, neurotrophins, p75NTR, Trk receptors
Introduction
Half a century ago, a polypeptide that induced neuronal growth was discovered and named nerve growth factor (NGF) (Levi-Montalcini, 1952). Several decades later, a series of other molecules with similar structure and functions were identified, and together they form a family of polypeptide growth factors called the neurotrophins (NTs). Besides NGF, this family comprises brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and NT-4/5, all of which are present in all tetrapods, except NT-4/5, which has not been found in birds (Hallböök, 1999). In teleosts, two additional neurotrophins closely related to NGF, namely NT-6 and NT-7, have been identified (Götz et al. 1994; Lai et al. 1998; Nilsson et al. 1998). NTs probably originated from the duplication of an ancestral gene, which gave rise to two intermediate genes that then produced NGF and NT-3, and BDNF and NT-4/5, respectively (Hallböök, 1999).
NTs bind to two kinds of receptors with dissociation constants of 10−9 m and 10−11 m that denominate low- and high-affinity receptors, respectively (for references see Lewin & Barde, 1996; Friedman & Greene, 1999). The low-affinity receptor is p75NTR. It belongs to the tumour necrosis factor receptor superfamily, and serves as a pan-neurotrophin receptor (Rodríguez-Tebar et al. 1990, 1992; Hempstead, 2002). The p75NTR locus produces two proteins, a full-length protein and a short variant lacking a segment of the extracellular domain (Dechant & Barde, 1997; von Schack et al. 2001). The functional role of p75NTR has not yet been fully elucidated (Bothwell, 1996; Lee et al. 2001; Roux & Barker, 2002). It is assumed to function as a co-receptor for the high-affinity receptors (for references see Esposito et al. 2001; Roux & Barker, 2002) and as a mediator of the pro-apoptotic programmes induced by NGF depending on the physiological or developmental stage of the cells (Carter & Lewin, 1997; Casaccia-Bonnefil et al. 1998; Kuner & Hertel, 1998; Meldolesi et al. 2000; Miller & Kaplan, 2001; Chao & Bothwell, 2002; Kendal et al. 2002). Moreover, it mediates the migration of Schwann cells (Bentley & Lee, 2000), and is involved in cell fate decisions in some non-nervous cells such as macrophages (Caroleo et al. 2001) and vascular smooth muscle cells (Wang et al. 2001). p75NTR also interacts with proteins that promote mitotic cycle arrest, thereby mediating a role of NTs in the cell cycle (Lopez-Sanchez & Frade, 2002).
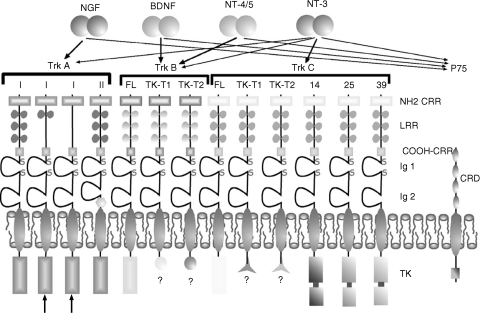
The protein tyrosine kinase Trk receptors TrkA (gp140trkA), TrkB (gp145trkB) and TrkC (gp145trkC) act as specific, high-affinity neurotrophin receptors (Meakin & Shooter, 1992; Glass & Yancopoulos, 1993; Barbacid, 1995; Lewin & Barde, 1996; Huang & Reichardt, 2001). These Trk receptors have an extracellular domain, which binds the different neurotrophins, and a cytosolic domain whose tyrosine-kinase activity is essential for signal transduction. Variants of Trks with insertions in either the extracellular domain or the tyrosine kinase domain have been identified for TrkA (Barker et al. 1993; Shelton et al. 1995) and TrkC (Lamballe et al. 1993; Valenzuela et al. 1993; Shelton et al. 1995). In addition, truncated receptors lacking the kinase domain have been described for TrkB and TrkC, but not for TrkA (Middelmas et al. 1991; Valenzuela et al. 1993; Tsoulfas et al. 1996). Variants of the extracellular domain of TrkA have also been detected in certain tissues (Dubus et al. 2000). Each member of the Trk family shows preferential ligand bindings among neurotrophins (Ip et al. 1993). TrkA is the preferred receptor for NGF (Kaplan et al. 1991; Klein et al. 1991), but has a lower efficiency for NT-3 or NT4/5 binding. TrkB is bound by BDNF and NT-4 and, to a lesser extent, by NT-3 (Klein et al. 1991; Ip et al. 1992). TrkC has a unique ligand, NT-3 (Lamballe et al. 1991) (Fig. 1).
Fig. 1.
Schematic representation of the structure of Trk and p75NTR neurotrophin receptors, and of the neurotrophins that bind each of them: thick arrows represent the primary receptor–ligand pairing; thin arrows indicate other binding possibilities. Two different isoforms of TrkA have been isolated, and termed TrkAI and TrkAII. Three TrkAI isoforms are known (shown at the left of the figure). In the thymus, two specific TrkAI isoforms have been isolated (arrows at bottom left), which show total or partial deletion of amino cysteine-rich regions of the extracellular domain. TrkAII differs from TrkAI in that it carries a small insertion of six amino acids next to the transmembrane domain (hexagon). With regard to TrkB and TrkC, full-length (FL), tyrosine kinase truncated (TK-T1 and TK-T2), and tyrosine kinase inserted (14, 25 and 39, equivalent to the number of amino acids forming the insertion) isoforms have been isolated. NH2 CRR, amino cysteine-rich regions; COOH-CRR, carboxy cysteine-rich regions; CRD, cysteine-rich domains; LRR, leukine-rich region; TK, tyrosine kinase domain.
As occurs with NTs, the genes codifying for the Trk receptors probably also originate from a common ancestral gene (van Kesteren et al. 1998; Hallböök, 1999). Thus both neurotrophins and Trk receptors are present in all vertebrates and their sequences are highly conserved during phylogeny.
The actions of NTs in the nervous system have been well studied and extensively reviewed (Fariñas, 1999; Huang & Reichardt, 2001), although the concept that the role of NTs is confined to cells of the nervous system is being reconsidered (Tessarollo, 1998). Thus detailed studies have revealed significant actions of neurotrophins in a wide variety of tissues outside the nervous system, especially in the immune system (Otten & Gadient, 1995; Tessarollo, 1998; Aloe et al. 1999; Aloe, 2001; Sariola, 2001).
It is now well established that NGF is involved in the normal pattern of sympathetic innervation of the lymphoid organs (Kannan et al. 1994, 1996), because they concentrate NGF conveyed by the lymphoid vessels from the sources (Carlson et al. 1995, 1998). However, other functions of NTs in the lymphoid organs are less clear. The aim of this review is to compile and discuss current data about the occurrence and distribution of NTs and their receptors in the lymphoid organs and immunocompetent cells, focusing on the possible functional significance of these molecules as modulators of the immune system in health and disease.
Presence of the neurotrophins and their receptors in the immune system (Table 1)
Table 1.
Localization of neurotrophin receptors in lymphoid organs of vertebrates
| Tissue | TrkA | TrkB | TrkC | p75 |
|---|---|---|---|---|
| Bone marrow | Erythroblasts1 | Erythroblasts1 | Megacariocytes1 | Dendritic cells2,4 |
| Neutrophils1 | Promyelocytes1 | |||
| Megacariocytes1 | ||||
| Haematopoietic cells2 | ||||
| Thymus | Dendritic cells5,6,7 | Macrophages8 | Stromal cells9,10 | Dendritic cells7 |
| Epithelial cells2,5,6,7 | Dendritic cells9,10 | Epithelial cells5,11,12,13 | ||
| Thymocytes2 | ||||
| Bursa of Fabricius | Epithelial cells9,14 | Dendritic cells14 | Epithelial cells14 | |
| Spleen | Macrophages2,15 | Stromal cells10 | Follicular dendritic cells6 | |
| Lymphocytes2 | ||||
| Lymphoid nodes | Dendritic cells5,16 | Dendritic cells16 | Dendritic cells6 | |
| Macrophages2 | Lymphocytes17 | |||
| Peyer's patches | Dendritic cells18 | Macrophages18 | ||
| Epithelial cells18 | ||||
| Palatine tonsils | Dendritic cells6,19 | Macrophages19 | Interdigitating dendritic cells19 | Dendritic cells6 |
| Epithelial cells6,19 | ||||
| Cecal tonsil | Epithelial cells20 | Macrophages20 | Macrophages20 | |
| Dendritic cells 20 | Dendritic cells20 |
Detailed studies carried out during the last decade about the tissue distribution and cellular localization of NT receptors have revealed they are present in cell subpopulations of primary and secondary lymphoid organs, as well as in some kinds of immunocompetent cells (see Aloe et al. 1999). Therefore, both these tissues and cells are potential targets for NTs. Interestingly, in all vertebrate species examined, from humans to fishes, NTs and/or their receptors have been detected in lymphoid organs (Ciriaco et al. 1996; Hannestad et al. 1997, 2000). Recently, a major contribution to understanding the in vivo functions of NTs has been provided by the study of the phenotype of mice lacking NTs or functional NT receptors (García-Suárez et al. 2000a, 2002; Ruberti et al. 2000).
Thymus
In the thymus, mRNAs for all NTs and their receptors have been detected (Timmusk et al. 1993; Laurenzi et al. 1994; Lomen-Hoerth & Shooter, 1995; Labouyrie et al. 1997; Parrens et al. 1998). Nevertheless, identification of the cells expressing each of them has not yet been fully accomplished, and it has still not been proved whether or not mechanisms of autocrinia or paracrinia exist within the organ.
In mammals, TrkA is mainly localized in subcapsular and medullar epithelial cells (Shibayama & Koizumi, 1996; Hannestad et al. 1997; Labouyrie et al. 1997; Parrens et al. 1998, 1999; García-Suárez et al. 2000b, 2001; Yoon et al. 2003), but is not expressed by thymocytes (Maroder et al. 1996; Hannestad et al. 1997; Labouyrie et al. 1997; Parrens et al. 1999; Levanti et al. 2001) (Fig. 2). The thymic TrkA seems to be functional as NGF administration produces epithelial cell hypertrophy in vivo (Abramchik et al. 1988) and increases IL-6 transcription in epithelial cells in vitro (Screpanti et al. 1992). Recently, Dubus et al. (2000) identified thymus-specific TrkA variants lacking leucine-rich motifs of the extracellular domain, which have been implicated in modulating NGF binding (Fig. 1). Interestingly, in malignant thymic epithelial cell tumours the expression of TrkA is lost (Parrens et al. 1998), and p75NTR is expressed (Parrens et al. 1999). Furthermore, during thymus regeneration of the rat thymus following acute-induced involution of the organ, the expression of TrkA mRNA and TrkA is enhanced in cells that normally express it (Yoon et al. 2003).
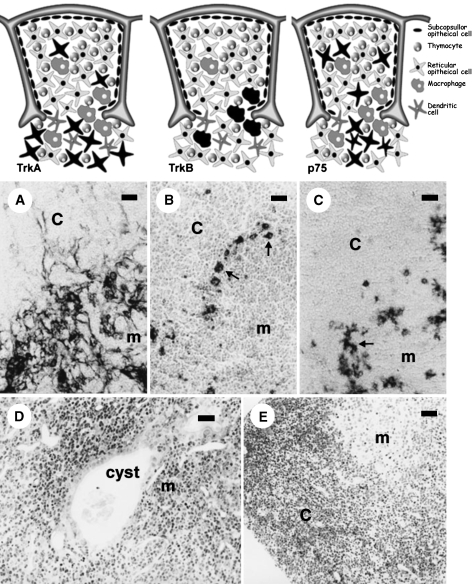
Fig. 2.
The upper pictures illustrate the localization of TrkA-(left), TrkB-(middle) and p75NTR (right)-positive cells in the rodent thymus; receptor-expressing cells are represented in black. Below are shown the corresponding TrkA (A), TrkB (B) and p75NTR (C) immunostained sections. The cells expressing TrkA are subcapsular and medullar thymic epithelial cells (mouse); those showing TrkB immunoreactivity, concentrated at the cortico-medullary border (arrows), are macrophages (rat), and p75NTR immunoreactivity is confined to a subpopulation of medullar thymic epithelial cells (arrows, rat). The lower images correspond to morphological aspects of functionally trkA- and trkB-deficient mice. The trkA-kinase –/– mouse thymus (D) is characterized by disorganization of the thymic architecture and the presence of medullar endodermic cysts containing amorphous material and cell debris, whereas the trkB-kinase –/– mouse (E) shows images of apoptotic lymphocyte death, especially in the cortex. c, cortical; m, medullar. Scale bar = 5 µm.
Regarding TrkB, the occurrence of mRNA for both truncated (Lomen-Hoerth & Shooter, 1995) and full-length isoforms (Laurenzi et al. 1994; Maroder et al. 1996; García-Suárez et al. 2002) has been reported. At the protein level, TrkB has been detected in thymocytes (Maroder et al. 1996; Besser & Wank, 1999; García-Suárez et al. 2002), as well as in stromal cells identified as ED1+ and F4/80+ macrophages in the rat and mouse, respectively (García-Suárez et al. 1998, 2002). In addition, TrkB expression has also been detected in morphologically identified macrophages of the bovine thymus (Levanti et al. 2001).
To our knowledge, TrkC has not been detected in the mammalian thymus at the protein level. p75NTR mRNA is also present in the thymus, primarily located on the stroma (Lomen-Hoerth & Shooter, 1995) in both medullar epithelial cells and dendritic cells (Parrens et al. 1998, 1999; García-Suárez et al. 2000b, 2001). Interestingly, exogenously administered NGF, or experimentally induced increased NGF plasma levels with 4-methylcatechol, have proven to induce a shift in p75NTR expression from medullar epithelial cells to macrophages. In fact, in control animals p75NTR expression was restricted to epithelial cells, whereas in the treated animals it disappeared from epithelial cells and was expressed in macrophages (García-Suárez et al. 2000b).
Trk-like proteins have also been detected in the thymus of vertebrates other than mammals (Baig & Khan, 1996; Heinrich & Lum, 2000). In the pigeon, TrkA-like expression was observed in medullar and a subpopulation of cortical epithelial cells, TrkB-like in medullar dendritic cells and cortical macrophages, and TrkC-like in scattered clusters of medullar epithelial cells, including Hassal's corpuscles (Ciriaco et al. 1996). Recently, expression of Trk-like proteins was observed in the thymus of the teleost Dicentrarchus labrax (Hannestad et al. 2000). Thus the presence of neurotrophins and their receptors in the thymus appears to be a feature common to most vertebrates.
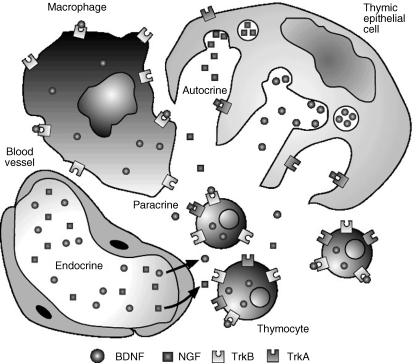
NGF is present in the thymus, mostly in the medulla, and is probably synthesized locally (Katoh-Semba et al. 1993; Aloe et al. 1997; Turrini et al. 2001). Since T cells are known to produce NGF (Ehrhard et al. 1993b; Santambrogio et al. 1994; Lambiase et al. 1997), it could be hypothesized that this neurotrophin acts in a paracrine manner on the epithelial cells expressing TrkA. Such an action could account for the trophic and maturational dependency of thymic epithelial cells on thymocytes (Ritter & Boyd, 1993; Shores et al. 1994; Hollander et al. 1995; Haynes & Hale, 1998) (Fig. 4).
Fig. 4.
Schematic representation of the possible sources and targets of NGF and BDNF in the mammalian thymus.
BDNF mRNA is present in the thymus (Laurenzi et al. 1994; Yamamoto et al. 1996; Timmusk et al. 1999), where it is expressed by stromal cells (Maroder et al. 1996), and BDNF signalling through TrkB receptors present on immature thymocytes seems to be necessary for thymocyte survival at certain developmental stages (Maroder et al. 1996) (Fig. 4). The thymus also contains mRNA and protein for NT-3 (Laurenzi et al. 1994; Katoh-Semba et al. 1996) and NT-4/5 (Timmusk et al. 1993; Laurenzi et al. 1994), but the cellular source of these polypeptides, as well as their role in thymic function, remains to be established.
Bursa of Fabricius
The bursa of Fabricius is a unique lymphoid organ present in birds, which provides the microenvironment for B-lymphocyte maturation and differentiation (Glick, 1991; Glick & Olah, 1993). Studies from our group have demonstrated the occurrence of Trk-like proteins in the pigeon bursa (Ciriaco et al. 1996, 1997). TrkA-like and TrkC-like proteins were found in the so-called follicle-associated and interfollicular epithelia, whereas TrkB-like protein was present in the bursal secretory dendritic cells. The bursa of Fabricius contains high levels of NGF during development (Ernfors et al. 1988), and this neurotrophin has been reported to increase the size of bursal lymphoid follicles (Bracci-Laudiero et al. 1991), and to reduce bursal cell mortality in vitro (Bracci-Laudiero et al. 1993a).
Spleen
The spleen contains detectable amounts of mRNA for all neurotrophins (Maisonpierre et al. 1990; Timmusk et al. 1993; Laurenzi et al. 1994; Yamamoto et al. 1996), but at the protein level only NT-3 has been detected in this organ (Zhou & Rush, 1993; Katoh-Semba et al. 1996). As for the receptors, p75NTR as well as Trks have been detected at the mRNA level in human and rat spleen (Laurenzi et al. 1994; Lomen-Hoerth & Shooter, 1995; Yamamoto et al. 1996).
In humans, follicular dendritic cells express both p75NTR and TrkA (Labouyrie et al. 1997). By contrast, in the rat spleen p75NTR expression has been localized to dendritic cells in the PALS (periarteriolar lymphatic sheath, Pérez-Pérez et al. 2003), and no TrkA expression has been reported to date. TrkB has been detected in immunohistochemically identified macrophage subpopulations of human (Shibayama & Koizumi, 1996), rat (Pérez-Pérez et al. 1999) and mouse (M. Pérez-Pérez et al. unpubl. obs.) macrophages (Fig. 3).
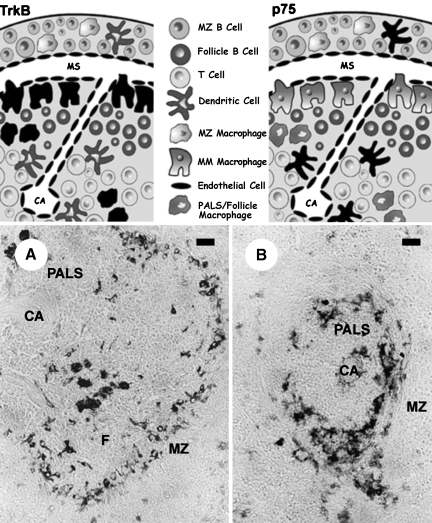
Fig. 3.
The upper pictures illustrate the localization of TrkB-(left) and p75NTR (right)-positive cells in the rat spleen; receptor-expressing cells are represented in black. Below are shown the corresponding TrkB (A) and p75NTR (B) immunostained sections. TrkB-immunoreactive cells include MMM and scattered white pulp macrophages, whereas p75NTR-positive cells are a subpopulation of dendritic cells. PALS, periarteriolar lymphatic sheath; F, follicle; MZ, marginal zone; CA, central arteriole; MM, marginal metallophilic. Scale bar = 5 µm.
Lymph nodes, palatine tonsils and Peyer's patches
In human palatine tonsils and lymph nodes, p75NTR is present in follicular dendritic cells and dendritic cells, as well as in periarteriolar macrophages (Pezzati et al. 1992; Hannestad et al. 1995; García-Suárez et al. 1997; Labouyrie et al. 1997). As for TrkA, it has been found in cryptic tonsilar epithelium, dendritic cells and interdigitated reticular cells (Hannestad et al. 1995; García-Suárez et al. 1997; Labouyrie et al. 1997). The same results applied to bovine lymphoid organs (Levanti et al. 1997, 2001)
In the pigeon caecal tonsil, a secondary gut-associated lymphoid organ, TrkA-like has been found in the intestinal epithelium, whereas TrkB-like and TrkC-like have been detected in macrophage-dendritic cells. By contrast, BDNF-like and NT-3-like occur in the intestinal epithelium covering the lymphoid tissue, mainly in endocrine cells. Conversely, NGF-like has never been detected in this organ (Hannestad et al. 1998). In fishes (D. labrax), Trk-like proteins are present in the head-kidney and spleen (Hannestad et al. 2000).
Neurotrophins and immunocompetent cells
Lymphocytes
The possibility of neurotrophins acting on lymphocytes was first reported by Dean et al. (1987), who observed that NGF increased the blastogenic response of mouse spleen cells. This observation, which suggested that lymphocytes (and presumably other immunocompetent cells) expressed neurotrophin receptors, was followed by the demonstration that these cells also synthesized and released neurotrophins, which led to the proposal that there might be autocrine and paracrine actions of neurotrophins on these cells (Fig. 4). Interestingly, the expression of both neurotrophins and their receptors by lymphocytes is frequently dependent on cell activation (Kerschensteiner et al. 1999; Moalem et al. 2000).
Expression of both NGF and TrkA is induced by mitogen activation in CD4+ T cells (Ehrhard et al. 1993b), and this TrkA receptor seems to be functional because NGF administration to antigen-stimulated CD4+ T cells induces expression of c-fos (Ehrhard et al. 1994). Both CD4+ and CD8+ T cells produce NGF, which is increased after antigenic stimulation in the Th2 subset (Santambrogio et al. 1994; see Van Eden et al. 2002, for a review of the Th1 and Th2 cells). In additon, unstimulated human CD4+ Th1 and Th2 cells, but not Th0, express both NGF and TrkA (Lambiase et al. 1997); and Th1 cells express full-length TrkB and low levels of TrkC (Besser & Wank, 1999), and CD4+ and CD8+ T cells transcribe BDNF mRNA and produce bioactive BDNF (Braun et al. 1999; Kerschensteiner et al. 1999), NT-3 and NT-4/5 (Moalem et al. 2000). By contrast, the expression of p75NTR by T cells is controversial (Kittur et al. 1992; Ehrhard et al. 1993b).
In B cells, TrkA (Melamed et al. 1996; Torcia et al. 1996; D’Onofrio et al. 2000) and p75NTR (Brodie et al. 1996) expression has been reported. However, according to Schenone et al. (1996), B cells do not express mRNA or protein for either p75NTR or TrkA; these authors reported TrkB mRNA and protein expression by B cells, and demonstrated activation of these TrkB receptors by BDNF. Discrepancies between these results may be due to the activation state of these cells. Recently, the occurrence of TrkB on B cells has been confirmed (Besser & Wank, 1999; D’Onofrio et al. 2000), and the expression of TrkC suggested (D’Onofrio et al. 2000). As for neurotrophin production, B cells produce NGF (Santambrogio et al. 1994; Torcia et al. 1996) and NT-3 (Besser & Wank, 1999). Activated T cells also produce BDNF (Kerschensteiner et al. 1999). Interestingly, NGF appears to be involved in B-cell survival because it is able to rescue these cells from induced apoptosis (Kronfeld et al. 2002).
In summary, NGF/TrkA, and possibly other NT/receptor systems, seem to have a role in both T- and B-cell physiology. Furthermore, each lymphocyte subset appears to express a characteristic array of NTs and their receptors.
Monocyte-macrophage cells
Monocytes express TrkA, and this expression increases after activation, whereas it is down-regulated during differentiation towards tissue macrophages (Ehrhard et al. 1993a). As for NTs, NGF, BDNF and NT-4/5 are expressed by macrophages (Schober et al. 1998; Besser & Wank, 1999; Boven et al. 1999; Braun et al. 1999; Caroleo et al. 2001). A recent paper demonstrates that both NGF and BDNF influence the cytokine expression pattern in peripheral blood mononuclear cells, as well as in antigen-specific T cells, modulating the production of interleukin-4, and transforming growth factor-β, tumour necrosis factor-α and γ-interferon (Bayas et al. 2003).
Other cells
In bone marrow, transcripts for both p75NTR and all Trks are present in stromal adventitial reticular cells (Cattoretti et al. 1993; Labouyrie et al. 1999), whereas haematopoietic cells express one or several Trk receptor proteins, but not p75NTR (Chevalier et al. 1994; Labouyrie et al. 1999; Simone et al. 1999). Moreover, NGF is probably produced by bone marrow stromal cells (Auffray et al. 1996; Grills & Schuijers, 1998). Bone marrow cells also express TrkB (but not TrkC) as well as low levels of BDNF and NT-4/5 (but not NGF or NT-3) (Laurenzi et al. 1998).
Granulocytes express NGF, BDNF and NT-4/5, but not NT-3 (Laurenzi et al. 1998). Indirect evidence also suggests the occurrence of TrkA receptors in neutrophils (Kannan et al. 1991, 1992, 1993), and eosinophils (Hamada et al. 1996), which in turn produce and release NGF (Solomon et al. 1998; Kobayashi et al. 2002).
Regarding the basophil/mast cell lineage, basophils express functional TrkA receptors, but neither TrkB nor TrkC were detected in basophils (Burgi et al. 1996). Mast cells express TrkA (Horigome et al. 1993; Tam et al. 1997; Welker et al. 1998) and produce NGF (Leon et al. 1994), BDNF and NT-3 (Tam et al. 1997).
Data about the effects of NTs on immunocompetent cells are heterogeneous and most of them refer to NGF. Table 2 summarizes the most relevant ones.
Table 2. Immunomodulatory roles of neurotrophins.
| Cell type | Action | Neurotrophin | Species |
|---|---|---|---|
| B-lymphocytes | Proliferation1, 2, 3; stimulation of antibody synthesis2, 4, 5, 6, 7 | NGF | Human, rat |
| Differentiation into plasma cells2; expression of IL-2 | NGF | Human | |
| receptors3, 8; induction of CGRP synthesis14 Survival9, 10 | NGF | Human, mouse | |
| Activation of Trks and signalling molecules11, 12, 13 | NGF, BDNF | Human | |
| T-lymphocytes | Proliferation1, 2 | NGF | Human, rat |
| T-cell-dependent antibody synthesis6 | NGF | Rat | |
| Expression of IL-2 receptors8 | NGF | Human | |
| TrkB phosphorilation, survival (thymocytes)15 | BDNF | Mouse | |
| Transcriptional activation of c-fos15, 16 | NGF, BDNF | Human, mouse | |
| Monocytes/macrophages | Monocytic differentiation17; stimulation of phagocytosis, parasite killing and IL-1β production23; increase in TNF-α production25; increase in Fcχ receptor expression27 | NGF | Mouse |
| Chemotaxis21 | NGF, NT-3 | Mouse | |
| Increase in nitric oxide secretion24, 25 | NT-3 | Mouse | |
| Survival18, 19, 20; increase in oxidative burst22 | NGF | Human | |
| Increase in cathepsin S expression26 | NGF | Human | |
| Neutrophils | Differentiation17 | NGF | Human, mouse |
| Survival28, 29; chemotaxis30, 31; enhancement of phagocytosis and superoxide production28 | NGF | Mouse | |
| Eosinophils | Differentiation32; survival33; chemotaxis33; release of inflamatory mediators34; increase in cytotoxic activity33; suppression of leukotriene C4 production35 | NGF | Human |
| Basophils | Differentiation32, 36, 37, 38 survival 33; activation40; increased histamine release41, 42; enhanced production of lipid mediators41, 42; stimulation of IL-13 secretion43; modulation of IgE-mediated responses43 | NGF | Human |
| Mast cells | Proliferation44, 45; degranulation, mediator release54, 55, 56, 57, 58, 59; survival49, 50, 51, 52; chemotaxis53 | NGF | Rat |
| Differentiation17, 28, 32, 46, 47, 48 | NGF | Human, mouse | |
| Others | Proliferation and differentiation of haematopoietic cells60, 61 | NGF | Human |
| Shape change of platelets62 | NGF | Rabbit | |
| Increase of vascular permeability63 | NGF | Rat |
The immune phenotype of functionally deficient TrkA and TrkB mice
Transgenic mice for NTs and their receptors have added greatly to our knowledge of the role of neurotrophins in nervous system development (see Fariñas, 1999). Occasionally, these animal models have also been used to address the question of the function of neurotrophins in other organs. The thymus of mice with a tyrosine kinase-deficient trkA gene product (Smeyne et al. 1994) was recently the subject of in-depth studies in our laboratory. The thymus of these animals showed numerous epithelial cell islands, thymic cysts with endodermal lining, and a much lower density of thymocytes than age-matched controls (García-Suárez et al. 2000a, 2001). This suggests that functional TrkA is necessary for the normal differentiation of the thymic epithelial primordium, and that NGF signalling through this receptor is probably important for thymic organogenesis. However, it is still uncertain whether this abnormal thymic epithelium retains the capacity to promote positive selection and to provide a suitable microenvironment for thymocytes maturation, although the low thymocyte density seems likely to result in partial or total loss of such capacities. I. Silos-Santiago (pers. commun.) demonstrated severe T- and B-cell depletion in these animals.
In functionally deficient TrkB mice, studied at postnatal day 15, the thymus showed an increase in the number of pyknotic nuclei, suggestive of apoptotic lymphocytes, especially in the cortical area. Ultrastructurally, both lymphocytes and stromal cells were strongly altered. Changes in lymphocytes consisted of abnormal morphology, fragmentation or absence of the nucleus and accumulation of cytoplasmic electron-dense bodies, which probably represented nuclear fragments with condensed chromatin. The macrophages contained numerous secondary lysosomes, whereas the epithelial cells showed cytoplasmic inclusions and vacuoles, without apparent changes in the nuclei. The TUNEL method confirmed the massive apoptosis of cortical lymphocytes in these animals. These data suggest that the TrkB ligands are involved in promoting cell survival, especially of the cortical thymocytes (García-Suárez et al. 2002). Finally, the structure of the thymus in animals lacking the gene coding for p75NTR was consistent with normality (García-Suárez et al. 2001).
Taken together, these results lend support to the idea that in the mammalian thymus both the NGF/TrkA and the BDNF/TrkB systems play important roles in the development and maintenance of epithelial cells and thymocytes, respectively, and that both ligand–receptor complexes are involved in the intercellular communication between these two main cell types of the thymus.
For lymphoid organs, data are still fragmentary. Results from our laboratory indicate that the spleen of newborn mice deficient in p75 shows structural changes consisting of an absence of innervating sympathetic fibers as well as a lack of incipient white pulp areas around the arterioles (Pérez-Pérez et al. 2003). By contrast, functional TrkA- or TrkB-deficient mice appear structurally normal (M. Pérez-Pérez et al. unpubl. obs.), although the possibility of a functional deficit of B cells should be addressed in further studies. Ruberti et al. (2000) observed increased cell death in the spleen of NGF-deficient adult mice.
The lymphoid organs, and especially the thymus, are richly innervated by sensory and vegetative nerve fibres that decrease with ageing (for references see Bellinger et al. 1997; Cavallotti et al. 1999, 2002). It is possible that some of the structural changes observed in the NT-receptor-deficient animals could be related to a decrease in innervation, but this has yet to be investigated.
Pathologies associated with changes in the neurotrophin system
It is well known that the nervous and immune systems interact in both health and disease, although the importance of these interactions is still a matter of debate (Kinoshita & Hato, 2001; Sternberg, 2001). Different hypotheses aimed at explaining, at least partially, the pathogenesis of several diseases have emerged on the basis of current knowledge of the role of NTs in the immune system. Some authors have suggested that NGF, and probably other NTs as well, acts as a hormone that is liberated into the bloodstream at times of stress, and in this way is able to act on immune cells throughout the body. This theory is based on the observation that NGF levels in plasma increase during stress and immunological diseases (Bonini et al. 1999). However, the fact that immune cells express both NT signalling receptors and NTs themselves suggests that they probably act on a local level and over short distances. Nevertheless, the NT/receptor complexes are likely to participate in the highly complex network of intercellular communications made up of cytokines, growth factors, neuropeptides and hormones (Mentlein & Kendall, 2000). Interleukines and other cytokines act as intermediaries in most of the inflammatory and immune actions of NTs in some diseases. There is an abundance of data on the influences of NTs on the production of other cytokines, and the influence of these on the synthesis of NTs (Aloe & Fiore, 1998; Turrini et al. 1998; Aloe et al. 1999) or their receptors (Besser & Wank, 1999) in different tissues. Nevertheless, little of this information applies to immune system cells (Screpanti et al. 1993; Marshall et al. 1999).
The inflammatory role of NGF is well known and its levels increase during inflammation, allergies and diseases of the immune system (Otten et al. 2000; Stanisz & Stanisz, 2000). Particularly important is the role of NGF in inflammatory hyperalgesia (Mendell et al. 1999; Shu & Mendell, 1999). This effect is probably due to a direct action of NGF on mast cells and sensory neurones (Woolf et al. 1996). Local production of NGF by immune cells, stimulated by IL-1β, in turn stimulated by TNFα, is probably the source of NGF (Woolf et al. 1997). Progressive tactile hyperalgesia elicited by repeated touch stimulation during inflammation is also NGF-dependent (Ma & Woolf, 1997). It has also been demonstrated recently that BDNF and NT-4/5 acting through TrkB, but not NT-3/TrkC, regulate nociceptive response to noxious heat as does NGF through TrkA (Shu et al. 1999).
In asthma and other allergic diseases, NGF levels are increased (Bonini et al. 1996; Lambiase et al. 1997; Sanico et al. 1999; Renz, 2001; de Vries et al. 2002). The bronchial hyper-reactivity of asthma is accompanied by an increase in NGF, probably produced by mononuclear cells, which enhances local Th2 responses, thereby increasing the production of IL-4, IL-5, IgG1 and IgE, but not IFN-γ nor IgG2 (Braun et al. 1998). Some of these responses may be mediated by p75NTR acting on Th2 cells (Tokuoka et al. 2001). Moreover, systemic NGF administration increases histamine-induced bronchial hyper-reactivity; this effect is probably mediated by tachykinins because it is abolished by a neurokinin-1 receptor antagonist, and may be exerted indirectly, via macrophages or mast cells (de Vries et al. 1999). Increased local levels of BDNF have recently been detected in allergic asthma (Braun et al. 1999).
During the development of atherosclerotic lesions in rats, there is induction of NTs and their receptors in the vascular smooth cells, whereas in the established lesions only the expression of p75NTR remains, because the activation of this receptor is an inductor of the smooth cell apoptosis observed in those lesions (Wang et al. 2000). In man, NGF decreases in the atherosclerotic lesions whereas the expression of p75NTR is increased (Chaldakov et al. 2001).
In arthritis, NGF levels are elevated in both serum (Dicou et al. 1993) and synovial fluid (Aloe et al. 1992; Aloe & Tuveri, 1997; Halliday et al. 1998), and this increase is higher in spondyloarthritis than in rheumatoid arthritis (Dicou et al. 1996). In the knee joints of arthritic mice, IL-1β (but not TNFα) increases NGF, and NGF seems to increase TNFα (Aloe & Fiori, 1998). NGF serum levels are also higher than normal in systemic lupus erythematosus (Bracci-Laudiero et al. 1993b; Dicou et al. 1993) as well as in a murine lupus model (Bracci-Laudiero et al. 1996). Interestingly, NGF has been used successfully in the treatment of chronic vasculitic ulcers associated with rheumatoid arthritis due to the keratinocyte proliferation and the vascular neoangiogenesis promoted by this molecule (Tuveri et al. 2000).
Post-infectious and autoimmune diseases (Riikonen et al. 1998), fibromyalgia (Giovengo et al. 1999) and chronic daily headache (Sarchielli et al. 2001) course with increased levels of NGF in the cerebrospinal fluid. Interestingly, NGF receptors are up-regulated in experimental autoimmune encephalomyelitis (Oderfeld-Nowak et al. 2001), and a gene therapy approach has been used experimentally, with good results, to down-regulate the expression of p75NTR (Soilu-Hanninen et al. 2000).
As for skin, in psoriatic keratinocytes NGF levels are increased, and NGF acts as a mitogen for these cells and as a T-cell activator (Raychaudhuri et al. 1998). In AIDS, patients with Kaposi's sarcoma show higher NGF levels than patients without Kaposi's sarcoma, and also higher than in those with non-AIDS Kaposi, and these tumour cells express TrkA and proliferate when exposed to NGF (Pica et al. 1998). In prurigo nodularis NGF is overexpressed in the skin (Johansson et al. 2002).
It is possible that studies of transgenic mice will contribute greatly to our understanding of the possible role of NTs in the immune system, and the importance of these growth factors in health and disease (García-Suárez et al. 2000a, 2001, 2002). This is the case for an extremely rare disorder called congenital insensitivity to pain with anhidrosis (CIPA), which has recently been shown to be caused by a mutation in the trkA gene (Indo et al. 1996; Mardy et al. 1999; Kobayashi et al. 2002). Patients with this disorder show neuronal deficits similar to those of trkA knockout mice. Furthermore, despite their normal serum immunoglobulin levels, they show frequent infections, especially osteomyelitis, indicating a possible defect in B-cell function. These findings are of interest, taking into account the aforementioned data on the possible role of the NGF/TrkA system in B cells. Unpublished data by I. Silos-Santiago and colleagues demonstrate that mice lacking functioning trkA have a strong immunodeficiency affecting both T and B cells.
Concluding remarks and future directions
Through their widespread expression in the immune organs and immunocompetent cells, NTs are candidate molecules for regulating immune as well as neuroimmune interactions. Accurate studies in transgenic and knockout mice, especially in adult surviving animals, are revealing hitherto unknown roles of NTs in vivo. This will open up new perspectives for a potential therapeutic use of NTs when pathologies are due to the absence, increased or defective production of NTs, or by mutations in their receptors.
Based on the available data mentioned in this review, it seems likely that NTs may be involved in immune pathologies. Thus an altered concentration of circulating or tissular NGF levels is associated with autoimmune inflammatory diseases, allergic diseases and parasitic infections. Furthermore, NTs, or dysregulation of NT receptor expression, may be involved in regulating the growth, differentiation and apoptosis of some kinds of non-neuronal tumours (see for a review Rubin & Segal, 2003), such as pancreatic ductal adenocarcinoma (Miknyoczki et al. 1999), melanoma (Innominato et al. 2001), prostate cancer (Satoh et al. 2001) and lung cancer (Ricci et al. 2001).
In the past few years, NTs and in particular NGF have been used with varying degrees of success in a variety of disorders, including peripheral metabolic and toxic neuropathies (Pradat et al. 2002; Apfel, 2002), spinal cord injuries (Blesch et al. 2002; Bregman et al. 2002; Murray et al. 2002), neurodegenative diseases (Batchelor et al. 1999; see also Dechant & Barde, 2002), and cutaneous (Matsuda et al. 1998) and corneal (Lambiase et al. 2000) wound repair. In tumours the Trk receptors are also viable molecular targets for medical intervention (see Ruggeri et al. 1999; Miknyoczki et al. 2002). Regarding diseases in which the immune system is particularly involved, NGF has proved to have useful effects in vasculitis-induced rheumatoid arthritis (Tuveri et al. 2000; Aloe, 2001) and is now being considered as a new therapeutic strategy in the blockade of NT overexpression during the allergic or inflammatory process. Nevertheless, it must be emphasized that there are serious pharmacological problems with the use of NTs in human therapy, especially because of the manner and site of administration. Virus transfer and the transplantation of engineered cells, which have been performed experimentally, may represent promising perspectives for NT delivery or NT-receptor blocking in the near future.
Acknowledgments
This study was supported by grants from the Spanish DGICYT (CC-99-SAF-0119-CO2O2) and University of Messina, Italy (PRA 1999). We wish to thank Mr Diego F. Monjil for his excellent technical assistance in preparing the figures.
References
- Abramchik SS, Yermakova VN, Kaliunov RM, Tanina RM, Tumilovich MK. The immunomodulatory effect of nerve growth factor. J. Neurosci. Res. 1988;19:349–356. doi: 10.1002/jnr.490190310. [DOI] [PubMed] [Google Scholar]
- Aloe L, Levi-Montalcini R. Mast cell increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J. Neuronimmunol. 1988;18:1–12. doi: 10.1016/0165-5728(88)90129-4. [DOI] [PubMed] [Google Scholar]
- Aloe L, De Simone R. NGF primed spleen cells injected in brain of developing rats differentiate into mast cells. Int. J. Dev. Neurosci. 1989;7:565–573. doi: 10.1016/0736-5748(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheumatism. 1992;35:351–355. doi: 10.1002/art.1780350315. [DOI] [PubMed] [Google Scholar]
- Aloe L, Micer A, Bracci-Lauderio L, Vigneti E, Turrini P. Presence of nerve growth factor in the thymus of prenatal, postnatal and pregnant rats. Thymus. 1997;24:221–231. [PubMed] [Google Scholar]
- Aloe L, Tuveri MA. Nerve growth factor and autoimmune rheumatic diseases. Clinics Exp. Rheumatol. 1997;15:433–438. [PubMed] [Google Scholar]
- Aloe L, Fiore M. Neuroinflammatory implications of Schistosoma mansoni infection: new information from the mouse model. Parasitol. Today. 1998;14:314–318. doi: 10.1016/s0169-4758(98)01283-6. [DOI] [PubMed] [Google Scholar]
- Aloe L, de Simone M, Properzi F. Nerve growth factor: a neurotrophin with activity on cells of the immune system. Microscope Res. Technique. 1999;45:285–291. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<285::AID-JEMT12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Aloe L. Nerve growth factor and neuroimmune responses: basic and clinical observations. Arch. Physiol. Biochem. 2001;109:354–356. doi: 10.1076/apab.109.4.354.4235. [DOI] [PubMed] [Google Scholar]
- Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does future hold? Int. Rev. Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- Auffray I, Chevalier S, Froger J, Izac B, Vainchenke W, Gascasn H, et al. Nerve growth factor is involved in the supportive effect by bone marrow-derived stromal cells of the factor-dependent human cell line UT-7. Blood. 1996;88:1608–1618. [PubMed] [Google Scholar]
- Baig MA, Khan MA. The induction of neurotrophin and TRK receptor mRNA expression during early avian embryogenesis. Int. J. Dev Neuroscience. 1996;14:55–60. doi: 10.1016/0736-5748(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann. New York Acad. Sciences. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J. Biol. Chem. 1993;268:15150–15157. [PubMed] [Google Scholar]
- Barouch R, Appel E, Kazimirsky G, Brodie C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J. Neuroimmunol. 2001a;112:72–77. doi: 10.1016/s0165-5728(00)00408-2. [DOI] [PubMed] [Google Scholar]
- Barouch R, Kazimirsky G, Appel E, Brodie C. Nerve growth factor regulates TNF-alpha production in mouse macrophages via MAP kinase activation. J. Leukaemia Biol. 2001b;69:1019–1026. [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs GA, Donnan GA, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayas A, Kruse N, Moriabadi NF, Weber F, Hummel V, Wohleben G, et al. Modulation of cytokine mRNA expression by brain-derived neurotrophic factor and nerve growth factor in human immune cells. Neurosci. Lett. 2003;335:155–158. doi: 10.1016/s0304-3940(02)01152-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Felten SY, Ackerman KD, Lorton D, Madden KS, Felten DL. Noradrenergic sympathetic innervation of lymphoid organs during development, aging and autoimmune disease. In: Amenta F, editor. Aging of the Autonomic Nervous System. Boca Raton: CRC Press; 1997. pp. 243–284. [Google Scholar]
- Bentley CA, Lee KF. p75 is important for axon growth and Schwann cell migration during development. J. Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser M, Wank R. Cutting-edge: clonally restricted production of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J. Immunol. 1999;162:6303–6306. [PubMed] [Google Scholar]
- Bischoff SC, Dahinden DC. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662–2669. [PubMed] [Google Scholar]
- Blesch A, Lu P, Tuszynski MH. Neurotrophic factor, gene therapy, and neural stem cells for spinal cord repair. Brain Res. Bull. 2002;57:833–838. doi: 10.1016/s0361-9230(01)00774-2. [DOI] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, et al. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl Acad. Sci. USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Bonini S, Levi-Schaffer F, Aloe L. Nerve growth factor: an important molecule in allergic inflammation and tissue remodelling. Int. Arch. Allergy Immunol. 1999;118:159–162. doi: 10.1159/000024055. [DOI] [PubMed] [Google Scholar]
- Bothwell M. p75NTR: a receptor after all. Science. 1996;272:506–507. doi: 10.1126/science.272.5261.506. [DOI] [PubMed] [Google Scholar]
- Boven LA, Middel J, Portegies P, Verhoef J, Jansen GH, Nottet HS. Overexpression of nerve growth factor and basic fibroblast growth factor in AIDS dementia complex. J. Neuroimmunol. 1999;97:154–162. doi: 10.1016/s0165-5728(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Boyle MDP, Lawman MJP, Gee AP, Young M. Nerve growth factor: a chemotactic factor for polymorphonuclear leukocytes in vivo. J. Immunol. 1985;134:564–568. [PubMed] [Google Scholar]
- Bracci-Laudiero L, Vigneti E, Aloe L. In vivo and in vitro effect of NGF on bursa of Fabricius cells during chick embryo development. Int. J. Neurosci. 1991;59:189–198. doi: 10.3109/00207459108985462. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Vigneti E, Iannicola C, Aloe L. NGF retards apoptosis in chick embryo bursal cell in vitro. Differentiation. 1993a;53:61–66. doi: 10.1111/j.1432-0436.1993.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Aloe L, Levi-Montalcini R, Galeazzi M, Schilter D, et al. Increased levels of NGF in sera of systemic lupus erythematosus patients. Neuroreport. 1993b;4:563–565. doi: 10.1097/00001756-199305000-00025. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Aloe L, Stenfors C, Tirassa P, Theodorsson E, Lundberg T. Nerve growth factor stimulates production of neuropeptide Y in human lymphocytes. Neuroreport. 1996;7:485–488. doi: 10.1097/00001756-199601310-00026. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Aloe L, Buanne P, Finn A, Stenfors C, Vigneti E, et al. NGF modulates CGRP synthesis in human B-lymphocytes: a possible anti-inflammatory action of NGF? J. Neuroimmunol. 2002;123:58–65. doi: 10.1016/s0165-5728(01)00475-1. [DOI] [PubMed] [Google Scholar]
- Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, et al. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, et al. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respiratory Cell Mol. Biol. 1999;21:537–546. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- Brodie C, Gelfand EW. Functional nerve growth factor receptors on human B lymphocytes. Interactions with IL-2. J. Immunol. 1992;148:3492–3497. [PubMed] [Google Scholar]
- Brodie C, Oshiba A, Renz H, Bradley K, Gelfand EW. Nerve growth factor and anti-CD40 provide opposite signals for the production of IgE in interleukin-4-treated lymphocytes. Eur. J. Immunol. 1996;26:171–178. doi: 10.1002/eji.1830260127. [DOI] [PubMed] [Google Scholar]
- Bruni A, Bigon E, Boarato E, Mietto L, Leon A, Toffano G. Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS Lett. 1982;138:190–192. doi: 10.1016/0014-5793(82)80438-9. [DOI] [PubMed] [Google Scholar]
- Bullock ED, Johnson EM., Jr Nerve growth factor induces the expression of certain cytokine and bcl-2 in mast cells. Potential role in survival promotion. J. Biol. Chem. 1996;271:27500–27508. doi: 10.1074/jbc.271.44.27500. [DOI] [PubMed] [Google Scholar]
- Burgi B, Brunner T, Dahinden CA. The degradation products of the C5a anaphylatoxin C5adesarg retains basophil-activating properties. Eur. J. Immunol. 1994;24:1583–1589. doi: 10.1002/eji.1830240720. [DOI] [PubMed] [Google Scholar]
- Burgi B, Otten UH, Ochensberger B, Rihs S, Heese K, Ehrhard PB, et al. Basophil priming by neurotrophic factors. Activation through the trk receptor. J. Immunol. 1996;157:5582–5588. [PubMed] [Google Scholar]
- Caneva L, Soligo D, Cattoretti G, De Harven E, Deliliers GL. Immuno-electron microscopy characterization of human bone marrow stromal cells with anti-NGFR antibodies. Blood Cells Mol Dis. 1995;21:73–85. doi: 10.1006/bcmd.1995.0011. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Albers KM, Beiting DJ, Parish M, Conner JM, Davis BM. NGF modulates sympathetic innervation of lymphoid tissues. J. Neurosci. 1995;15:5892–5899. doi: 10.1523/JNEUROSCI.15-09-05892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Johnson S, Parrish ME, Cass WA. Development of immune hyperinnervation in NGF-transgenic mice. Exp. Neurol. 1998;149:209–220. doi: 10.1006/exnr.1997.6711. [DOI] [PubMed] [Google Scholar]
- Caroleo MC, Costa N, Bracci-Laudiero L, Aloe L. Human monocyte/macrophages activated by exposure to LPS overexpress NGF and NGF receptors. J. Neuroimmunol. 2001;113:193–201. doi: 10.1016/s0165-5728(00)00441-0. [DOI] [PubMed] [Google Scholar]
- Carter BD, Lewin GR. Neurotrophins live or let die: Does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Kong H, Chao MV. Neurotrophins: the biological paradox of survival factors eliciting apoptosis. Cellular Death Differentiation. 1998;5:357–364. doi: 10.1038/sj.cdd.4400377. [DOI] [PubMed] [Google Scholar]
- Cattoreti G, Schiro R, Orazi A, Soligo D, Colombo M. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–1738. [PubMed] [Google Scholar]
- Cavallotti C, Artico M, Cavallotti D. Occurrence of adrenergic nerve fibers and of adrenaline in thymus gland of juvenile and aged rats. Immunol. Lett. 1999;70:53–62. doi: 10.1016/s0165-2478(99)00127-3. [DOI] [PubMed] [Google Scholar]
- Cavallotti D, Artico M, Iannetti G, Cavallotti C. Occurrence of adrenergic nerve fibers in human thymus during immune response. Neurochem. Int. 2002;40:211–221. doi: 10.1016/s0197-0186(01)00074-2. [DOI] [PubMed] [Google Scholar]
- Chaldakov GN, Stankulov IS, Fiore M, Ghenev PI, Aloe L. Nerve growth factor levels and mast cell distribution in human coronary atherosclerosis. Atherosclerosis. 2001;159:57–66. doi: 10.1016/s0021-9150(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Chao M, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Chesa PG, Rettig WJ, Thomson TM, Old LJ, Melamed MR. Immunohistochemical analysis of nerve growth factor receptor in normal and malignant human tissues. J. Histochem. Cytochem. 1988;36:383–389. doi: 10.1177/36.4.2831267. [DOI] [PubMed] [Google Scholar]
- Chevalier S, Praloran V, Smith C, Macgrogan D, Ip NY, Yancopoulos GD, et al. Expression and functionality of the trkA proto-oncogene product/NGF receptor in undifferentiated hematopoietic cells. Blood. 1994;83:1479–1485. [PubMed] [Google Scholar]
- Ciriaco E, Dall’Aglio C, Hannestad J, Huerta JJ, Laura R, Germana G, Vega JA. Localization of Trk neurotrophin receptor-like proteins in avian primary lymphoid organs (thymus and bursa of Fabricius) J. Neuroimmunol. 1996;69:73–78. doi: 10.1016/0165-5728(96)00062-8. [DOI] [PubMed] [Google Scholar]
- Ciriaco E, García-Suárez O, Ricci A, Abbate F, Piedimonte G, Vega JA. Trk-like proteins during post-hatching growth of the avian bursa of Fabricius. Vet. Immunol. Immunopathol. 1997;55:313–320. doi: 10.1016/s0165-2427(96)05714-5. [DOI] [PubMed] [Google Scholar]
- D’Onofrio M, de Grazia U, Morrone S, Cuomo L, Spinsanti P, Frati L, et al. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur. Cytokine Network. 2000;11:283–291. [PubMed] [Google Scholar]
- Dean DH, Hiramoto RN, Ghanta VK. Modulation of immune responses. A possible role for murine salivary epidermal and nerve growth factors. J. Periodontol. 1987;58:498–500. doi: 10.1902/jop.1987.58.7.498. [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA. Signalling through the neurotrophin receptor p75NTR. Current Opinion Neurobiol. 1997;7:413–418. doi: 10.1016/s0959-4388(97)80071-2. [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75 (NTR): novel functions and implications for diseases of the nervous system. Nature Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Dicou E, Masson C, Jabbour W, Nerriere V. Increased frequency of NGF in sera of rheumatoid arthritis and systemic lupus erythematosus patients. Neuroreport. 1993;5:321–324. doi: 10.1097/00001756-199312000-00036. [DOI] [PubMed] [Google Scholar]
- Dicou E, Perrot S, Menkes CJ, Masson C, Nerriere V. Nerve growth factor (NGF) autoantibodies and NGF in the synovial fluid: implications in spondylarthropathies. Autoimmunity. 1996;24:1–9. doi: 10.3109/08916939608995352. [DOI] [PubMed] [Google Scholar]
- Dubus P, Parrens M, El-Mokhtari Y, Ferrer J, Groppi A, Merlio JP. Identification of novel trkA variants with deletions in leucine-rich motifs of the extracellular domain. J. Neuroimmunol. 2000;107:42–49. doi: 10.1016/s0165-5728(00)00257-5. [DOI] [PubMed] [Google Scholar]
- Ehrhard PB, Ganter U, Bauer J, Otten U. Expression of functional trk protooncogene in human monocytes. Proc. Natl Acad. Sci. USA. 1993a;90:5423–5427. doi: 10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc. Natl Acad. Sci. USA. 1993b;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhard PB, Erb P, Graumann U, Schmutz B, Otten U. Expression of functional trk tyrosine kinase receptors after T cell activation. J. Immunol. 1994;152:2705–2709. [PubMed] [Google Scholar]
- Ernfors P, Hallbook F, Ebendal T, Shooter EM, Radeke MJ, Misko TP, et al. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1988;1:983–996. doi: 10.1016/0896-6273(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, et al. The cytoplasmic and transmembrane domains of p75 and TrkA receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Fariñas I. Neurotrophin actions during the development of the peripheral nervous system. Microsc. Res. Techniques. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Franklin RA, Brodie C, Melamed I, Terada N, Lucas JJ, Gelfand EW. Nerve growth factor induces activation of MAP-kinase and p90rsk in human B lymphocytes. J. Immunol. 1995;154:4965–4972. [PubMed] [Google Scholar]
- Friedman WJ, Greene LA. Neurotrophins signaling via Trks and p75. Exp. Cell Res. 1999;253:131–142. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- Garaci E, Caroleo MC, Aloe L, Aquaro S, Piacentini M, Costa N, et al. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc. Natl Acad. Sci. USA. 1999;96:14013–14018. doi: 10.1073/pnas.96.24.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Suarez O, Hannestad J, Esteban I, Martinez Del Valle M, Naves FJ, Vega JA. Neurotrophin receptor-like protein immunoreactivity in human lymph nodes. Anat. Record. 1997;249:226–232. doi: 10.1002/(SICI)1097-0185(199710)249:2<226::AID-AR9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- García-Suárez O, Hannestad J, Esteban I, Sainz R, Naves FJ, Vega JA. Expression of the TrkB neurotrophin receptor by thymic macrophages. Immunology. 1998;94:235–241. doi: 10.1046/j.1365-2567.1998.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Suárez O, Germanà A, Hannestad J, Ciriaco E, Laurà R, Naves J, et al. TrkA is necessary for the normal development of the murine thymus. J. Neuroimmunol. 2000a;108:11–21. doi: 10.1016/s0165-5728(00)00251-4. [DOI] [PubMed] [Google Scholar]
- García-Suárez O, Germanà A, Hannestad J, Pérez-Pérez Esteban I, Naves FJ, et al. Changes in the expression of the nerve growth factor receptors TrkA and p75LNFR in the rat thymus with ageing and increased nerve growth factor plama levels. Cell Tissue Res. 2000b;301:225–234. doi: 10.1007/s004419900133. [DOI] [PubMed] [Google Scholar]
- García-Suárez O, Germana A, Hannestad J, Ciriaco E, Silos-Santiago I, Germanà G, et al. Involvement of the NGF receptors (TrkA and p75lngfr) in the development and maintenance of the thymus. Italian J. Anat. Embryol. 2001;106:279–285. [PubMed] [Google Scholar]
- García-Suárez O, Blanco-Gelaz MA, López ML, Germanà A, Cabo R, Diaz-Esnal B, et al. Massive lymphocyte apoptosis in the thymus of functionally deficient TrkB mice. J. Neuroimmunol. 2002;129:25–34. doi: 10.1016/s0165-5728(02)00166-2. [DOI] [PubMed] [Google Scholar]
- Gee AP, Boyle MD, Munger KL, Lawman MJ, Young M. Nerve growth factor: stimulation of polymorphonuclear leukocyte chemotaxis in vitro. Proc. Natl Acad. Sci. USA. 1983;80:7215–7218. doi: 10.1073/pnas.80.23.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J. Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- Glass DJ, Yancopoulos GD. The neurotrophins and their receptors. Trends Cell Biol. 1993;3:262–268. doi: 10.1016/0962-8924(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Glick B. Historical perspective: the bursa of Fabricius and its influence on B-cell development, past and present. Vet. Immunol. Immunopathol. 1991;30:3–12. doi: 10.1016/0165-2427(91)90003-u. [DOI] [PubMed] [Google Scholar]
- Glick B, Olah J. A bursal secretory dendritic cell and its contributions to the microenvironment of the developing bursal follicle. Res. Immunol. 1993;144:446–447. doi: 10.1016/0923-2494(93)80127-k. [DOI] [PubMed] [Google Scholar]
- Götz R, Koster R, Winkler C, Raulf F, Lottspeich F, Schartl M, Thoenen H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- Grills BL, Schuijers JA. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthopaedica Scand. 1998;69:415–419. doi: 10.3109/17453679808999059. [DOI] [PubMed] [Google Scholar]
- Gudat F, Laubscher A, Otten U, Pletscher A. Shape changes induced by biologically active peptides and NGF in blood platelets of rabbits. Br. J. Pharmacol. 1981;74:533–538. doi: 10.1111/j.1476-5381.1981.tb10461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallböök F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Current Opinion Neurobiol. 1999;9:616–621. doi: 10.1016/S0959-4388(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem. Res. 1998;23:919–922. doi: 10.1023/a:1022475432077. [DOI] [PubMed] [Google Scholar]
- Hamada A, Watanabe N, Ohtomo H, Matsuda H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br. J. Haematol. 1996;93:299–302. doi: 10.1046/j.1365-2141.1996.5151055.x. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Levanti B, Vega JA. Distribution of neurotrophin receptors in human palatine tonsils: an immunohistochemical study. J. Neuroinmmunol. 1995;58:131–137. doi: 10.1016/0165-5728(95)00014-s. [DOI] [PubMed] [Google Scholar]
- Hannestad J, García-Suárez O, Huerta JJ, Esteban I, Naves FJ, Vega JA. TrkA neurotrophin receptor protein in the rat and human thymus. Anat. Record. 1997;249:373–379. doi: 10.1002/(SICI)1097-0185(199711)249:3<373::AID-AR8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Germana A, Catania S, Laura R, Ciriaco E, Vega JA. Neurotrophins and their receptors in the pigeon caecal tonsil. An immunohistochemical study. Vet. Immunol. Immunopathol. 1998;61:359–367. doi: 10.1016/s0165-2427(97)00145-1. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Marino F, Germana A, Catania S, Abbate F, Ciriaco E, et al. Trk neurotrophin receptor-like proteins in the teleost Dicentrarchus labrax. Cell Tissue Res. 2000;300:1–9. doi: 10.1007/s004410000181. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Hale LP. The human thymus: a chimeric organ of central and peripheral lymphoid components. Immunol. Res. 1998;18:61–78. doi: 10.1007/BF02788750. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Lum T. Fish neurotrophins and Trk receptors. Int. J. Dev. Neurosci. 2000;18:1–27. doi: 10.1016/s0736-5748(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. The many faces of p75NTR. Current Opinion Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Hollander GA, Wang B, Nuchogiannopoulou A, plantenburg PP, Van Ewijk W, Burakoff SJ, et al. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373:350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- Horigome K, Pryor JC, Bullock ED, Johson EM. Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J. Biol. Chem. 1993;268:1481–1487. [PubMed] [Google Scholar]
- Horigome K, Bullock ED, Johnson EM., Jr Effectcs of nerve growth factor on rat peritoneal mast cells. Survival promotion and intermediate gene-early gene induction. J. Biol. Chem. 1994;269:2695–2702. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo Y, Tsurata M, Karim MA, Ohta K, Kawano T, Tonoki H, et al. Mutations in the TrkA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genet. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Innominato PF, Libbrecht L, Van Den Oord JJ. Expression of neurotrophins and their receptors in pigment cell lesion of the skin. J. Pathol. 2001;194:95–100. doi: 10.1002/path.861. [DOI] [PubMed] [Google Scholar]
- Ip NY, Ibanez CF, Nye SH, Mcclain J, Jones PF, Gies DR, et al. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl Acad. Sci. USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Greene LA, et al. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Johansson O, Liang Y, Emtestam L. Increased nerve growth factor- and tyrosine kinase A-like immunoreactivities in prurigo nodularis skin – an exploration of the cause of neurohyperplasia. Arch. Dermatol. Res. 2002;293:614–619. doi: 10.1007/s00403-001-0285-8. [DOI] [PubMed] [Google Scholar]
- Kanbe N, Kurosawa M, Miyachi Y, Kanbe M, Saitoh H, Matsuda H. Nerve growth factor prevents apoptosis of cord blood-derived human cultured mast cells synergistically with stem cell factor. Clin. Exp. Allergy. 2000;30:1113–1120. doi: 10.1046/j.1365-2222.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Ushio H, Koyama H, Okada M, Oikawa M, Yoshihara T, et al. 2.5S nerve growth factor enhances survival, phagocytosis, and superoxide production of murine neutrophils. Blood. 1991;77:1320–1325. [PubMed] [Google Scholar]
- Kannan Y, Usami K, Okada M, Shimizu S, Matsuda H. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem. Biophys. Res. Commun. 1992;186:1050–1056. doi: 10.1016/0006-291x(92)90853-d. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Matsuda H, Ushio K, Kawamoto K, Shimada Y. Murine granulocyte-macrophage and mast cell colony formation promoted by nerve growth factor. Int. Arch. Allergy Immunol. 1993;102:362–367. doi: 10.1159/000236584. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Stead RH, Goldsimith CH, Bienenstock J. Lymphoid tissues induce NGF-dependet and NGF-independent neurite outgrowth from rat superior cervical ganglia explants in culture. J. Neurosci. Res. 1994;37:374–383. doi: 10.1002/jnr.490370309. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Bienenstock J, Ohta M, Stanisz AM, Stead RH. Nerve growth factor and cytokines mediate lymphoid tissue-induced neurite outgrowth from mouse superior cervical ganglia in vitro. J. Immunol. 1996;175:313–320. [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Kashiwamata S, Kato K. An acceleration of age-related increases in levels of the beta-subunit of nerve growth factor in selected tissues form senescence-accelerated mice (SAM-P/8) J. Mol. Neurosci. 1993;4:107–115. doi: 10.1007/BF02782123. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Kaisho Y, Shintani A, Nagahama M, Kato K. Tissue distribution and immunocytochemical localization of neurotrophin-3 in the brain and peripheral tissues of rats. J. Neuroschem. 1996;66:330–337. doi: 10.1046/j.1471-4159.1996.66010330.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trk protooncogene receptor. Blood. 1995;86:4638–4644. [PubMed] [Google Scholar]
- Kawamoto K, Aoki J, Tanaka A, Itakura A, Hosono H, Arai H, et al. Nerve growth factor activates mast cells through the collaborative interaction with lysophosphatidylserine expressed on the membrane surface of activated platelets. J. Immunol. 2002;168:6412–6419. doi: 10.4049/jimmunol.168.12.6412. [DOI] [PubMed] [Google Scholar]
- Kendall SE, Goldhawk DE, Kubu C, Barker PA, Verdi JM. Expression analysis of a novel p75NTR signaling protein, which regulates cell cycle progression and apoptosis. Mechanism Dev. 2002;117:187–200. doi: 10.1016/s0925-4773(02)00204-6. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren RE, Fainzilber M, Hauser G, van Minnen J, Vreugdenhil E, Smit AB, et al. Early evolutionary origin of the neurotrophin receptor family. EMBO J. 1998;17:2534–2542. doi: 10.1093/emboj/17.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H, Yoshida A, Ishioka C, Mikawa H. Stimulation of Ig production and growth of human lymphoblastoid B-cell lines by nerve growth factor. Immunology. 1991a;72:451–452. [PMC free article] [PubMed] [Google Scholar]
- Kimata H, Yoshida A, Ishioka C, Kusunoki T, Hosoi T, Mikawa H. Nerve growth factor specifically induces human IgG4 production. Eur. J. Immunol. 1991b;21:137–141. doi: 10.1002/eji.1830210121. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Hato F. Cellular and molecular interactions of thymus with endocrine organs and nervous system. Cellular Mol. Biol. 2001;47:103–117. [PubMed] [Google Scholar]
- Kittur SD, Song L, Endo H, Adler WH. Nerve growth factor receptor gene expression in human peripheral blood lymphocytes in aging. J. Neurosci. Res. 1992;32:444–448. doi: 10.1002/jnr.490320316. [DOI] [PubMed] [Google Scholar]
- Klein R, Jing S, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogen encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Mizisin AP. Nerve growth factor and neurotrophin-3 promote chemotaxis of mouse macrophages in vitro. Neurosci. Letter. 2001;305:157–160. doi: 10.1016/s0304-3940(01)01854-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. doi: 10.1182/blood.v99.6.2214. [DOI] [PubMed] [Google Scholar]
- Kronfeld I, Kazimirsky G, Gelfand EW, Brodie C. NGF rescues human B lymphocytes from anti-IgM induced apoptosis by activation of PKCζ. Eur. J. Immunol. 2002;32:136–143. doi: 10.1002/1521-4141(200201)32:1<136::AID-IMMU136>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kuner P, Hertel C. NGF induces apoptosis in a human neuroblastoma cell line expressing the neurotrophin receptor p75NTR. J. Neurosci. Res. 1998;54:465–474. doi: 10.1002/(SICI)1097-4547(19981115)54:4<465::AID-JNR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- La Sala A, Corinti S, Federici M, Saragovi HU, Girolomoni G. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J. Leukocyte Biol. 2000;68:104–110. [PubMed] [Google Scholar]
- Labouyrie E, Parrens M, de Mascarel A, Vbloch B, Merlio JP. Distribution of NGF receptors in normal and pathological human lymphoid tissues. J. Neuroimmunol. 1997;77:161–173. doi: 10.1016/s0165-5728(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, et al. Expression of neurotrophins and their receptors in human bone marrow. Am. J. Pathol. 1999;154:405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Fu WY, Ip FCF IPNY. Cloning and expression of a novel neurotrophin, NT-7. Mol. Cell Neurosci. 1998;11:64–76. doi: 10.1006/mcne.1998.0666. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M. TrkC a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Tapley P, Barbacid M. TrkC encodes multiple neurotrophin-3 receptors with distinct biological properties, and substrate specifities. EMBO J. 1993;12:3083–3094. doi: 10.1002/j.1460-2075.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Bracci-Lauderio L, Bonini S, Starace G, Délios MM, de Carli M, et al. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immunol. 1997;100:408–414. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Manni L, Bonnini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analysis of rat and human corneas. Invest. Ophthalmol. Vision Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- Laurenzi MA, Barbany G, Timmusk T, Lindgren JA, Persson H. Expression of mRNA encoding neurotrophins and neurotrophin receptors in rat thymus, spleen tissues and immunocompetent cells. Regulation of neurotrophin-4 mRNA expression by mitogens and leukotriene B4. Eur. J. Biochem. 1994;223:733–741. doi: 10.1111/j.1432-1033.1994.tb19047.x. [DOI] [PubMed] [Google Scholar]
- Laurenzi MA, Beccari T, Stenke L, Sjolinder M, Stinchi S, Lindgren JA. Expression of mRNA encoding neurotrophins and neurotrophin receptors in human granulocytes and bone marrow cells-enhanced neurotrophin-4 expression induced by LTB4. J. Leukocyte Biol. 1998;64:228–234. doi: 10.1002/jlb.64.2.228. [DOI] [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Current Opinion Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, et al. Mast cells synthesize, store and release nerve growth factor. Proc. Natl Acad. Sci. USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanti B, Hannestad J, Esteban I, Ciriaco E, Germana G, Vega JA. Neurotrophin receptor-like proteins in Peyer's patches. Anat. Record. 1997;249:365–372. doi: 10.1002/(SICI)1097-0185(199711)249:3<365::AID-AR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Levanti MB, Germana A, Catania S, Germana GP, Gauna-Anasco L, Vega JA, et al. Neurotrophin receptor-like proteins in the bovine (Bos taurus) lymphoid organs, with special reference to thymus and spleen. Anat. Histol. Embryol. 2001;30:193–198. doi: 10.1046/j.1439-0264.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. Effects of mouse tumor transplantation on the nervous system. Ann. New York Acad. Sci. 1952;55:330–343. doi: 10.1111/j.1749-6632.1952.tb26548.x. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde Y-A. Physiology of neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Liuzzo JP, Petancesca SS, Devi LA. Neurotrophic factors regulate cathepsin S in macrophages and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Mol. Med. 1999;5:334–343. [PMC free article] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Shooter EM. Widespread neurotrophin receptor expression in the immune system and other non-neuronal rat tissues. J. Neurochem. 1995;64:1780–1789. doi: 10.1046/j.1471-4159.1995.64041780.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez N, Frade JM. Control of the cell cycle by neurotrophins: lessons from the p75 neurotrophin receptor. Histol. Histopathol. 2002;17:1227–1237. doi: 10.14670/HH-17.1227. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. The progressive tactile hyperalgesia induced by peripheral inflammation is nerve growth factor dependent. Neuroreport. 1997;8:807–810. doi: 10.1097/00001756-199703030-00001. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Bellusco L, Squinto S, Ip NY, Furt ME, Lindsay RM, et al. Neurotrophin-3. A neurotrophin factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Manning PT, Russel JH, Simmons B, Johnson EM., Jr Protection from guanethidine-induced neuronal destruction by NGF: effects of NGF on immune function. Brain Res. 1985;340:61–69. doi: 10.1016/0006-8993(85)90773-5. [DOI] [PubMed] [Google Scholar]
- Mardy S, Muira Y, Endo F, Matsuda I, Sztriha L, Frossard P, et al. Congenital insensitivity to pain which anhidrosis: novel mutations in the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor. Am. J. Human Genet. 1999;64:1570–1579. doi: 10.1086/302422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroder M, Bellavia D, Meco D, Napolitano M, Stigliano A, Alesse E, et al. Expression of trkB neurotrophin receptor during T cell development. J. Immunol. 1996;157:2864–2872. [PubMed] [Google Scholar]
- Marshall JS, Stead RH, Mcsharry C, Nielsen L, Bienenstock J. The role of mast cell degranulation products in mast cell hyperplasia. I. Mechanism of action of nerve growth factor. J. Immunol. 1990;144:1886–1892. [PubMed] [Google Scholar]
- Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J. Immunol. 1999;162:4271–4276. [PubMed] [Google Scholar]
- Matsuda H, Coughlin MD, Bienenstock J, Denburg JA. Nerve growth factor promotes human hematopoietic colony growth and differentiation. Proc. Natl Acad. Sci. USA. 1988a;85:6508–6512. doi: 10.1073/pnas.85.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Switzer J, Coughlin MD, Bienenstock J, Denburg JA. Human basophilic cell differentiation promoted by 2.5S nerve growth factor. Int. Arch. Allergy Appl. Immunol. 1988b;86:453–457. doi: 10.1159/000234634. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, et al. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J. Exp. Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J. Exp. Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek W, Weskamp G, Erne P, Otten U. Nerve growth factor induces mast cell degranulation without chaining intracellular calcium. FEBS Lett. 1986;198:315–320. doi: 10.1016/0014-5793(86)80428-8. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Melamed I, Kelleher CA, Franklin RA, Brodie C, Hempstead B, Kaplan D, et al. Nerve growth factor signal transduction in human B lymphocytes is mediated by gp140trk. Eur. J. Immunol. 1996;26:1985–1992. doi: 10.1002/eji.1830260903. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Sciorati C, Clementi E. The p75 receptor: first insights into the transduction mechanisms leading to either cell death or survival. Trends Pharmacol. Sci. 2000;21:242–243. doi: 10.1016/s0165-6147(00)01497-8. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc. Res. Techniques. 1999;45:252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Kendall MD. The brain and thymus have much in common: a functional analysis of their microenvironments. Immunol. Today. 2000;21:133–140. doi: 10.1016/s0167-5699(99)01557-1. [DOI] [PubMed] [Google Scholar]
- Middelmas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full length and two truncated receptors. Mol. Cell. Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miknyoczki SJ, Lang D, Huang L, Klei-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behaviour. Int. J. Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Miknyoczki SJ, Wang W, Chang H, Dobrzanski P, Ruggeri BA, Dionne CA, et al. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin. Cancer Res. 2002;8:1924–1931. [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- Miura K, Saini SS, Gauvreau G, Macglashan DW., Jr Differences in functional consequences and signal transduction induced by IL-3, IL-5, and nerve growth factor in human basophils. J. Immunol. 2001;167:2282–2291. doi: 10.4049/jimmunol.167.4.2282. [DOI] [PubMed] [Google Scholar]
- Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, et al. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J. Autoimmunity. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- Murray M, Kim D, Liu Y, Tobias C, Tessler A, Fischer I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res. Rev. 2002;40:292–300. doi: 10.1016/s0165-0173(02)00211-4. [DOI] [PubMed] [Google Scholar]
- Nilsson AS, Fainzilber M, Falck P, Ibanez CF. Neurotrophin-7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998;424:285–290. doi: 10.1016/s0014-5793(98)00192-6. [DOI] [PubMed] [Google Scholar]
- Oderfeld-Nowak B, Zaremba M, Micera A, Aloe L. The upregulation of nerve growth factor receptors in reactive astrocytes of rat spinal cord during experimental autoimmune encephalomyelitis. Neurosci. Lett. 2001;308:165–168. doi: 10.1016/s0304-3940(01)02001-8. [DOI] [PubMed] [Google Scholar]
- Otten U, Baumann B, Girad J. Nerve growth factor induces plasma extravasation in rat skin. Eur. J. Pharmacol. 1984;106:199–201. doi: 10.1016/0014-2999(84)90697-6. [DOI] [PubMed] [Google Scholar]
- Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl Acad. Sci. USA. 1989;86:10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U, Gadient RA. Neurotrophins and cytokines-intermediaries between the immune and nervous systems. Int. J. Dev Neurosci. 1995;13:147–151. doi: 10.1016/0736-5748(95)00016-a. [DOI] [PubMed] [Google Scholar]
- Otten U, Marz P, Heese K, Hock C, Kunz D, Rose-John S. Cytokines and neurotrophins interact in normal and diseased states. Ann. New York Acad. Sci. 2000;917:322–330. doi: 10.1111/j.1749-6632.2000.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Parrens M, Labouyrie E, Groppi A, Dubus P, Carles D, Velly JF, et al. Expression of NGF receptors in normal and pathological human thymus. J. Neuroimmunol. 1998;85:11–21. doi: 10.1016/s0165-5728(97)00242-7. [DOI] [PubMed] [Google Scholar]
- Parrens M, Dubus P, Groppi A, Velly JF, Labouyrie E, De Mascarel A, et al. Differential expression of NGF receptors in human thymic epithelial tumors. Pathol. Res. Prac. 1999;195:549–553. doi: 10.1016/S0344-0338(99)80004-1. [DOI] [PubMed] [Google Scholar]
- Pearce L, Thompson A. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J. Physiol. 1986;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez M, Esteban I, García-Suárez O, Hannestad J, Naves FJ, Vega JA. Expression of neurotrophin recetor TrkB in rat spleen macrophages. Cell Tissue Res. 1999;298:75–84. doi: 10.1007/s004419900089. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez M, García-Suárez O, Esteban I, Germanà A, Naves FJ, Vega JA. p75NTR in the Spleen: Age-Dependent Changes, Effect of NGF and 4-Methylcatechol Treatment, and Structural Changes in p75NTR-Deficient Mice. Anat. Record. 2003;270:117–128. doi: 10.1002/ar.a.10010. [DOI] [PubMed] [Google Scholar]
- Pescarmona E, Pisacane A, Pignatelli E, Baroni CD. Expression of epidermal and nerve growth factor receptors in human thymus and thymomas. Histopathology. 1993;23:39–44. doi: 10.1111/j.1365-2559.1993.tb01181.x. [DOI] [PubMed] [Google Scholar]
- Pezzati P, Stainsz AM, Marshall JS, Bienenstock J, Stead RH. Expression of nerve growth factor receptor immunoreactivity on follicular dendritic cells from human mucosa associated lymphoid tissues. Immunology. 1992;76:485–490. [PMC free article] [PubMed] [Google Scholar]
- Pica F, Volpi A, Barillari G, Fraschetti M, Franzese O, Vullo V, et al. Detection of high nerve growth factor serum levels in AIDS-related and -unrelated Kaposi's sarcoma patients. AIDS. 1998;12:2025–2029. doi: 10.1097/00002030-199815000-00014. [DOI] [PubMed] [Google Scholar]
- Pradat PF, Kennel P, Naimi-Sadaoui S, Finiels S, Scherman D, Orsini C, et al. Viral and non-viral gene therapy partically prevents experimental cisplatin-induced neuroapthy. Gene Therapy. 2002;9:1333–1339. doi: 10.1038/sj.gt.3301801. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Dermato-Venereol. 1998;78:84–86. doi: 10.1080/000155598433368. [DOI] [PubMed] [Google Scholar]
- Renz H. The role of the neurotrophins in bronchial asthma. Eur. J. Pharmacol. 2001;429:231–237. doi: 10.1016/s0014-2999(01)01322-x. [DOI] [PubMed] [Google Scholar]
- Ricci A, Greco S, Mariotta S, Felici L, Bronzetti E, Cavazzana A, et al. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respiratory Cell Mol. Biol. 2001;25:439–446. doi: 10.1165/ajrcmb.25.4.4470. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Soderstrom S, Korhonen LT, Lindholm DB. Overstimulation of nerve growth factors in postinfectious and autoimmune diseases. Pediatric Neurol. 1998;18:231–235. doi: 10.1016/s0887-8994(97)00204-x. [DOI] [PubMed] [Google Scholar]
- Ritter MA, Boyd RL. Development in the thymus: it takes two to tango. Immunol. Today. 1993;14:462–468. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tebar A, Dechant G, Götz G, Barde YA. Binding of neurotrophin 3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tebar A, Dechant G, Barde Y. Binding of brain derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin recepotor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, Gonfloni S, et al. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 2000;20:2589–2601. doi: 10.1523/JNEUROSCI.20-07-02589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JB, Segal RA. Growth, survival and migration: the Trk to cancer. Cancer Treatment Res. 2003;115:1–18. doi: 10.1007/0-306-48158-8_1. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Miknyoczki SJ, Singh J, Hundkins RL. Role of neurotrophin–trk interactions in oncology: the anti-tumor efficacy of potent and selective trk tyrosine kinase inhibitors in pre-clinical tumor models. Current Med. Chem. 1999;6:845–857. [PubMed] [Google Scholar]
- Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int. Arch. Allergy Immunol. 1999;118:154–158. doi: 10.1159/000024054. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Benedetti M, Chao MV, Muzaffar R, Kuling K, Gabellini N, et al. Nerve growth factor production by lymphocytes. J. Immunol. 1994;153:4488–4495. [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001;57:132–134. doi: 10.1212/wnl.57.1.132. [DOI] [PubMed] [Google Scholar]
- Sariola H. The neurotrophic factors in non-neuronal tissues. Cellular Mol. Life Sci. 2001;58:1061–1066. doi: 10.1007/PL00000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh F, Mimata H, Nomura T, Fujita Y, Shin T, Sakamoto S, et al. Autocrine expression of neurotrophins and their receptors in prostate cancer. Int. J. Urol. 2001;8:S28–S34. doi: 10.1046/j.1442-2042.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood. 2000;95:2052–2058. [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nature Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Schenone A, Gill JS, Zacharias DA, Windebank AJ. Expression of high- and low-affinity neurotrophin receptors on human transformed B lymphocytes. J. Neuroimmunol. 1996;64:141–149. doi: 10.1016/0165-5728(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Schober A, Huber K, Fey J, Unsicker K. Distincts populations of macrophages in the adult rat adrenal gland: a subpopulation with neurotrophin-4-like immunoreactivity. Cell Tissue Res. 1998;291:365–373. doi: 10.1007/s004410051006. [DOI] [PubMed] [Google Scholar]
- Screpanti I, Meco S, Scarpa S, Morrone S, Frati L, Gulino A, et al. Neuromodulatory loop mediated by nerve growth factor and interleukin-6 in thymic stromal cell cultures. Proc. Natl Acad. Sci. USA. 1992;89:3209–3212. doi: 10.1073/pnas.89.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I, Modesti A, Gulino A. Heterogeneity of thymic stromal cells and thymocyte differentiation: a cell culture approach. J. Cell Sci. 1993;105:601–606. doi: 10.1242/jcs.105.3.601. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Philips HS, et al. Human trks: molecular, cloning, tissues distribution, and expression of extramolecular domain inmunoadhesins. J. Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am. J. Pathol. 1996;148:1807–1817. [PMC free article] [PubMed] [Google Scholar]
- Shores EW, Van Ewijk W, Singer A. Maturation of medullary thymic epithelium requires thymocytes expressing fully assembled CD3-TCR complexes. Int. Immunol. 1994;6:1393–1402. doi: 10.1093/intimm/6.9.1393. [DOI] [PubMed] [Google Scholar]
- Shu XQ, Llinas A, Mendell LM. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. Pain. 1999;80:463–470. doi: 10.1016/S0304-3959(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Neurotrophins and hyperalgesia. Proc. Natl Acad. Sci. USA. 1999;96:7693–7696. doi: 10.1073/pnas.96.14.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone MD, De Santis S, Vigneti E, Papa G, Amadori S, Aloe L. Nerve growth factor: a survey of activity on immune and hematopoietic cells. Hematol. Oncol. 1999;17:1–10. doi: 10.1002/(sici)1099-1069(199903)17:1<1::aid-hon635>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sin AZ, Roche EM, Togias A, Lichtenstein LM, Schroeder JT. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J. Allergy Clin. Immunol. 2001;108:387–393. doi: 10.1067/mai.2001.117459. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin SA, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Epa R, Shipham K, Butzkueven H, Bucci T, Barret G, et al. Treatment of experimental autoimmune encephalomyelitis with antisense oligonucleotides against the low affinity neurotrophin receptor. J. Neurosci. Res. 2000;59:712–721. doi: 10.1002/(SICI)1097-4547(20000315)59:6<712::AID-JNR3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Solomon A, Aloe L, Pe’Er J, Frucht-Pery J, Bonini S, Bonini S, et al. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J. Allergy Clin. Immunol. 1998;102:454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Stanisz AM, Stanisz JA. Nerve growth factor and neuroimmune interactions in inflammatory diseases. Ann. New York Acad. Sci. 2000;917:268–272. doi: 10.1111/j.1749-6632.2000.tb05392.x. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neuroendocrine regulation of autoimmune/inflammatory disease. J. Endocrinol. 2001;169:429–435. doi: 10.1677/joe.0.1690429. [DOI] [PubMed] [Google Scholar]
- Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, et al. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 1996;88:4630–4637. [PubMed] [Google Scholar]
- Susaki Y, Tanaka A, Honda E, Matsuda H. Nerve growth factor modulates Fc gamma receptor expression on murine macrophages J774A.1 cells. J. Vet. Med. Sci. 1998;60:87–91. doi: 10.1292/jvms.60.87. [DOI] [PubMed] [Google Scholar]
- Takafuji S, Bischoff SC, De Weck AL, Dahinden CA. Opposing effects of tumor necrosis factor-alpha and nerve growth factor upon leukotriene C4 production by human eosinophils triggered with N-formyl-methionyl-leucyl-phenylalanine. Eur. J. Immunol. 1992;22:969–974. doi: 10.1002/eji.1830220414. [DOI] [PubMed] [Google Scholar]
- Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807–1820. [PubMed] [Google Scholar]
- Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–137. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Thorpe LW, Pérez-Polo JR. The influence of nerve growth factor on the in vitro proliferative response of rat spleen lymphocytes. J. Neurosci. Res. 1987;18:134–139. doi: 10.1002/jnr.490180120. [DOI] [PubMed] [Google Scholar]
- Thorpe LW, Werrbach-Perez K, Perez-Polo JR. Effects of nerve growth factor on the expression of interleukin-2 receptors on cultured human lymphocytes. Ann. New York Acad. Sci. 1987;496:310–311. doi: 10.1111/j.1749-6632.1987.tb35781.x. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur. J. Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Lendahl U, Metsis M. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. J. Biol. Chem. 1999;274:1078–1084. [PubMed] [Google Scholar]
- Tokuoka S, Takahashi Y, Masuda T, Tanaka H, Furukawa S, Nagai H. Disruption of antigen-induced airway inflammation and airway hyper-responsiveness in low affinity neurotrophin receptor p75 gene deficient mice. Br. J. Pharmacol. 2001;134:1580–1586. doi: 10.1038/sj.bjp.0704411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torcia M, Bracci-Lauderio L, Lucibello M, Nencioni L, Labardi D, et al. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Tsoulfas P, Stepehens RM, Kaplan DR, Parada LR. TrkC isoforms with insert in the kinase domain show impaired signaling responses. J. Biol. Chem. 1996;271:5691–5697. doi: 10.1074/jbc.271.10.5691. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Switzer J, Bienenstock J, Denburg JA. Interactions of hemopoietic cytokines on differentiation of HL-60 cells. Nerve growth factor is a basophilic lineage-specific co-factor. Int. Arch. Allergy Appl. Immunology. 1990;91:15–21. doi: 10.1159/000235083. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Wong D, Dolovich J, Bienenstock J, Marshall J, Denburg JA. Synergistic effects of nerve growth factor and granulocyte-macrophage colony-stimulating factor on human basophilic cell differentiation. Blood. 1991;77:971–979. [PubMed] [Google Scholar]
- Turrini P, Tirassa P, Vigneti E, Aloe L. A role of the thymus and thymosin-alpha1 in brain NGF levels receptor expression. J. Neuroimmunol. 1998;82:64–72. doi: 10.1016/S0165-5728(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Turrini P, Sacaría ML, Aloe L. Presence and posible functional role of nerve growth factor in the thymus. Cell. Mol. Biol. 2001;47:55–64. [PubMed] [Google Scholar]
- Tuveri M, Generini S, Matucci-Cerinic AM, Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356:1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Maisonpierre PC, Glass Dj Rojas E, Nuñez L, Kong Y, et al. Alternative forms of rat TrkC with different functional capacities. Neuron. 1993;10:963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Van Eden W, Van Der Zee R, Van Kooten P, Berlo SE, Cobelens PM, Kavelaars A, et al. Balancing the immune system. Ann. Reumatol. Dis. 2002;61(Suppl. II):ii25–ii28. doi: 10.1136/ard.61.suppl_2.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A, Dessing MC, Engels F, Henricks PA, Nijkamp FP. Nerve growth factor induces a neurokinin-1 receptor- mediated airway hyperresponsiveness in guinea pigs. Am. J. Respirative Crit. Care Med. 1999;159:1541–1544. doi: 10.1164/ajrccm.159.5.9808058. [DOI] [PubMed] [Google Scholar]
- de Vries A, Engels F, Henricks PA, Leusink-Muis T, Fischer A, Nijkamp FP. Antibodies directed against nerve growth factor inhibit the acute bronchoconstriction due to allergen challenge in guinea-pigs. Clin. Exp. Allergy. 2002;32:325–328. doi: 10.1046/j.1365-2222.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Bauer JH, Li Y, Shao Z, Zetoune FS, Cattaneo E, Vincenz C. Characterization of a p75 (NTR) apoptotic signalling pathway using a novel cellular model. J. Biol. Chem. 2001;276:33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- Wang S, Bray P, Mccaffrey T, March K, Hempstead BL, Kraemer R. p75NTR mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am. J. Pathol. 2000;157:1247–1258. doi: 10.1016/S0002-9440(10)64640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker P, Grabbe J, Grutzkau A, Henz BM. Effects of nerve growht factor (NGF) and other fibroblast-derived growth factors on immature mast cells (HMC-1) Immunology. 1998;94:310–317. [PMC free article] [PubMed] [Google Scholar]
- Welker P, Grabbe J, Gibbs B, Zuberbier T, Henz BM. Nerve growth factor-beta induces mast cell marker expression during in vitro culture of human umbilical cord blood cells. Immunology. 2000;99:418–426. doi: 10.1046/j.1365-2567.2000.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J. Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Brain J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of mRNA for neurotrophic factors (NGF, BDNF, NT-3 and GDNF) and their receptors p75NGFR, TrkA, TrkB, and TrkC in the adult human peripheral nervous system and nonneuroral tissues. Neurochem. Res. 1996;21:929–993. doi: 10.1007/BF02532343. [DOI] [PubMed] [Google Scholar]
- Yoon S, Lee HW, Baek SY, Kim BS, Kim JB, Lee SA. Upregulation of TrkA neurotrophin receptor expression in the thymic subcapsular, periseptal, perivascular, and cortical epithelial cells during thymus regeneration. Histochem. Cell Biol. 2003;119:55–68. doi: 10.1007/s00418-002-0486-z. [DOI] [PubMed] [Google Scholar]
- Zhou X-F, Rush R. Localization of neurotrophin-3-like immunoreactivity in peripheral tissues of the rat. Brain Res. 1993;621:189–199. doi: 10.1016/0006-8993(93)90106-w. [DOI] [PubMed] [Google Scholar]