Abstract
The concept of stem cells within the adult brain is not new. However, only recently have scientific techniques become sufficiently advanced to identify them although this remains problematic and the technology is still developing. Nevertheless, it is now generally recognized that stem cells are restricted to two germinal regions within the intact brain. From here they can migrate to specific destinations where they integrate with existing circuitry. Their identity remains controversial but a growing body of evidence suggests it may have an astrocytic phenotype. Within the germinal regions the stem cells are confined to a niche environment and are capable of responding to environmental signals generated locally in an autocrine or paracrine fashion. The niche environment is also modulated by more generalized systemic and physiological activity. These observations are exciting in their own right and form the basis of this review. They are also beginning to alter how we think about neural injury and disease and to impact on the development of novel therapies.
Keywords: adult, astrocyte, dentate gyrus, hippocampus, neural, review, stem cell niche, stem cell, subventricular zone
The concept of adult neural stem cells is not new
The concept that no new neurons were formed in the adult brain did not begin to change until the 1960s when Joseph Altman suggested that ‘the neogenesis of neurons in the adult might arise from non-differentiated precursors such as ependymal cells’ (Altman, 1962). Using light microscopic analysis of [3H]-thymidine-labelled cells he identified newborn cells in restricted regions of the adult brain (Altman, 1966) but was unable to confirm their neuronal identity. Two decades later investigations of the seasonal variation of song repertoire in Serinus canaria by Nottebohm and colleagues led to the conclusive demonstration of telencephalic neuronal replacement in the adult avian brain (Graziadei & Graziadei, 1979a; Goldman & Nottebohm, 1983).
Further evidence suggested that stem cells might be present in the adult mammalian brain. First, olfactory epithelium had been demonstrated to retain the ability to generate olfactory neurons in adult mammals (Graziadei & Graziadei, 1979b). Second, the demonstration of epidermal growth factor (EGF) and EGF-receptor immunoreactivity in the adult CNS suggested that mitogen-responsive cells may exist within the mammalian neuropil (Fallon et al. 1984; Birecree et al. 1991). This was confirmed in two studies published in 1992. The first isolated cells from the striatum of the adult mouse brain and demonstrated that they were responsive to the mitogenic agent EGF, a molecule known to be important in embryonic neuronal development. These EGF-responsive cells gave rise to progeny expressing neuronal and glial phenotypes thereby demonstrating multipotentiality (Reynolds & Weiss, 1992). The second study isolated cells from the whole forebrain that were responsive to both EGF and fibroblast growth factor (FGF) and generated cells expressing both glial and neuronal morphology (Richards et al. 1992).
The adult mammalian brain contains defined germinal zones
The evidence of ongoing neurogenesis in the adult conflicted with the prevailing classical view that neurogenic germinal centres disappear in the early postnatal period. Instead the data suggested that germinal activity persists in the adult brain. Subsequent studies identified the subventricular region of the forebrain as an area containing proliferating neuronal precursors that migrate to the olfactory bulb where they differentiate into granule and periglomerular interneurons (Morshead & van der Kooy, 1992; Lois & Alvarez-Buylla, 1993; Luskin, 1993; Vescovi et al. 1993; Morshead et al. 1994). Ultrastructural data demonstrating mitotic neuronal precursors in the granule cell layer of the adult hippocampus (Kaplan & Bell, 1984) led to a reappraisal of the germinal potential of the adult dentate gyrus and the subsequent demonstration that it also contained adult neural stem cells (Cameron et al. 1993b; Gage et al. 1995, 1998). These cells reside in the subgranular layer (SGL) and migrate the short distance into the granule cell layer where they differentiate into granule cell projection neurons.
The forebrain germinal region has been described in detail (Morshead & van der, 1992; Doetsch & Alvarez-Buylla, 1996; Lois et al. 1996; Doetsch et al. 1997, 1999, 2002). Fate-specified neuroblasts destined to differentiate into olfactory interneurons within the olfactory bulb migrate in the rostral migratory stream (RMS) as chains using cell–cell interaction mediated by polysialylated neural cell adhesion molecule (PSA-NCAM). They are ensheathed by the processes of cells expressing an immature astrocytic phenotype: Glial Fibrillary Acid Protein (GFAP), vimentin- and nestin-positive, β-tubulin- and PSA-NCAM negative. These cells undergo asymmetric division to generate olfactory interneurons and are capable of repopulating a depleted subventricular zone (SVZ) population. They also give rise to multipotent progenitors in vitro. A third population of cells form focal clusters closely associated with the chains of migrating neuroblasts but not present in the RMS. These cells have immature ultrastructural and phenotypic characteristics, being nestin positive but not staining with any other immunohistochemical markers. They constitute a transit amplifying population and are highly mitotic giving rise to migrating neuroblasts. Similarly, in the hippocampus subgranular zone astrocytes give rise to a precursor population that does not express a glial or neuronal phenotype but does express PSA-NCAM. These cells in turn give rise to granule neurons (Seri et al. 2001).
Neuronal precursors are capable of migration out of the germinal zones
The migration of neuronal precursors has been most accurately described in the SVZ of the forebrain (Lois & Alvarez-Buylla, 1994; Doetsch & Alvarez-Buylla, 1996; Lois et al. 1996). SVZ neuroblasts migrate tangentially long distances through a network of interconnecting pathways distributed within the wall of the lateral ventricle. These pathways become confluent at the rostral margin of the lateral ventricular wall to form the RMS projecting to the olfactory bulb (Doetsch & Alvarez-Buylla, 1996; Doetsch et al. 1997). The region-specific expression of PSA-NCAM (Bonfanti et al. 1992) appears to be associated with migration of newly generated adult neuroblasts in both the SVZ (Doetsch & Alvarez-Buylla, 1996) and hippocampus (Seki, 1993; Kuhn et al. 1996). Mutations of the NCAM gene (Tomasiewicz et al. 1993; Cremer et al. 1994) or enzymatic removal of PSA (Tomasiewicz et al. 1993) hamper migration of SVZ cells along the RMS.
Proliferation of neuronal precursors is confined to germinal regions which are conserved between species
Although the proliferation kinetics of adult neural stem cells are poorly understood, labelling studies in the forebrain germinal region suggest that between 16 and 35% of the SVZ population are proliferating (Morshead & van der Kooy, 1992). Within this population the most actively dividing cells appear to be the transit amplifying population (Doetsch et al. 1997). In the adult hippocampal germinal region approximately one new neuron for every 2000 granule cells already present is born every day (Kempermann et al. 1997a). In mice this process of adding to the granule cell population appears to continue until approximately 6 months of age, i.e. adulthood, after which the population remains stable (Kempermann et al. 1998). Although there is a decline in neurogenesis in the aging brain the process does continue (Kuhn et al. 1996; Maslov et al. 2004). Interestingly, bromodeoxyuridine (BrdU) labelling of newborn cells suggests that age-related decline of activity within adult neurogenic regions may be regionally specified and is more pronounced in the hippocampus (Kuhn et al. 1996).
The need for mitogens in the proliferative process of adult progenitors was recognized from their early identification and isolation (Reynolds & Weiss, 1992; Richards et al. 1992). EGF and basic FGF (bFGF) have been used alone or in combination (Richards et al. 1992; Luskin, 1993; Lois et al. 1996). Dose–response studies have determined that the optimal concentration of mitogens for survival effects is 20 ng mL−1 culture media (Ray et al. 1993). Lineage analyses suggest that EGF- and FGF-responsive multipotent progenitors in the adult CNS derive from a common precursor (Luskin, 1993; Vescovi et al. 1993; Craig et al. 1996; Palmer et al. 1995; Doetsch et al. 1999; Gritti et al. 1999). Hence the combined use of EGF and FGF confers no additional mitogenic effect in vitro compared with either mitogen alone (Gritti et al. 1999), although the mitogenic effect on the germinal population in vivo may be regionally specified (Kuhn et al. 1997; Hitoshi et al. 2002).
Studies of proliferation kinetics within the embryonic forebrain germinal zone suggest that FGF-responsive stem cells give rise to separate FGF- and EGF-responsive progeny (Tropepe et al. 1999; Martens et al. 2000). In the adult, EGF-responsive stem-like cells were initially thought to correspond to the slowly dividing astrocytic stem cell (Morshead et al. 1994). Further investigation has demonstrated that mitogens act on the more rapidly dividing transit amplifying cells that express EGF and FGF receptors (Gritti et al. 1999; Doetsch et al. 2002).
Similar subventricular and hippocampal adult neurogenic regions have been identified in primates (Pencea et al. 2001a; Kornack & Rakic, 2001) and humans (Eriksson et al. 1998; Pincus et al. 1998; Johansson et al. 1999; Kukekov et al. 1999; Roy et al. 2000; Arsenijevic et al. 2001; Nunes et al. 2003; Westerlund et al. 2003; Sanai et al. 2004). Thus, it has now been confirmed that adult neurogenesis is present in all major vertebrate taxa (Garcia-Verdugo et al. 2002).
A growing body of evidence suggests that stem cells in the adult brain are astrocytes
The human adult neural stem cell demonstrates an astrocytic ultrastructural morphology and expresses vimentin and GFAP (Sanai et al. 2004). Furthermore, ablation of GFAP-expressing astrocytes prevents neurosphere formation from SVZ tissue, suggesting that SVZ astrocytes are the primary adult stem cell (Imura et al. 2003; Morshead et al. 2003).
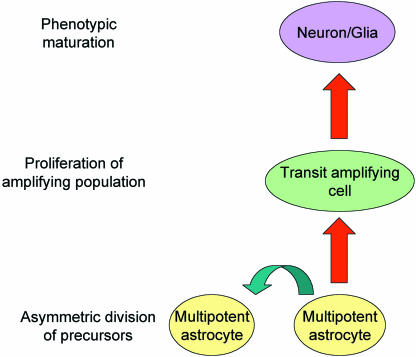
These observations are consistent with lineage data that have identified germinal precursors as astrocytes in both the SVZ (Doetsch et al. 1999) and dentate gyrus of the hippocampus (Seri et al. 2001). These stem cell-like astrocytes divide asymmetrically to maintain a population of slowly dividing, multipotent, self-renewing cells within the germinal region, while giving rise to a population of more rapidly dividing transit amplifying or precursor cells. This transient population gives rise to fate-specified neuroblasts, which in turn differentiate into functionally mature neurons when they reach their target (Fig. 1).
Fig. 1.
In the adult brain, stem cell-like astrocytes divide asymmetrically to maintain a population of slowly dividing precursors in the germinal niche. A transient population of rapidly dividing cells can also be generated that can migrate away from the germinal niche and differentiate into functionally mature neurons and glia.
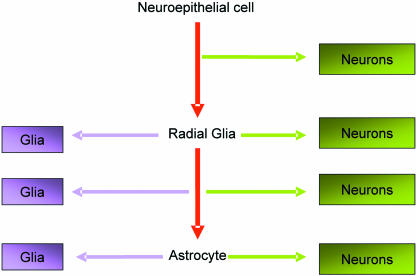
These observations coupled with data suggesting radial glia act as stem cells in the foetal brain have led to a novel hypothesis of neural stem cell lineage (see Alvarez-Buylla et al. 2001; Doetsch, 2003; Merkle et al. 2004 and references therein). According to this unified lineage hypothesis the embryonic neuroepithelial stem cell gives rise to the radial glial stem cell in the embryo from which the postulated astrocytic stem cell in the adult brain is derived (Fig. 2) (Alvarez-Buylla et al. 2001).
Fig. 2.
Neural epithelial cells evolve into radial glia which give rise to astrocytes. Both radial glia and astrocytes divide asymmetrically to produce both glia and neurons. These cells may be produced directly or via a transit amplifying population. In humans and other mammalian species the radial glia are lost in the perinatal period. It is now thought that they transform into a subpopulation of astrocytes that retain the ability to produce neurons and glia.
Stem cells are regulated by local environmental cues
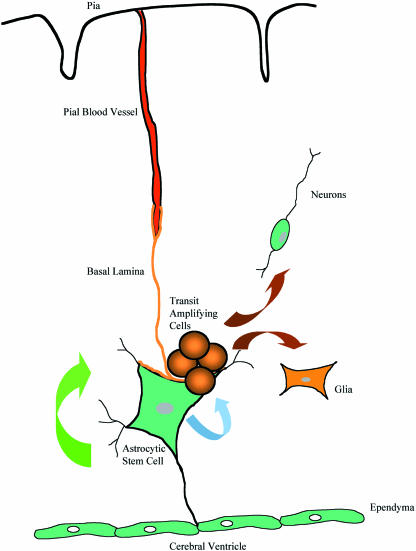
The growing recognition that adult neural stem cells have a glial morphology together with the characterization of the cellular architecture of the germinal regions has focused attention on how the environment or niche regulates stem cell activity (Fig. 3) (Fuchs et al. 2004). Homotopic transplantation of adult hippocampal and SVZ precursors resulted in new interneurons within the dentate granule cell layer of the hippocampus and the olfactory bulb, respectively. Implants into non-germinal regions failed to generate new neurons although glial cells were generated (Lois & Alvarez-Buylla, 1994; Gage et al. 1995; Doetsch & Alvarez-Buylla, 1996; Suhonen et al. 1996; Herrera et al. 1999). The suggestion that the niche microenvironment can be instructive in the fate specification of neural stem cells is an hypothesis that receives further support from heterotopic implants of adult stem cells. Hippocampal progenitors implanted into the neurogenic SVZ produce phenotypically appropriate tyrosine hydroxylase cells in the olfactory bulb (Suhonen et al. 1996). This phenotype does not occur in the hippocampus, suggesting that soluble factors and cell–cell contact in the niche may have an instructive role.
Fig. 3.
Current concept of the stem cell niche. The multipotent stem cell-like astrocytes are closely opposed to the ventricular lining and basal lamina associated with the pial microvasculature. Asymmetric division gives rise to self-renewal (green arrow) and a transit amplifying population (blue arrow). These cells can migrate out of the germinal niche and differentiate into neurons and glia (brown arrows).
Elaboration of the mechanism of chain migration demonstrated that the astrocytic stem cells were in intimate contact with the transit amplifying cells and the neuroblasts (Doetsch et al. 1997). This direct astrocyte–stem cell interaction facilitates proliferation and neuronal fate specification of progenitors in both the forebrain germinal region (Lim & Alvarez-Buylla, 1999) and hippocampus (Song et al. 2002), an ability that, on the part of astrocytes, is regionally specified in both adult and neonatal environments (Lim & Alvarez-Buylla, 1999; Song et al. 2002).
In the adult brain the neurogenic niche is juxtaposed to the basal lamina of the neural microvasculature (Mercier et al. 2002). As a result, neurogenesis and angiogenesis are intimately related (Louissaint et al. 2002). In the subgranular germinal zone of the hippocampus clusters of neurogenic precursors are also spatially and temporally associated with angiogenesis (Palmer et al. 2000). Within this niche, neurogenic cells express vascular endothelial growth factor (VEGF) receptors, which co-localize with the immature neuronal marker double-cortin (Dcx) (Jin et al. 2002). Intraventricular infusion of VEGF stimulates proliferation of both SVZ and hippocampal progenitors. Further evidence of a functional relationship between stem cells and vascular endothelial cells comes from the observation that endothelial cells release unidentified soluble factors that regulate proliferation of the stem cells and play an instructive role in neuronal fate specification (Shen et al. 2004). Thus, in the germinal regions astrocytes function as primary precursors and also participate in the generation and maintenance of the microenvironment that regulates their self-renewal, migration and fate specification.
Identifying the signal cascades that mediate the changes in the stem cell niche and its occupants has become a major field of investigation (Table 1). The involvement and interaction of the many different molecules is not fully understood and it is not clear whether all of these play a physiological role in the regulation of neurogenesis or whether these factors act directly on stem cells or through secondary signals (Lie et al. 2004).
Table 1.
Various factors affecting neurogenesis
| Factor | Effects | Reference(s) |
|---|---|---|
| Epidermal growth factor (EGF) | • Increases proliferation of cells in SVZ | (Craig et al. 1996; Kuhn et al. 1997) |
| • Decreases cell migration to olfactory bulb | ||
| • Influences the fate of the cells in the SGZ, causing more precursors to be restricted to the glial fate, away from neuronal differentiation | ||
| • Promotes division and differentiation of astrocytes but is not a lineage restriction factor | (Johe et al. 1996) | |
| • Overexpression of the EGF receptor in vivo may cause induction of a fate shift from neurons to glia rather than simply promote astrocytic differentiation | (Lillien 1995; Burrows et al. 1997) | |
| Fibroblast growth factor (FGF) | • A mitogenic agent for neuroepithelial precursors rather than a survival agent | (Murphy et al. 1990; Drago et al. 1991) |
| • Stimulates the differentiation of the neuroepithelial cells into mature neurons and glia | ||
| • Survival factor for postmitotic neurons | (Walicke & Baird, 1988) | |
| • May keep cells in the cell cycle, actively repressing differentiation. May decrease the time spent in G1, thereby increasing the number of proliferative divisions | (Palmer et al. 1995) | |
| • Intraventricular infusion increases cortical size, as well as neuronal number in rat embryos | (Vaccarino et al. 1999) | |
| • Much more effective in increasing neuronal-cell number compared with EGF | (Richards et al. 1992) | |
| • After injury, FGF-2 expression is up-regulated to promote neurogenesis, gene delivery of FGF-2 to null mutant mice (and wild-type) produced an elevation in neurogenesis even though the basal rate of neurogenesis was the same in wild-type and FGF-2-null mutant mice | (Yoshimura et al. 2001, 2003) | |
| • Enhanced expression by gene delivery, attenuated hippocampal cell loss following lesion | ||
| • Subcutaneous delivery induces proliferation of SVZ/SEZ precursors in adult animals | (Wagner et al. 1999b) | |
| Brain-derived neurotrophic factor (BDNF) | • Ventricular administration to adult mice causes an increased mitotic activity and neuronal number in the olfactory bulb | (Benraiss et al. 2001; Pencea et al. 2001b; Zigova et al. 1998) |
| • Increased number of neurons in both neurogenic zones and regions that normally do not form neurons | ||
| • Transfection of NSCs to secrete BDNF improved survival, while antisense treatment decreased survival of cerebellar neurons and was lethal to hippocampal stem cells | (Rubio et al. 1999) | |
| • Plays an important role in the regulation of the basal level of neurogenesis in dentate gyrus of adult mice | (Lee et al. 2002) | |
| Platelet-derived growth factor (PDGF) | • Expression of receptor increases during differentiation, low levels found in uncommitted cells | (Erlandsson et al. 2001) |
| • Stimulates cell division in vitro and maintains neuronal precursor proliferation | ||
| • Evidence to suggest a role for PDGF in oligodendrocyte differentiation | (Ibarrola et al. 1996) | |
| Ciliary neurotrophic factor (CNTF) | • Administration of CNTF, and LIF to neural stem cells in vitro causes an almost exclusive differentiation into astrocytes | (Johe et al. 1996) |
| • Injection of CNTF into the adult mouse forebrain enhances self-renewal of neural stem cells in vivo, in the absence or presence of EGF. Analysis of EGF-responsive neural stem cells proliferating in vitro found that CNTF inhibits lineage restriction of neural stem cells to glial progenitors, which in turn results in enhanced expansion of stem cell number | (Shimazaki et al. 2001) | |
| Leukaemia inhibitory factor (LIF) | • Required for the long-term growth of EGF responsive neural stem cells after more than 30 population doublings | (Wright et al. 2003) |
| • Maintains the proliferative capability and stem cell properties of ES cells | (Smith et al. 1988) | |
| • LIF receptor decifient animals lack astrocytes and have fewer adult NSCs (due to decreased cell proliferation) so signalling through the LIFR may promote astrocyte differentiation and inhibit neuronal differentiation | (Bartlett et al. 1998) | |
| • Signalling later on through the LIFR, may be required for long-term self-renewal of NSCs | (Shimazaki et al. 2001) | |
| Insulin-like growth factor (IGF-1) | • Physical exercise-induced increase in hippocampal neurogenesis is mediated by the increased uptake of IGF-1 into brain from serum | (Trejo et al. 2001) |
| • Peripheral infusion can increase adult neurogenesis | (Aberg et al. 2000) | |
| • Can reverse the aging-related decline in neurogenesis | (Lichtenwalner et al. 2001) | |
| Thyroid hormone | Nurr1 (a transcription factor of the thyroid hormone/retinoic acid nuclear receptor superfamily) induces neuronal differentiation and confers competence to respond to extrinsic signals that induce dopaminergic fate | (Wagner et al. 1999a) |
| • Agents such as thyroid hormone and retinoic acid increase the number of cells in the adult brain displaying postmitotic neuronal markers | (Palmer et al. 1997) | |
| • Instructive in restricting neuronal precursors to the oligodendrocyte lineage | (Johe et al. 1996) |
In the mature brain, as in other tissues, stem cells and their niches are retained in specialized regions in which developmental processes can occur for the life of the animal (Fuchs et al. 2004). Many embryonic developmental signals and morphogens appear to be conserved in adult neurogenic regions including Notch, Eph/ephrins, Bone morphogenetic proteins (BMPs), Noggin and Sonic hedgehog (Shh). Notch 1 and its ligand Jagged are expressed in adult neurogenic regions as is the downstream effector Hes5 (Stump et al. 2002). Activation of notch appears to reduce proliferation and neuronal differentiation in the postnatal SVZ (Chambers et al. 2001). Vascular endothelium also modulates the notch signalling cascade with activation of Hes1 (Shen et al. 2004). Similarly, Eph/ephrins localize to the SVZ astrocytes and modulate their proliferation and migration (Conover et al. 2000) while Patched, the Shh receptor, is expressed in adult hippocampal progenitors where Shh itself stimulates proliferation in a dose-dependent manner (Lai et al. 2003). BMPs are expressed in the adult SVZ in the same region as Shh. During development BMPs promote astrocyte differentiation (Gross et al. 1996) and thus may have a role in limiting neurogenesis in the adult SVZ (Lim et al. 2000). BMPs are antagonized by Noggin, which is locally expressed in ependymal cells (Lim et al. 2000). Based on this current evidence it seems that major developmental signalling pathways are conserved into adulthood but spatially restricted to germinal niches where they appear to regulate multiple aspects of precursor proliferation and differentiation.
The stem cell niche is responsive to physiological and environmental signals that affect the whole animal
Neurogenesis dynamically responds to a variety of macro-environmental or hormonal stimuli such as free physical activity, learning, enriched housing or stress (Table 2).
Table 2.
Various speculations for the role of neurogenesis in the germinal centres
| Area | Viewpoints | References |
|---|---|---|
| SVZ | Important in the ability of a rodent mother to recognize and nurture her offspring, as it is shown that a release of prolactin in pregnancy is associated with increased SVZ neurogenesis | (Shingo et al. 2003) |
| Newborn interneurons may play a part in sharpening the response of neighbouring cells to odours | (Gheusi et al. 2000) | |
| SGZ | Spur the formation of connections between new and existing neurons, increasing the brain's capacity to process and store novel information | (Gage, 2000, 2002) |
| Adult neurogenesis may provide survival advantages in an inhospitable environment | ||
| Local generation of new neurons in structures could participate in the formation or integration of new memories | ||
| Neuronal replacement in the adult exists to keep circuits functionally young, able to master skills in the way that young brains do and so prolong the health and reproductive fitness of the animal | (Nottebohm, 2002) | |
| New hippocampal neurons may participate in the processing of memory in the hippocampus. Administration of a drug that kills dividing cells reduces the number of neurons born in the hippocampus and impairs the hippocampus-dependent memory task, the association of temporally separated stimuli | (Shors et al. 2001) | |
| New cells in the dentate gyrus may play a role in hippocampal modulation of the hypothalamo-pituitary-adrenal axis response to stress | (Herman et al. 1989) | |
| During adult hippocampal neurogenesis, as during embryogenesis, new but immature neurons are generated in surplus and are then selected into functional circuits | (Kempermann et al. 2003) |
Stress and its concomitant increase in glucocorticoid levels have been shown to inhibit adult neurogenesis by suppressing cell proliferation in the hippocampal SGL (Cameron & Gould, 1994; Gould et al. 1997). Adrenalectomy increases the proliferation of granule cell precursors and, ultimately, the production of immature granule neurons (Cameron & Gould, 1994; Cameron & McKay, 1999), showing that there is an inhibitory role of glucocorticoids on neurogenesis, which is reversed by their systemic application. However, very few [3H]thymidine-labelled mitotic cells in the dentate gyrus express glucocorticoid and mineralocorticoid receptors (Cameron et al. 1993a), suggesting that adrenal steroids do not act directly on granule cell progenitors in the adult rat dentate gyrus.
Voluntary exercise can not only double the number of proliferative cells and increase the survival of the new neurons, but also selectively increases the amplitude of long-term potentiation in the dentate gyrus (van Praag et al. 1999). This is not the case in the CA1 region of the same animals, showing a functional correlate for these new neurons in the brain. Physical exercise seems to increase circulating levels of IGF-1 which in turn enhance hippocampal BDNF levels and sensitivity to afferent stimulation (Carro et al. 2000). However, prolonged physical exercise has been shown to have a negative effect on progenitor proliferation in the dentate gyrus (Naylor et al. 2004). Exercise-induced activity in the hypothalamic–pituitary–adrenal axis leads to increased plasma corticosteroid levels, suggesting a stress response as a possible mechanism. The link with neurogenesis and hippocampal brain-derived neurotrophic factor (BDNF) was also found to be elevated by dietary restriction (Lee et al. 2000). Active learning has also been shown to promote hippocampal neurogenesis (Gould et al. 1999), where the generation of new neurons appears necessary to encode memory formation (Shors et al. 2001). An enriched environment has also been shown to increase neurogenesis and spatial learning ability in mice, although via a different mechanism than exercise. Adult mice exposed to an enriched housing environment have a significantly greater number of new neurons in the dentate gyrus in comparison with littermates housed in standard cages (Kempermann et al. 1997b) due to the increased survival of new neurons rather than increased proliferation. By contrast, exercise increases both survival and proliferation.
Studies over the past few years have identified steroid hormones and peptide hormones, e.g. prolactin, as potential regulators of adult neurogenesis. Ovariectomized rats were found to have significant reductions in the proliferation of hippocampal granule cell precursors, which was restored to normal with the administration of oestrogen (Tanapat et al. 1999). This effect was found to be mediated by serotonin. Serotonin depletion by p-chlorophenylalanine (PCPA) and ovariectomy together produce approximately the same decreases in the SGL as ovariectomy alone and administration of 5-HT restored cell proliferation decreased by ovariectomy (Banasr et al. 2001).
Shingo et al. (2003) found that prolactin mediates the stimulation of neuronal progenitors in the forebrain SVZ of female mice during pregnancy. In adult songbirds, testosterone induces the expression of VEGF, thereby increasing angiogenesis. The newly generated endothelial cells then stimulate neurogenesis by increasing the levels of BDNF in the neurogenic areas, which enhances the proliferation of progenitors (Louissaint et al. 2002).
The normal regulation of adult stem cell activity is thus dependent on the interaction between a variety of signals intrinsic to the niche environment and the influence of macroenvironmental factors on the organism as a whole. The complexity of these interactions underscores the likelihood that no one single factor or signal transduction pathway will be able to support stem cell behaviour in the absence of others. Neurogenesis is primarily a developmental process that involves the proliferation, migration and differentiation of primordial CNS stem cells. It is becoming clear that pieces of the embryonic developmental puzzle are retained for adult neurogenesis. The fundamental difference between developmental and adult neurogenesis is that new adult neurons undergo these processes in an already mature environment and therefore have to integrate into pre-existing circuits. For neurogenesis to be effective the new neurons must be able to integrate appropriately, display functional properties that are similar to the characteristics of the neurons lost, and be generated in numbers sufficient to replace those which are lost.
Emerging ideas about the identity and function of adult neural stem cells are already beginning to impact on how we think about neurological disease
Ongoing neurogenesis in the adult brain raises the possibility of reconstituting damaged or senescent circuits and functions in the CNS. However, any therapeutic potential can only be developed through improved understanding of the regulation of adult neurogenesis, the cellular and molecular mechanisms controlling neural cell fate determination, and the mechanisms of functional integration of these new neurons. Our increased understanding is already leading to the development of novel concepts about what a neural stem cell is and what its functions might be. These new ideas will in turn lead to new ways of thinking about neurological disease and how strategies for regeneration and repair may be developed in the future (Lindvall et al. 2004).
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302–318. doi: 10.1016/0014-4886(62)90040-7. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Arsenijevic Y, Villemure JG, Brunet JF, et al. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Bartlett PF, Brooker GJ, Faux CH, et al. Regulation of neural stem cell differentiation in the forebrain. Immunol Cell Biol. 1998;76:414–418. doi: 10.1046/j.1440-1711.1998.00762.x. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecree E, King LE, Jr, Nanney LB. Epidermal growth factor and its receptor in the developing human nervous system. Brain Res Dev Brain Res. 1991;60:145–154. doi: 10.1016/0165-3806(91)90043-i. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Olive S, Poulain DA, Theodosis DT. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience. 1992;49:419–436. doi: 10.1016/0306-4522(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993a;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993b;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der KD. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PF. Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc Natl Acad Sci USA. 1991;88:2199–2203. doi: 10.1073/pnas.88.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Erlandsson A, Enarsson M, Forsberg-Nilsson K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J Neurosci. 2001;21:3483–3491. doi: 10.1523/JNEUROSCI.21-10-03483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Seroogy KB, Loughlin SE, et al. Epidermal growth factor immunoreactive material in the central nervous system: location and development. Science. 1984;224:1107–1109. doi: 10.1126/science.6144184. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain Res Dev Brain Res. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979a;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979b;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, et al. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Tropepe V, Ekker M, van der KD. Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development. 2002;129:233–244. doi: 10.1242/dev.129.1.233. [DOI] [PubMed] [Google Scholar]
- Ibarrola N, Mayer-Proschel M, Rodriguez-Pena A, Noble M. Evidence for the existence of at least two timing mechanisms that contribute to oligodendrocyte generation in vitro. Dev Biol. 1996;180:1–21. doi: 10.1006/dbio.1996.0280. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The Predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997a;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997b;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–162. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl.):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Martens DJ, Tropepe V, van der Kooy D. Separate proliferation kinetics of fibroblast growth factor-responsive and epidermal growth factor-responsive neural stem cells within the embryonic forebrain germinal zone. J Neurosci. 2000;20:1085–1095. doi: 10.1523/JNEUROSCI.20-03-01085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;10:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead M, Garcia AD, Sofroniew MV, van der KD. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Murphy M, Drago J, Bartlett PF. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. J Neurosci Res. 1990;25:463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2004 doi: 10.1152/jn.01085.2004. in press. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001a;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001b;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, et al. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Rubio F, Kokaia Z, Arco A, et al. BDNF gene transfer to the mammalian brain using CNS-derived neural precursors. Gene Ther. 1999;6:1851–1866. doi: 10.1038/sj.gt.3301028. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Seki T. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. AY. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002;114:153–159. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz H, Ono K, Yee D, et al. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der KD. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, et al. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Wagner J, Akerud P, Castro DS, et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999a;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999b;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke PA, Baird A. Trophic effects of fibroblast growth factor on neural tissue. Prog Brain Res. 1988;78:333–338. doi: 10.1016/s0079-6123(08)60301-5. [DOI] [PubMed] [Google Scholar]
- Westerlund U, Moe MC, Varghese M, et al. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res. 2003;289:378–383. doi: 10.1016/s0014-4827(03)00291-x. [DOI] [PubMed] [Google Scholar]
- Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN. Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J Neurochem. 2003;86:179–195. doi: 10.1046/j.1471-4159.2003.01826.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Teramoto T, Whalen MJ, et al. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112:1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]