Abstract
Many organisms are able to regenerate lost or damaged body parts that are structural and functional replicates of the original. Eventually these become fully integrated into pre-existing tissues. However, with the exception of deer, mammals have lost this ability. Each spring deer shed antlers that were used for fighting and display during the previous mating season. Their loss is triggered by a fall in circulating testosterone levels, a hormonal change that is linked to an increase in day length. A complex ‘blastema-like’ structure or ‘antler-bud’ then forms; however, unlike the regenerative process in the newt, most evidence (albeit indirect) suggests that this does not involve reversal of the differentiated state but is stem cell based. The subsequent re-growth of antlers during the spring and summer months is spectacular and represents one of the fastest rates of organogenesis in the animal kingdom. Longitudinal growth involves endochondral ossification in the tip of each antler branch and bone growth around the antler shaft is by intramembranous ossification. As androgen concentrations rise in late summer, longitudinal growth stops, the skin (velvet) covering the antler is lost and antlers are ‘polished’ in preparation for the mating season. Although the timing of the antler growth cycle is clearly closely linked to circulating testosterone, oestrogen may be a key cellular regulator, as it is in the skeleton of other male mammals. We still know very little about the molecular machinery required for antler regeneration, although there is evidence that developmental signalling pathways with pleiotropic functions are important and that novel ‘antler-specific’ molecules may not exist. Identifying these pathways and factors, deciphering their interactions and how they are regulated by environmental cues could have an important impact on human health if this knowledge is applied to the engineering of new human tissues and organs.
Keywords: antler, bone, deer, regeneration
Introduction
Deer antlers are one of the animal kingdom's most dramatic examples of male prowess and thus since ancient times have been held in great regard by humans. However, antlers also provide a model for studying two unique processes: the development of a complete appendage that is delayed until puberty and mammalian organ regeneration. No other mammal can naturally regenerate any lost organ, let alone anything as large and complex as an antler, e.g. the antlers of a 200-kg adult red deer may weigh as much 30 kg but take only 3 months to grow. By contrast, animals that have retained the capacity to regenerate are found in most other phyla and a variety of these are studied by regeneration biologists. These include planaria, hydra, urodele amphibians, Xenopus and zebrafish (Brockes, 1997; Fujisawa, 2003; Nye et al. 2003; Poss et al. 2003; Sanchez Alvarado, 2003; Slack et al. 2004). In fact, it has been proposed that only by studying a variety of examples of natural regeneration can we develop our understanding of why some animals regenerate and others do not (Brockes, 2004). However, despite their obvious convenience as experimental models, these are not mammals and although some mouse strains have been shown to have an increased capacity for repair (Heber-Katz et al. 2004), they are unable to regenerate whole organs. This is why the mechanisms that underlie antler regeneration should continue to be investigated, notwithstanding the limitations of deer as an animal model. This argument made most persuasively by Richard Goss, the regeneration biologist who pioneered antler research in the late twentieth century (Goss, 1995). Antler research can help us understand why regenerative ability has been lost in mammals and take us further towards a ‘holy grail’ of modern human medicine: the ability to regenerate organs that have been removed through trauma or excision.
The diverse anatomy of antlers
Deer are hoofed, ruminant mammals in the Cervidae family (order Artiodactyl) and are among the most graceful and attractive of animals. This family consists of 17 genera and about 53 species. Deer are native to all parts of the world except Antarctica, Australia, central and southern Africa, Madagascar and New Zealand, and have adapted to virtually every land habitat, from dry deserts to woodlands, prairies, marshes and Arctic regions. Deer are the only animals that grow antlers, which are composed of skin, nerves, blood vessels, fibrous tissue, cartilage and bone, and thus should not be confused with horns, which are a keratinized tissue that grow from their base under the control of underlying mesenchymal cells.
Except for the reindeer (Rangifer tarandus), antlers develop only in male deer and in most species this occurs in the spring of the animal's second year of life. As with the developing limb, antlers have three axes: a proximal–distal axis, an anterior–posterior axis and a dorsal–ventral axis (see Fig. 2A; Li & Suttie, 2001). Antlers come in all shapes and sizes from small un-branched antlers of only a few centimetres in length, as seen on the pudu (Pudu puda), the smallest of the deer species, to elaborately branched racks or palmate headpieces of impressive proportions in species such as the moose and elk. The moose (Alces spp.) is the largest deer (an adult stag's body weight can reach 800 kg) and these animals possess the largest antlers. Elk (Cervus canadenesis) (Fig. 1A) also carry impressive sets of antlers that can extend to 1.5 m in length. The antlers of more diminutive adult fallow bucks (Dama dama) are also very spectacular (Fig. 1B) curving majestically outward and upward. The species studied by us is the red deer (Cervus elapus), the antlers of which have a more ‘classic’ shape, depicted in artworks throughout history (Fig. 1C).
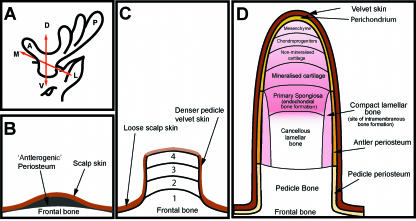
Fig. 2.
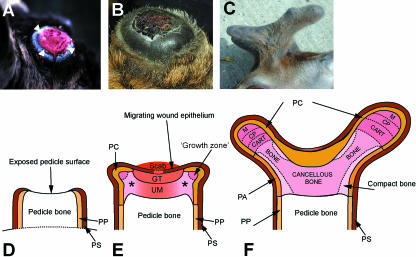
(A) Schematic diagram to show the three axes of the antler development: A-P, anterior-posterior axis; D-V, dorso-ventral axis; M-L, medio-lateral axis. (B,C) Schematic diagrams illustrating three stages of antler development. (B) Antlerogenic periosteum is present in the embryo and after birth as a localized thickening of the periosteum of the frontal bone. (C) Development of the pedicle occurs through four stages: 1, intramembranous ossification; 2, transitional ossification; 3, pedicle endochondral ossification; 4, antler endochondral ossification and velvet skin formation. (D) Longitudial section through a growing primary antler illustrating the main anatomical regions. Endochondral bone growth occurs at the distal tip while bone forms by intramembranous ossification around the antler shaft.
Fig. 1.
The diverse anatomy of antlers. (A) Moose (Alces spp.). >(B) Fallow deer (Dama dama). (C) Red deer (Cervus elapus). B and C are reproduced courtesy of Dr John Fletcher, Reediehill Deer Farm, Auchtermuchty, Fife, UK.
Development of primary antlers
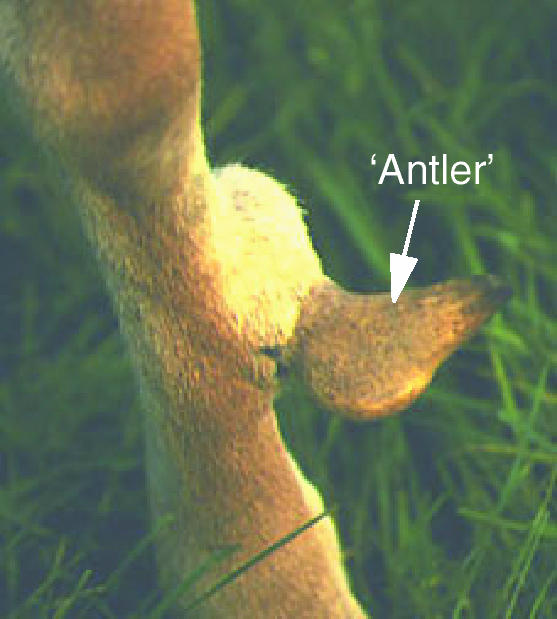
Antlers develop from pedicles, permanent bony protuberances on the frontal bone. As the fawn approaches puberty (at approximately 5–7 months old in the red deer), a collection of determined periosteal cells located in the distal parts of the cristae externae of the frontal bones are activated by rising androgen levels in the blood. This specialized periosteum (Fig. 2B) was originally described as ‘antlerogenic periosteum’ (AP) by Richard Goss (Goss & Powel, 1985). The AP contains specific binding sites for testosterone (Li & Suttie, 1998) although testosterone does not appear to have direct effects on cultured AP cells whereas IGF-I stimulates their proliferation (Li et al. 1999; Sadighi et al. 2001). Trabecular bone is then formed beneath the periosteum and a pedicle develops (Sempere et al. 1983; Suttie et al. 1984, 1988, 1991). Normally the antlerogenic periosteum of female deer remains quiescent because the hormonal requirements necessary for pedicle formation are not present. However, female deer can be induced to form pedicles if injected with androgens (Kierdorf et al. 1995).
Li & Suttie (1994) have undertaken detailed histological analyses of pedicle formation and have identified four ossification stages. The first is the intramembranous ossification stage (1 in Fig. 2C), and this is where the initial antlerogenic cells start to proliferate and differentiate into osteoblasts. These osteoblasts form trabecular bone in cellular periosteum. The second stage is transitional ossification, which takes place when the pedicle has reached 5–10 mm in height (2 in Fig. 2C). Osseocartilaginous tissue is formed by antlerogenic cells at the apical surface, which have undergone a change in differentiation pathway to form chondrocytes. Pedicle endochondral ossification is the third stage, when chondrogenesis takes place in the pedicle alone (3 in Fig. 2C). The fourth and final stage is termed antler endochondral ossification. Here the antlerogenic cells maintain their chondrogenic differentiation pathway until the first antler has been fully formed (4 in Fig. 2C). The onset of antler formation is coincident with the appearance of ‘shiny’ velvet skin covering the distal end of the pedicle. These un-branched antlers (described as ‘spikers’ in the deer industry) then elongate as a result of an endochondral process in the distal tip (Fig. 2D; Chapman, 1975). Because the cellular anatomy of endochondral ossification is very similar in the primary and regenerating antlers it will be described in a later section.
Growth of the first antler continues until the autumn rutting season approaches when there is another increase in circulating testosterone. This endocrine change is associated with a cessation in longitudinal growth, the antler bone becomes fully mineralized and the overlying velvet is shed exposing bare bone. This leaves the single un-branched antler attached to the pedicle until it is cast the following spring. This inhibition of longitudinal bone growth in response to an increase in sex steroids is similar to that which occurs at puberty in humans (Riggs et al. 2002). In fact it is now known that in the male skeleton many of the effects of testosterone are indirect, following its conversion to oestrogen by the enzyme aromatase (Riggs et al. 2002). That oestrogen may be an important regulator of antler growth was first demonstrated by Goss (1968), who found that injection of oestrogen inhibited growth of regenerating antlers and promoted premature ossification and shedding of the velvet skin. Bubenik et al. (1975b) subsequently showed that administration of an oestrogen antagonist had an inhibitory effect on antler bone formation. More recently, Bubenick et al. (2005) have provided evidence that oestrogen is actually synthesized by antler tissues in deer stags. It has also been demonstrated that the seasonal antler cycle in female reindeer is regulated by oestradiol, in this case synthesized by the ovary (Lincoln & Tyler, 1999).
Transplantation experiments demonstrated that the tissue from which the first antler develops is antlerogenic periosteum (Fig. 2B). Hartwig (1967) showed that moving this tissue to another region of the frontal bone resulted in an antler forming at the new location but not at the original site. It was subsequently shown that growth of antler structures can be induced by transplantation of this periosteum to a heterotopic location (Fig. 3) overlying the metacarpal bone. Furthermore, these ‘mini-antlers’ are influenced by the same hormonal cues as antlers on the skull, e.g. they shed their velvet skin at the appropriate time of year (Hartwig & Schrudde, 1974; Goss & Powel, 1985). Structures resembling antlers, or pedicle-antlers, have also been generated when AP was transplanted over the calvariae of nude mice (Li et al. 2001). Studies using a cell lineage tracer described by Li and Suttie in their review of antlerogenic periosteum provide further evidence that pedicles and antlers are derived from this tissue (Li & Suttie, 2001). Taken together, these studies led Li & Suttie (2001) to suggest that, like some embryonic tissues, AP has full self-differentiating capacity. Interestingly, the cellular layer of AP is very rich in glycogen (Li & Suttie, 1998), a characteristic of fetal osteoblasts.
Fig. 3.
Growth of an ‘antler’ from transplanted antlerogenic periosteum. Antlerogenic periosteum was transplanted from the frontal bone and grafted onto the metacarpal bone of a young fallow deer. Photograph courtesy of Uwe and Horst Kierdorf, Justus Liebig University of Giessen, Germany.
Unfortunately, there is a paucity of information on the local molecular mechanisms involved in antler and pedicle development. In vitro studies have shown that insulin-like growth factor I (IGF-I) may be an important systemic regulator of pedicle formation as it stimulates proliferation of antlerogeneic cells from all four ossification stages. Retinoic acid (RA) is also likely to play a role because application of RA to the developing pedicle increased the growth rate of the first antler and this was suggested to occur via an increase in the proliferation of periosteal cells (Kierdorf & Bartos, 1999). Recently, Barling and colleagues (Barling et al. 2004a,b) have undertaken a series of studies aimed at identifying growth factors and their receptors in the skin and underlying bone of primary antlers. Growth factors identified include epidermal growth factor (EGF) and the EGF receptor, fibroblast growth factor 2 (FGF-2) and the FGF receptors, FGFR1, FGFR2 and FGFR3, bone morphogenetics proteins (BMPs) 2, 4 and 14 and the BMP receptors BMPR1B and ACTRII. They hypothesize that these growth factors signal between the osseocartilagenous and skin compartments of the primary antler (Barling et al. 2004b). The spatial and temporal differences in the localization of these growth factors showed that their distribution in skin of the primary antler resembles that described in adult skin of other species, whereas their distribution in bone and cartilage resembles that in the fetal skeleton.
Identifying the pathways that regulate the prenatal development of antler primordiae should also be a priority for future study. In the embryo, localized thickenings of the periosteum of the frontal bone can be observed at sites of future pedicle/antler development (Fig. 2B; Lincoln, 1973; Li & Suttie, 2001). These primordial pedicles enlarge between 55 and 150 days of gestation but regress at later stages. This tissue specification is likely to involve interactions between mesenchyme and the overlying epithelial cell layer, which Li & Suttie (2001) described as resembling the apical ectodermal ridge in the developing limb. It is noteworthy that development of the mammary gland, which, like antlerogenic periosteum, is regulated by sex steroids, involves epithelial–mesenchymal interactions (Foley et al. 2001). One of the molecules that mediates epithelial–mesenchymal interactions in the developing mammary gland is parathyroid hormone-related peptide (PTHrP). Although the role of PTHrP in the development of antlerogenic periosteum has not been investigated, there is evidence that it plays an important role in both the developing and the regenerating antler. Barling et al. (2004a) have recently identified the PTHrP and the PTH/PTHrP receptor in both velvet skin and underlying mesenchymal tissues of red deer primary antlers. We have also found that PTHrP is widely expressed in tissues of regenerating red deer antlers and that its synthesis is regulated by TGF-β (Faucheux et al. 2004). Clearly a major challenge lies in characterizing the pathways and factors that: (i) define where and how AP forms during fetal life, (ii) enable AP to ‘survive’ until the systemic environment is appropriate for antler development and (iii) activate expansion of progenitor cells in the perisoteum. The potential role of periosteum in regenerating antlers will be discussed in more detail in the next section.
Systemic regulation of the repeated cycles of antler regeneration
Because the primary function of antlers is to enable stags to protect and retain harems of females (does), their growth is linked to the annual breeding cycle and its associated fluctuations in sex hormone concentrations. In temperate species such as the red deer, it is a change in day length that regulates reproductive activity, although there are endogenous rhythms of antler growth (West & Nordan, 1976). Studies in orchidectomized (castrated) stags and in stags administered exogenous hormones have demonstrated that sex steroids are most important at the start and at the end of the antler growth cycle. Incredibly, Aristotle was the first scientist to describe the effects of castration on antier growth!
Antlers are normally cast in spring when testosterone levels are low. Castration of stags in late winter/early spring will lead to premature casting of antlers (Jaczewski et al. 1976), whereas administration of exogenous sex steroids at this time will prevent casting and regeneration (Fletcher, 1978). The rapid growth of antlers occurs during the early summer when there is a plentiful food supply and stags live sedentary lives while ‘testosterone depleted’ (Goss, 1983). Because antler regeneration takes place at a time when deer reproductive organs are inactive it was proposed many years ago that there must also be a non-gonadal factor involved and this was named ‘antler growth stimulus’ (AGS) (Wislocki, 1943). IGF-I synthesized in the liver is the most likely candidate as IGF-I concentrations are high during the period of rapid antler growth (Suttie et al. 1985), there are IGF receptors in the antler's growing tip (Elliott et al. 1992, 1993) and IGFs promote proliferation of antler cells (Price et al. 1994; Sadighi et al. 1994). Changes in concentrations of other hormones are associated with the antler growth cycle, including 1,25(OH)2D3 (Van der Eems et al. 1988; Sempere et al. 1989), thyroid hormones (Shi & Barrell, 1994), cortisol (Bubenik et al. 1975a; Suttie et al. 1995) and prolactin (Sempere et al. 1983; Suttie et al. 1984), although their function remains poorly understood.
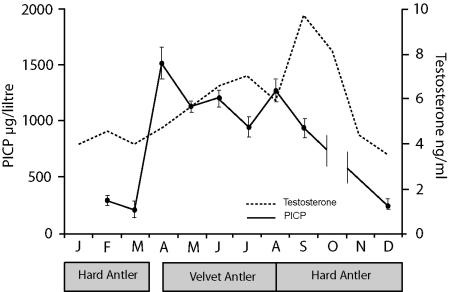
Antler growth places an enormous demand for mineral on the skeleton (sufficient mineral is needed to enable a 30-kg bone to grow in ∼3 months), and to meet this demand cyclical reversible osteoporosis occurs at other skeletal sites (Bubenik, 1983). This bone loss is greatest in non-weight-bearing bones such as the rib. Measuring biochemical markers of bone cell activity in the circulation has shown how bone turnover increases dramatically at the time of rapid antler growth. Figure 4 shows the results of a study in which we measured changes in circulating levels of the carboxyterminal pro-peptide of type I collagen, a marker of osteoblast activity (Eriksen et al. 1993), at different times of year. This showed that levels were ten times higher during the period of antler regeneration. Baksi & Newbury (1988) have also shown changes in serum osteocalcin and hydroxyproline associated with antler growth.
Fig. 4.
Changes in circulating concentrations of testosterone and the carboxy-terminal pro-peptide of type I collagen (PICP) during the antler growth cycle in red deer stags. Serum samples were collected at post-mortem from deer stags killed for venison at different times of year. PICP was measured by radioimmunoassay (Orion, Diagnostica, Finland) and the values shown are the mean ± SEM. This illustrates that antler regeneration is associated with significant changes in bone turnover. The testosterone graph is adapted from Muir et al. (1988).
Atlhough low levels of sex steroids are ‘permissive’ for regeneration, high levels appear to act as a ‘brake’, as discussed previously in the context of the developing antlers (Goss, 1968). Therefore, as the breeding season approaches and testosterone levels increase, endochondral growth stops, the antlers become fully calcified, and the velvet skin covering then thins, becomes ‘dry’ and is then shed (Fig. 5A) to expose the antler as a solid bone that remains firmly attached to the pedicle. The seasonal surge in testosterone also causes a behavioural change in the deer. Even before shedding their velvet, bucks begin to establish positions in the social hierarchy (Fig. 5B). Testosterone levels peak in late autumn when sexual activity is intense (Lincoln, 1971) and then decline again as spring approaches.
Fig. 5.
The consequences of the rise in concentrations of circulating testosterone in late summer. (A) Shedding of velvet skin. (B) A pair of boxing stags. (Courtesy of Dr John Fletcher, Reediehill Deer Farm, Auchtermuchty, Fife, UK).
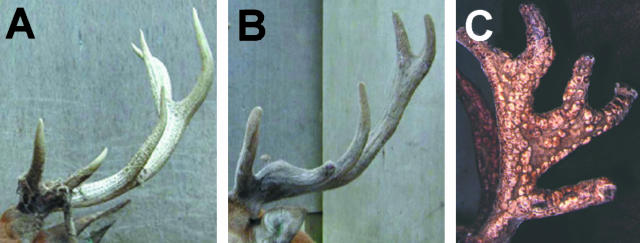
In red deer, castration during the period of antler growth will delay velvet shedding and the antlers do not fully mineralize (Fig. 6B). In some more phylogenetically evolved deer species the growth of castrated antlers is quite aberrant, e.g. fallow deer develop peculiar bony protuberances that Goss (1983) classified as ‘antleromas’ (Fig. 6C). Recently, Kierdorf et al. (2004) described in detail the structure of the antler of castrated fallow deer and presented evidence to suggest that these protuberances (Fig. 6C) are in fact benign tumour growths with comparable histological changes to osteomas. Roe deer also develop large benign tumours on castrated antlers in which intradermal bone has been observed. The link between regeneration and cancer was highlighted in a review by Brockes (1998), and led us to suggest previously (Price & Allen, 2004) that castrated antlers develop tumour-like structures because a mechanism has evolved in deer whereby sex steroids (acting directly or indirectly) normally limit cell cycle progression in antler progenitor cells. It is the remarkable capacity for growth and self-renewal of antler progenitor cells that underpins the capacity of antlers to regenerate, but this has a potential down-side: an increased risk of transformation. By contrast, lower organisms such as the newt have evolved a system whereby their cells can have an extended lifespan (and thus sustain regeneration) yet remain remarkably resistant to cancer (Tsonis, 1983). Humans and other mammals may have lost the ability to regenerate because the evolutionary benefit is outweighed by the increased risk of developing cancer.
Fig. 6.
The effects of castration on antler growth. (A) Antler of an intact red deer stag in early autumn. The velvet skin has been shed following the rise in circulating testosterone levels. (B) Antler of a red deer stag that was orchidectomized during the first month of antler growth; this results in retention of the velvet skin. (C) The antler of a castrated fallow deer. Numerous bony protuberances (‘antleromas’) can be observed over the antler surface. Photograph courtesy of Uwe and Hans Kierdorf, Justus Liebig University of Giessen, Germany.
Antler regeneration: casting of the old set of antlers
Casting of the stag's primary antlers marks the onset of an annual regenerative cycle that will continue throughout its life. In a 2-year-old animal the regenerated antlers are relatively unimpressive, but as the stag ages his antlers also become larger. Before the antlers are cast the skin covering the distal pedicle assumes the shiny appearance of antler velvet and the region becomes slightly ‘swollen’ (Fig. 7A). Antlers are then normally cast within a day or so of each other and this event is effected by osteoclasts resorbing bone in the distal pedicle (Goss et al. 1992). This resorption appears to be very tightly regulated because, unlike teeth, antlers do not start to ‘wobble’ in the days/hours before they are shed. Casting then leaves an exposed concave pedicle surface that rapidly fills with blood, although within hours a large scab forms to cover it (Fig. 7A). Kierdorf et al. (1993) have described the histological changes in this region and found that after casting osteoclasts continue to resorb bone in the distal pedicle, which creates a smooth surface. This is followed by a phase of bone formation that restores the portion of the pedicle that was lost with the cast antler.
Fig. 7.
Different stages of antler regeneration. (A) The pedicle immediately after the old antler has been cast. Note the ‘ring’ of regenerating tissue around the edge (arrowheads). (Courtesy of Dr John Fletcher, Reediehill Deer Farm, Auchtermuchty, Fife, UK). (B) The early antler bud ∼4 days after antler casting. A scab has now formed. Asterisk indicates the position of a future branch. (C) Antlers at ∼30 days of growth showing the anterior (brow) and posterior (main beam) tines (arrows). (D–F) Schematic diagrams of sections through the regenerating antler. PP, periosteum of pedicle; PS, pedicle skin; GT, ‘granulation’ tissue; PC, perichondrium; UM, undifferentiated mesenchymal tissue; PC, perichondrium; CP, chondroprogenitors; CART, cartilage; PA, periosteum of the antler. (D) Day zero. Casting leaves an exposed pedicle bone surface upon which a scab forms (as in B). (E) Antler bud at about days 9–10. The migrating wound epithelium has almost completely covered the pedicle surface. The underlying tissue has features of granulation tissue but also contains undifferentiated mesenchymal cells, so we describe this as ‘undifferentiated mesenchyme’. Growth centres have been established at the sites where branches will develop and chondrogeneis is evident beneath them (marked by an asterix). (F) Day 30. Longitudinal growth takes place in the distal tip of each branch. D is adapted from Li et al. (2005).
In most species of deer loss of the antler coincides with regeneration of its replacement. However, in reindeer and moose, antlers are cast in late autumn and yet the wound does not heal over the pedicle stump and re-growth does not take place until environmental conditions are appropriate several months later (this delay may have evolved to reduce the energy demands on the animal as a consequence of carrying heavy antlers during harsh winters). Goss also demonstrated experimentally that if the distal end of the pedicle is amputated in winter (a ‘non-permissive’ time of year) wound healing and regeneration was delayed until spring (Goss, 1972). These observations imply that antler regeneration is not triggered by the tissue repair mechanisms induced by casting, whereas components of the injury response pathway are known to be critical for inducing regeneration in the newt (Brockes et al. 2001). The phenomenon of double-headed antlers also demonstrates that injury is not an absolute requirement for antler regeneration. In this situation, new antlers develop from the base of the previous antlers that are retained on the pedicle (Kierdorf et al. 1994). That is not to say that there is a relationship between antler regeneration and wound repair; by definition the process of epimorphic regeneration requires wound healing to take place (Goss, 1972). For example, Goss (1972) showed that when skin was sutured over the stump following antler removal regeneration could not take place.
The cue for the initiation of deer antler regeneration is therefore most likely to be a systemic factor(s) whose synthesis is regulated by changes in the hypothalamic–pituitary axis. Regeneration could be initiated either when concentrations of a ‘permissive’ factor reach a threshold or when concentrations of a ‘repressor’ fall below a certain threshold. Testosterone is a strong candidate for being the ‘repressor’ as casting of antlers is known to be associated with a decrease in circulating concentrations (Suttie et al. 1995; Bubenik et al. 1997). One of the consequences of a decline in sex steroid concentrations (and or the number of receptors) could be induction of bone resorption at the antler–pedicle bone interface since sex steroids are known to inhibit osteoclast function in other species (Shevde et al. 2000). However, the role of sex steroids is likely to be more complex and may also involve regulation of stem cell populations in the pedicle tissues (this will be discussed in more detail in a later section).
Antler regeneration: formation of the antler bud
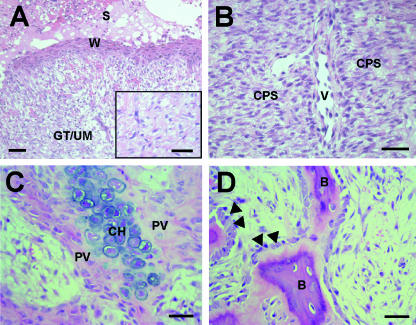
The anatomy of the early stages of antler regeneration has been described in a number of recent publications is illustrated here in Fig. 7 (Kierdoff et al. 2003; Li et al. 2004, 2005; Price, 2005). Immediately after the old antlers have been cast a raised ‘swollen’ ring of skin surrounds the distal end of the pedicle (Fig. 7A). This has a ‘shiny’ surface, characteristic of antler velvet skin. Within hours, the epidermis, known as ‘wound epithelium’, starts to migrate across the exposed surface of the pedicle bone and covers a mass of cells within loosely packed extracellular matrix tissue (Fig. 8A). These cells have not been well characterized; for example, it is not known whether they express similar markers to cells in the newt blastema. Li et al. (2004) describe the tissue in the centre of the pedicle as granulation tissue. However, we have shown that as early as 4 days after casting a significant proportion of cells stain positive for PTHrP (Faucheux et al. 2004), which leads us to assume that these are of mesenchymal origin.
Fig. 8.
Histology of the early regenerating antler. Haematoxylin and eosin (H&E)-stained undecalcified paraffin sections. (A) The migratory wound epithelium (W) overlying undifferentiated mesenchyme (UM) and granulation tissue (GT) in the centre of the antler bud at day 4. S, scab. Inset: a higher magnification view of mesenchymal tissue. (B) By day 9 there is a distinct zone of chondroprogenitors (CPs) and longitudinally aligned vascular channels (v). (C) Cartilage formation in the 9-day antler. Chondrocytes (CH) are surrounded by cartilage matrix and pervascular mesencymal tissue (PV). (D) Recent bone (B) formation. Osteoblasts are marked with arrowheads.
By days 9–10, epithelialization is complete and a number of zones can be distinguished histologically at anterior and posterior sites on the pedicle that mark the position where future antler branches will develop (Figs 7E and 8). Below the wound epithelium and perichondrium is a zone of proliferating mesenchymal cells, and more proximally chondrogenesis and bone formation takes place (Fig. 8C,D).
The structure which forms on the pedicle after antler casting has traditionally been called a ‘blastema’ (Fig. 7B) because blastema formation is a fundamental step in the process of epimorphic regeneration. Epimorphic regeneration is defined as ‘de novo development of appendages distal to the level of amputation’ (Goss, 1980), and is distinct from the processes of cellular regeneration turnover or tissue repair. However, the antler ‘blastema’ (Fig. 7B,E) is not a morphologically uniform structure like the blastema that forms following amputation of appendages in newts and other lower vertebrates (Brockes, 1997). This, together with evidence that antler regeneration may not involve reversal of the differentiated state, has recently led Li et al. (2004, 2005) to conclude that antler regeneration is not blastema-based. These authors define a blastema as being ‘formed from the de-differentiation of all cell lineages on the immediate amputation plane’ (Li et al. 2005). However, we would argue that the key issue is whether the undifferentiated cells in a blastema can regenerate a lost part, not whether they are formed by de-differentiation. For example, the regenerating tail of Xenopus involves formation of a structure that is generally described as a blastema, although there is no evidence that it forms by de-differentiation (Slack et al. 2004).
Within a few days after casting, distinct ‘growth zones’ can be identified in the early antler at sites where branches will eventually develop (Fig. 7E) (Price et al. 1994). These can be identified by the presence of proliferating cell nuclear antigen (PCNA)-positive cells (Price, 2005) that also synthesize PTHrP, which we have previously proposed is a marker of antler progenitor cells (Faucheux et al. 2004). We suggest that although the whole structure that forms on the pedicle should not be called a blastema, the individual growth zones do have some of the features that define a blastema, i.e. they consist of ‘a mass of undifferentiated cells that will develop into an organ or tissue that is present at a site of regeneration’. Therefore, until cells in the different regions of the early regenerating antler ‘blastema’ have been better characterized, we suggest that it is somewhat premature to conclude that antler regeneration is not blastema-based.
Because the early stages of antler regeneration have not been studied in any detail at a cellular level, we know little about the signalling pathways that are involved. As discussed above, PTHrP is localized in mesenchymal cells but it is also present in regenerating wound epithelium, suggesting that it may have multiple roles (Faucheux et al. 2004). In the same study we also identified TGF-β in the early antler and found that it up-regulates PTHrP synthesis by cultured blastema cells. There are several lines of evidence to suggest that retinoic acid (RA) is also likely to play a role during the early stages of regeneration: the RA-synthesizing enzyme RALDH2 is synthesized in the early antler and in situ hybridization studies have also shown that RARα, RABβ and RXRβ receptors are also expressed, although they are not each present in every cell type. For example, RARβ is specifically expressed in cells at sites of cartilage/bone formation in more proximal regions (Price & Allen, 2004). This demonstrates that the early antler bud is not a uniform structure and that, like PTHrP, RA may have multiple functions.
Another question in antler biology pertains to the source of cells which give rise to the regenerating antler Wislocki and Goss were of the view that migrating cells from the dermis of the pedicle were the source (Wislocki, 1943; Goss, 1972, 1984, 1995). However, a number of workers in this field currently take the view that regenerating antlers are derived mainly from a population of progenitor cells in the periosteum of the pedicle (Kierdorf & Kierdorf, 1992, 2000, 2001; Li et al. 2005). This tissue is derived from antlerogenic periosteum, which has convincingly been shown to be the origin of the pedicle and primary antlers (Hartwig, 1974; Goss & Powel, 1985; Kierdorf & Kierdorf, 2001). Li et al. (2005) concluded from their histological studies that antler regeneration is stem cell based with the periosteum being the source of these cells. However, before a cell can be defined as a true ‘stem’ cell it must be shown to have a capacity for self-renewal and to be capable of differentiation into specialized lineages. To date there is no evidence that cells from the pedicle of an adult deer have the capacity for self-renewal or can differentiate into anything other than chondrocytes and osteoblasts (Li et al. 1995). A number of approaches could be taken to address this issue: (1) the identification of stem cell markers in tissues of the pedicle, (2) the demonstration that cells cultured from the periosteum of the pedicle of regenerating antlers are pluripotential in vitro and in animal models in vivo, and, (3) the demonstration that periosteum cells stably transfected with a genetic marker [e.g. green fluorescent protein (GFP)] differentiate into different antler cell types in regenerating antlers in vivo.
Antler regeneration: cartilage and bone growth
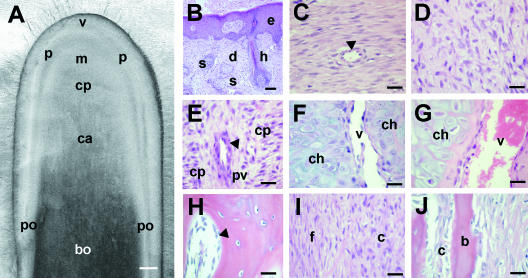
In the first month after casting, antlers grow relatively slowly; however, during the next 2 months longitudinal growth is very rapid and this rate of bone formation represents the fastest described in the mammalian kingdom (Goss, 1983). The longitudinal growth of the antler that occurs in the distal end of each branch (shown schematically in Fig. 7F and histologically in Fig. 9) was originally described as a process of modified endochondral ossification by Banks & Newbry (1983). These authors classified the antler tip as consisting of four zones, namely the zones of proliferation, maturation, hypertrophy and calcification, representing the spectrum of bone development.
Fig. 9.
Histology of the regenerating antler during rapid longitudinal growth. (A) Longitudinal tissue section of antler tip to show macroscopic appearance of regions: v, velvet skin; p, perichondrium; m, mesenchyme; cp, chondroprogenitor region; c, cartilage; bo, bone; po, periosteum. Scale bar = 0.5 cm. (B–J) H&E-stained undecalcified paraffin sections of the tissue regions shown in A. (B) Velvet skin. e, epidermis; d, dermis; h, hair follicle; s, sebaceous gland. (C) Fibrous perichondrium. A blood vessel is marked by an arrowhead. (D) Mesenchymal ‘growth zone’. (E) Chondroprogenitor (cp) region. As in the early antler bud, cells start to become aligned in ‘columns.’ However, the vascular spaces are relatively small (arrowhead). (F) Non-mineralized cartilage. Recently differentiated chondrocytes (ch) are arranged in trabeculae separated by larger vascular channels (v). (G) Mineralized cartilage region. Chondrocytes and the vascular channels (v) increase in size in this region. (H) Spongy bone in the mid shaft of the antler that has formed by endochondral ossification. Osteoblasts are marked with an arrowhead. (I) Fibrous (f) and cellular (c) layers of the antler periosteum. (J) Intramembranous bone formation (b) takes place beneath the cellular periosteum (c). Scale bar (B–J), 100 µm.
The mesencymal growth zone
Below the dermis of the velvet skin (Fig. 9B) is the perichondrium (Fig. 9C), which is continuous proximally with the periosteum that surrounds the shaft of the antler and is the site of intramembranous bone formation. There is an outer fibrous perichondrium where type I collagen mRNA and protein are highly expressed (Price & Faucheux, 2001) and an inner, more cellular zone (Fig. 9D). This region of the antler has been variously described by different authors (our group is also guilty of this), and the descriptors include ‘reserve mesenchyme’, ‘hyperplastic perichondrium’, ‘cellular periosteum’ and ‘mesenchyme’. For consistency we now describe this as ‘mesenchyme’ or ‘mesenchymal growth zone’ because cells in this region, like cells in this region of the primary antler, are actively dividing (Matich et al. 2003; Faucheux et al. 2004). In culture these cells proliferate rapidly as monolayers and synthesize type I but not type II collagen (our unpublished observations), and this reflects their in vivo phenotype (Price et al. 1996). However, unlike mesenchymal cells from the developing limb, they cannot be cultured as micromasses; they spread out to form monolayers and chondrogenesis is not initiated. Compared with cells in more proximal regions of the antler, cells in this region express only low levels of alkaline phosphatase and this reflects their undifferentiated state (Price et al. 1994; Price, 2005). However, although these cells will differentiate into chondrocytes in more proximal regions, they do express markers of the early osteoblast lineage (our unpublished observations) and in the presence of dexamethasone alkaline phosphatase expression will increase (Faucheux et al. 2001). This indicates that these cells are at least bi-potential, although we have preliminary evidence that they can also differentiate into adipocyte-like cells under the appropriate culture conditions.
In the mesenchymal growth zone there will be a tightly co-ordinated balance between cell growth, cell survival and differentiation into chondroprogenitors, with some signalling pathways inducing proliferation and others inducing differentiation. In recent years our group and others have made some progress in identifying the local factors that may play a role. Several years ago it was first shown that their proliferation in vitro is stimulated by IGF-I and IGF-II and IGF receptors are present in this region of the antler tip in vivo (Price et al. 1994; Sadighi et al. 1994). Furthermore, IGF-I and IGF-II were identified in a screen of antler extracts undertaken some years ago (Mundy et al. 2001). This is consistent with the hypothesis that IGF-I is likely to be the ‘antler growth stimulus’. We have found that FGF-2 also stimulates proliferation of mesenchymal cells from regenerating antlers (Price, 2005), although we have not yet tried immunolocalizing it in regenerating antlers. Members of the FGF family and their receptors have also been identified in the primary antler. A proteome analysis of red deer antler has been recently undertaken but surprisingly neither FGFs, IGFs, IGF binding proteins nor IGF receptors were identified (Park et al. 2004).
The BMP and TGF-β signalling pathways appear to induce a more differentiated state in mesenchymal cells, as our preliminary studies have shown that BMP-2 and TGFβ-1 inhibit the proliferation of mesenchymal cells whereas they induce ALP activity (Price, 2005). BMP-2 and BMP-4 have both been cloned from antlers (Feng et al. 1995, 1997) and Barling and collagues have identified BMP-2, BMP-4 and BMP-14 and their receptors in the primary antler (Barling, 2004b). We have recently immunolocalized TGF-β in regenerating antlers and it appears to act upstream of PTHrP, which, together with the PTH/PTHrP receptor, are synthesized by the majority of mesenchymal cells (Faucheux et al. 2004). Interestingly, we have found that PTHrP has no effect on the proliferation of mesenchymal cells (Faucheux & Price, 1999) and may maintain the undifferentiated state, although the functional significance of its abundance in this region requires further study. We have previously presented several lines of evidence that RA also plays a role in controlling mesenchymal cell growth and differentiation: first, the RA-synthesising enzyme RALDH2 can be immunolocalized in mesenchymal cells, and second, retinol, all-trans-RA and 9-cis-RA were identified in mesenchyme by HLPC and in vitro all-trans-RA dose dependently increased ALP activity in cultures of mesenchymal cells (Allen et al. 2002). However, the effects of RA in this region are likely to be very complex, as reflected in the distinct patterns of expression of the RA receptors RARα, RXRβ, RXRβ and RXRγ.
Antler cartilage
With the naked eye it is possible to identify where chondrogenesis starts in a longitudinal antler section; it is where the tissue becomes slightly darker in colour because of an increase in size of the vascular channels (Fig. 9A). It is also possible to distinguish that there is a distal region of non-mineralized cartilage whereas more proximally deposition of mineral takes place (Price et al. 1996). The vascularization of antler cartilage is the most striking difference between its anatomy and that of other hyaline cartilages. This abundant blood supply is required to meet the high metabolic demands imposed by rapid tissue regeneration. That the antler provides a valuable model for the study of angiogenesis was proposed recently by Clark et al. (2004), who have identified VEGF and the VEGF receptor in regenerating antler tissues.
Histologically, the boundary between mesenchyme and the zone of chondroprogenitors is not distinct in longitudinal sections, but is a region where cells start to become arranged into columns between which there are vascular spaces (Fig. 9F). Previously we have described this as a ‘zone of transition’ because cells at this site are at different stages of differentiation along the chondrocyte lineage (Price et al. 1996). Type IIA collagen mRNA identifies chondroprogenitors in the developing skeleton (Sandell et al. 1994) and this isoform is expressed in the distal antler cartilage whereas type IIB collagen mRNA and protein are expressed throughout the cartilage (Price et al. 1996). Type IIA collagen is not expressed in growth plate cartilage (Sandell et al. 1994), another reason why it is difficult to compare directly the process of endochondral ossification in a long bone with that in antlers. Neither is type I collagen found in the growth plate, whereas it is expressed by antler cells in the ‘transition zone’. Yet another difference between the matrix of antler and mammalian growth plate cartilage is the distribution of type X collagen; in the growth plate it is expressed only in proximal hypertrophic chondrocytes (Sandell et al. 1994) whereas in the antler type X collagen can be localized in the majority of chondrocytes.
Non-mineralized cartilage (Fig. 9F) provides us with an abundant source of cells for in vitro studies, and culturing them as a micromass helps to maintain the chondrocyte phenotype (Allen et al. 2002; Price, 2005). This provides a useful model for in vitro studies of chondrogenesis; however, as will be described below, we also use the model to study antler osteoclasts. To date we have focused on molecules that we have identified in antler cartilage in vivo. PTHrP can be immunolocalized in chondroprogenitors but not in fully differentiated chondrocytes (Faucheux et al. 2004), and in micromass cultures it inhibits differentiation, consistent with its role during limb development (Faucheux, 1999). Indian hedgehog (IHH) and TGFβ-1 are also expressed in antler chondroprogenitors, indicating that these molecules may act with PTHrP in a feedback loop to control differentiation. RA also controls chondrogenesis; both all-trans-RA and 9-cis-RA are present in antler cartilage, and in vitro RA inhibits proteoglycan synthesis by chondrocytes, an effect that requires RAR signalling (Faucheux, 1999; Allen et al. 2002). However, RXRβ may mediate the effects of RA in vivo because it is expressed throughout cartilage trabeculae.
Cartilage and bone resorption
The growth of antlers, like that of other rapidly developing bones, requires extensive remodelling of cartilage and bone and thus is dependent on the local formation of osteoclasts from circulating mononuclear progenitors. In addition to columns of chondrocytes and vascular spaces, antler cartilage contains perivascular tissue (Fig. 9F,G) and this has been identified as a site where cells of the osteoclast lineage differentiate (Faucheux, 1999; Faucheux et al. 2002; Szuwart et al. 2002). When digests are obtained from antler cartilage for in vitro studies this includes a fraction of osteoclast progenitors because cells with the phenotypic characteristics of mammalian osteoclasts will differentiate in micromass cultures of chondrocytes under the appropriate conditions (Faucheux, 1999). This system is now used routinely in our laboratory to study the mechanisms that regulate antler osteoclast function. Factors that will induce antler osteoclastogenesis in vitro include PTHrP, RANKL, M-CSF and RA (Allen et al. 2000; Faucheux et al. 2002). Because sex steroids are important regulators of bone resorption in other species (Riggs et al. 2002), we are currently exploring whether the cessation of antler bone growth induced by high concentrations of testosterone involves direct effects of sex steroids on osteoclast activity.
Antler bone formation
Although osteoclasts start to differentiate in the non-mineralized cartilage, the largest TRAP-positive cells are localized in the mineralized cartilage lower down the antler tip where extensive matrix resorption takes place. This results in the formation of a number of irregular trabeculae that are eventually replaced by woven bone and cancellous lamellar bone in the primary spongiosa (Fig. 9H) at the centre of the antler shaft (Banks & Newbry, 1983). Osteoblasts appear to differentiate from a population of osteoblast progenitors in perivascular tissue in cartilage; these cells synthesize type I collagen mRNA (Price et al. 1996), alkaline phosphatase (Price et al. 1994) and, as they mature, osteocalcin, a marker of the fully differentiated osteoblast phenotype (Allen et al. 2002). At the same time intramembranous ossification takes place around the antler shaft (Fig. 9J) and although we have not studied ALP expression at this site, osteocalcin can be immunolocalized in osteoblasts beneath the periosteum (Allen et al. 2002).
To date, our knowledge of the factors that may specifically regulate osteogenesis in regenerating antlers is derived from descriptive studies, although culturing these cells from explants of antler bone is straightforward and so there is great potential for further work in this area. As discussed recently by Kierdorf & Kierdorf (2004), the rapid rate of antler bone growth makes it an excellent model for investigating osteogenesis. PTHrP, acting through the PPR, is likely to play a role as both are present in osteoblasts at sites of endochondral and intramembranous bone formation. By contrast, we have only found IHH in osteoblasts in the midshaft of the antler, not at the periphery, which suggests a specific role in endochondral bone growth, consistent with the findings of knock-out studies in mice (St-Jacques et al. 1999). IHH mRNA has also been localized in antler cartilage and bone by in situ hybridization, following its identification in a cDNA library (Lord et al. 2004). TGFβ-1 is also present in antler osteoblasts (Price et al. 2004). Another molecule that is likely to regulate bone formation in antlers is RA given that it is found at relatively high levels in antler bone and RALDH2 is localized in osteoblast progenitors in antler cartilage as well as in more differentiated osteoblasts. The effects of RA on osteoblast lineage cells may be mediated by the RARα receptor because it is expressed in perivascular tissues where type I collagen mRNA-expressing cells are also located (Allen et al. 2002). All of the molecules discussed above have been shown to regulate osteoblast function in other species (Grigoriadis et al. 1986; Choong et al. 1993; Oliva et al. 1993; Slootweg et al. 1996; Park et al. 1997; St-Jacques et al. 1999; Kobayashi et al. 2002).
What is clear from the above is that the factors so far identified as being involved in antler regeneration have multiple functions. For example, both RA and PTHrP regulate chondrocyte, osteoclast and osteoblast differentiation. Our knowledge of the pathways involved remains in its infancy, but what is becoming apparent is that nature is conservative and that during antler regeneration a number of developmental pathways are recapitulated. In fact, studies in a diverse range of models are beginning to show pleiotropic functions for known genetic pathways in the context of regeneration (Sanchez Alvarado, 2004). The hunt for novel ‘antler factors’ has been going on for a number of years; it started in the 1990s with the generation of a cDNA library by Mundy and colleagues (Mundy, 2001) who were trying to identify novel bone anabolic agents. More recently, Lord et al. (2004) generated a large database of genes present in regenerating antlers and the first proteomic analysis of red deer antler has been reported (Park et al. 2004). These studies provide an invaluable resource, inform us of ‘what is there’ and lead to hypothesis-driven research. However, it is very likely that novel molecular pathways may not be uncovered in antlers. In our view the challenge lies instead in understanding the way that different signalling pathways interact, both with each other and with their local and systemic environment, to regenerate a structure as large and complex as an antler. Dissection of the function of these pathways requires the manipulation of gene expression in vivo and we have preliminary data to show that biolistic particle transfer can be used to transfect cells in the blastema (Price & Allen, 2004). However, retroviral/adenoviral approaches may also prove to be useful tools for studying gene function.
Conclusions
Deer antlers are not only spectacular creations of the natural world but also provide us with a unique model for regeneration research (although the limitations of using a large semi-wild animal with a seasonal pattern of regeneration and limited potential for genetic manipulation should not be underestimated). Regeneration of antlers is regulated by environmental and systemic cues, rather than by injury or amputation, and recent evidence suggests that it probably involves stem cells, not de-differentiation of mature cells. Therefore, antler regeneration does not utilize all of the same strategies as regeneration in the urodele amphibian. However, this makes the antler more, not less, relevant as a model for human regeneration because if we can understand how deer have adapted the normal mechanisms of development, cell renewal and repair to regenerate a complete appendage it may be possible to achieve the same outcome in diseased or damaged human tissues.
Acknowledgments
This work has been supported by grants from the Medical Research Council, the BBSRC and the Wellcome Trust. We thank our collaborators Dr Jeannine Danks, Professor Malcolm Maden, Professor Jeremy Brockes, Professor Mike Horton and Dr Monica Colitti for their contribution to this work.
References
- Allen SP, Maden M, Price JS. Retinoic acid regulates osteoclast and chondrocyte differentiation in deer antlers which express retinoic acid receptors in vivo. Bone. 2000;28:S84. [Google Scholar]
- Allen SP, Maden M, Price JS. A role for retinoic acid in regulating the regeneration of deer antlers. Dev Biol. 2002;251:409–423. doi: 10.1006/dbio.2002.0816. [DOI] [PubMed] [Google Scholar]
- Baksi SN, Newbury JW. Plasma calcemic hormones in mature female reindeer, Rangifer tarandus. Gen Comp Endocrinol. 1988;69:262–266. doi: 10.1016/0016-6480(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Banks JW, Newbry WJ. Antler development as a unique modification of mammalian endochondral ossification. In: Banks RD, editor. Antler Developments in Cervidae. Kingsville, TX: Cesar Kleburg Wildlife Research Institute; 1983. pp. 279–306. [Google Scholar]
- Barling PM, Liu H, Matich J, et al. Expression of PTHrP and the PTH/PTHrP receptor in growing red deer antler. Cell Biol Int. 2004a;28:661–673. doi: 10.1016/j.cellbi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Barling PM, Lai AK, Tong AST, Nicholoson LFB. The distribution of growth factors and their receptors in growing red deer antler. In: Suttie JM, editor. Advances in Antler Science and Product Technology. Vol. 2. New Zealand: 2004b. pp. 37–44. [Google Scholar]
- Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- Brockes JP. Regeneration and cancer. Biochim Biophys Acta. 1998;1377:M1–M11. doi: 10.1016/s0304-419x(97)00029-2. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. J Anat. 2001;199:3–11. doi: 10.1046/j.1469-7580.2001.19910003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. Inroduction: new directions in tissue repair and regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:741–856. [Google Scholar]
- Bubenik GA, Bubenik AB, Brown GM, Trenkle A, Wilson DI. Growth hormone and cortisol levels in the annual cycle of white-tailed deer (Odocoileus virginianus) Can J Physiol Pharmacol. 1975a;53:787–792. doi: 10.1139/y75-108. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Bubenik AB, Brown GM, Wilson DA. The role of sex hormones in the growth of antler bone tissue. I: Endocrine and metabolic effects of antiandrogen therapy. J Exp Zool. 1975b;194:349–358. doi: 10.1002/jez.1401940202. [DOI] [PubMed] [Google Scholar]
- Bubenik G. The endocrine regulation of the antler cycle. In: Brown R, editor. Antler Developmant in Cervidae. 1983. pp. 73–107. [Google Scholar]
- Bubenik GA, Miller KV, Lister AL, Osborn DA, Bartos L, van der Kraak GJ. Testosterone and estradiol concentrations in serum, velvet skin and growing antler bone of male white-tailed deer. J Exp Zoolog A Comp Exp Biol. 2005;JOJ3:186–192. doi: 10.1002/jez.a.139. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Schams D, White RJ, Rowell J, Blake J, Bartos L. Seasonal levels of reproductive hormones and their relationship to the antler cycle of male and female reindeer (Rangifer tarandus) Comp Biochem. 1997;116:269–77. doi: 10.1016/s0305-0491(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Chapman D. Antlers – bones of contention. Mammal Rev. 1975;5:121–172. [Google Scholar]
- Choong PFM, Martin TJ, Ng KW. Effects of ascorbic acid, calcitrol, and retinoic acid on the differentiation of preosteoblasts. J Orthop Res. 1993;11:638–647. doi: 10.1002/jor.1100110505. [DOI] [PubMed] [Google Scholar]
- Clark D, Haines SR, Lord EA, Wang W, Suttie JM. Antler and angiogenesis. In: Suttie JM, editor. Antler Science and Product Technology. Vol. 2 2004. [Google Scholar]
- Elliott JL, Oldham JM, Ambler GR, et al. Presence of insulin-like growth factor-I receptors and absence of growth hormone receptors in the antler tip. Endocrinology. 1992;130:2513–2520. doi: 10.1210/endo.130.5.1315246. [DOI] [PubMed] [Google Scholar]
- Elliott JL, Oldham JM, Ambler GR, et al. Receptors for insulin-like growth factor-II in the growing tip of the deer antler. J Endocrinol. 1993;138:233–242. doi: 10.1677/joe.0.1380233. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res. 1993;8:127–132. doi: 10.1002/jbmr.5650080202. [DOI] [PubMed] [Google Scholar]
- Faucheux C, Price JS. Parathyroid hormone-related peptide may play a role in deer antler regeneration. In: Danks J, Dacke C, Flik G, Gay C, editors. Calcium Metabolism: Comparative Endocrinology. Bristol: BioScientifica Ltd; 1999. pp. 131–138. [Google Scholar]
- Faucheux C, Nesbitt SA, Horton MA, Price JS. Cells in regenerating deer antler cartilage provide a microenvironment that supports osteoclast differentiation. J Exp Biol. 2001;204:443–455. doi: 10.1242/jeb.204.3.443. [DOI] [PubMed] [Google Scholar]
- Faucheux C, Horton MA, Price JS. Nuclear localization of type I parathyroid hormone/parathyroid hormone-related protein receptors in deer antler osteoclasts: evidence for parathyroid hormone-related protein and receptor activator of NF-kappaB-dependent effects on osteoclast formation in regenerating mammalian bone. J Bone Miner Res. 2002;17:455–464. doi: 10.1359/jbmr.2002.17.3.455. [DOI] [PubMed] [Google Scholar]
- Faucheux C, Nicholls BM, Allen S, Danks JA, Horton MA, Price JS. Recapitulation of the parathyroid hormone-related peptide–Indian hedgehog pathway in the regenerating deer antler. Dev Dyn. 2004;231:88–97. doi: 10.1002/dvdy.20117. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Esparza J, Harris MA, Mundy GR, Harris SE. Deer antler tissue contains two types of bone morphogenetic protein 4 mRNA transcripts. Biochim Biophys Acta. 1995;1263:163–168. doi: 10.1016/0167-4781(95)00106-q. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Ghosh-Choudhury N, Esparza J, Mundy GR, Harris SE. Bone morphogenetic protein 2 transcripts in rapidly developing deer antler tissue contain an extended 5′ non-coding region arising from a distal promoter. Biochim Biophys Acta. 1997;1350:47–52. doi: 10.1016/s0167-4781(96)00178-9. [DOI] [PubMed] [Google Scholar]
- Fletcher T. The induction of male sexual behavior in red deer (Cervus elaphus) by the administration of testosterone to hinds and estradiol-17beta to stags. Horm Behav. 1978;11:74–88. doi: 10.1016/0018-506x(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Foley J, Dann P, Hong J, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:513–525. doi: 10.1242/dev.128.4.513. [DOI] [PubMed] [Google Scholar]
- Fujisawa T. Hydra regeneration and epitheliopeptides. Dev Dyn. 2003;226:182–189. doi: 10.1002/dvdy.10221. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Inhibition of growth and shedding of antlers by sex hormones. Nature. 1968;220:83–85. doi: 10.1038/220083a0. [DOI] [PubMed] [Google Scholar]
- Goss R. Wound healing and antler regeneration. In: Maibach HI, Rovee DT, editors. Epidermal Wound Healing. Chicago: Year Book Med. Publ., Inc; 1972. pp. 219–228. [Google Scholar]
- Goss RJ. Clin Orthop. 1980. Prospects of regeneration in man; pp. 270–282. [PubMed] [Google Scholar]
- Goss RJ. Deer Antlers, Regeneration, Evolution and Function. New York: Academic Pres; 1983. [Google Scholar]
- Goss RJ. Photoperiodic control of antler cycles in deer. VI. Circannual rhythms on altered day lengths. J Exp Zool. 1984;230:265–271. doi: 10.1002/jez.1402300212. [DOI] [PubMed] [Google Scholar]
- Goss RJ, Powel RS. Induction of deer antlers by transplanted periosteum. I. Graft size and shape. J Exp Zool. 1985;235:359–373. doi: 10.1002/jez.1402350307. [DOI] [PubMed] [Google Scholar]
- Goss RJ, Van Praagh A, Brewer P. The mechanism of antler casting in the fallow deer. J Exp Zool. 1992;264:429–436. doi: 10.1002/jez.1402640408. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Future directions in antler research. Anat Rec. 1995;241:291–302. doi: 10.1002/ar.1092410302. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Petkovich PM, Rosenthal EE, Heersche JN. Modulation by retinoic acid of 1,25-dihydroxy vitamin-D3 effects on alkaline phosphatase activity and parathyroid hormone responsiveness in an osteoblast like osteosarcoma cell line. Endocrinology. 1986;119:932–939. doi: 10.1210/endo-119-2-932. [DOI] [PubMed] [Google Scholar]
- Hartwig H. Experimentelle untersuchungen zur entwicklungsphysiologie der stangenbildung beim reh (Capreolus capreolus L. 1758) Roux′ Arch Entwicklungsmech. 1967;158:358–384. doi: 10.1007/BF01380537. [DOI] [PubMed] [Google Scholar]
- Hartwig H, Schrudde J. Experimentelle Untersuchungen zur Bildung der primaren Stirnauswüchse beim Reh (Capreolus capreolus L.) Z Jagdwiss. 1974;20:1–13. [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004;359:785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaczewski Z, Doboszynska T, Krzywinski A. The induction of antler growth by amputation of the pedicle in red deer (Cervus elaphus L.) males castrated before puberty. Folia Biol (Krakow) 1976;24:299–307. [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H. State of determination of the antlerogenic tissues with special reference to double-head formation. In: Brown RD, editor. The Biology of Deer. New York: Springer; 1992. pp. 525–531. [Google Scholar]
- Kierdorf U, Schultz M, Fischer K. Effects of an antiandrogen treatment on the antler cycle of male fallow deer (Dama dama L.) J Exp Zool. 1993;266:195–205. doi: 10.1002/jez.1402660305. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H, Schultz M. The macroscopic and microscopic structure of double-head antlers and pedicle bone of cervidae (Mammalia, Artiodactyla) Anat Anz. 1994;176:251–257. doi: 10.1016/s0940-9602(11)80488-7. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H, Knuth S. Effects of castration on antler growth in fallow deer (Dama dama L.) J Exp Zool. 1995;273:33–43. doi: 10.1002/jez.1402730105. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Bartos L. Treatment of the growing pedicle with retinoic acid increased the size of the first antlers in fallow deer (Dama dama L.) Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:7–9. doi: 10.1016/s0742-8413(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H. Delayed ectopic antler growth and formation of a double-head antler in the metacarpal region of a fallow buck (Dama dama L.) following transplantation of antlerogenic periosteum. Anat Anz. 2000;182:365–370. doi: 10.1016/S0940-9602(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H. The role of the antlerogenic periosteum for pedicle and antler formation in deer. In: Sim JS, Sunwoo HH, Hudson RJ, Jeon BT, editors. Antler Science and Product Technology. Canada: Antler Science and Production Technology. Research Centre; 2001. pp. 33–52. [Google Scholar]
- Kierdoff U, Stoffels E, Stoffels D, Kierdoff H, Szuwart T, Clemen G. Histological studies of bone formation during pedicle restoration and early antler regeneration in roe deer and fallow deer. Anat Rec. 2003;273:741–751. doi: 10.1002/ar.a.10082. [DOI] [PubMed] [Google Scholar]
- Kierdoff U, Kierdoff H. Bone formation in antlers. In: Suttie JM, Haines SR, Li C, editors. Advances in Antler Science and Product Technology. 2004. pp. 55–63. [Google Scholar]
- Kierdorf U, Kierdorf H, Schultz M, Rolf HJ. Histological structure of antlers in castrated male fallow deer (Dama dama) Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1352–1362. doi: 10.1002/ar.a.20127. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chung UI, Schipani E, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- Li C, Suttie JM. Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus) Anat Rec. 1994;239:198–215. doi: 10.1002/ar.1092390211. [DOI] [PubMed] [Google Scholar]
- Li C, Waldrup KA, Corson ID, Littlejohn RP, Suttie JM. Histogenesis of antlerogenic tissues cultivated in diffusion chambers in vivo in red deer (Cervus elaphus) J Exp Zool. 1995;272:345–355. doi: 10.1002/jez.1402720504. [DOI] [PubMed] [Google Scholar]
- Li C, Suttie JM. Electron microscopic studies of antlerogenic cells from five developmental stages during pedicle and early antler formation in red deer (Cervus elaphus) Anat Rec. 1998;252:587–599. doi: 10.1002/(SICI)1097-0185(199812)252:4<587::AID-AR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Li C, Littlejohn RP, Suttie JM. Effects of insulin-like growth factor 1 and testosterone on the proliferation of antlerogenic cells in vitro. J Exp Zool. 1999;284:82–90. [PubMed] [Google Scholar]
- Li C, Harris AJ, Suttie JM. Tissue interactions and antlerogenesis: new findings revealed by a xenograft approach. J Exp Zool. 2001;290:18–30. doi: 10.1002/jez.1032. [DOI] [PubMed] [Google Scholar]
- Li C, Suttie JM. Deer antlerogenic periosteum: a piece of postnatally retained embryonic tissue? Anat Embryol (Berl) 2001;204:375–388. doi: 10.1007/s004290100204. [DOI] [PubMed] [Google Scholar]
- Li C, Suttie JM, Clark DE. Morphological observation of antler regeneration in red deer (Cervus elaphus) J Morph. 2004;262:731–740. doi: 10.1002/jmor.10273. [DOI] [PubMed] [Google Scholar]
- Li C, Suttie JM, Clark DE. Histological examination of antler regeneration in red deer (Cervus elaphus) Anat Rec A Discov Mol Cell Evol Biol. 2005 doi: 10.1002/ar.a.20148. [DOI] [PubMed] [Google Scholar]
- Lincoln GA. Puberty in a seasonally breeding male, the red deer stag (Cervus elaphus L.) J Reprod Fertil. 1971;25:41–54. doi: 10.1530/jrf.0.0250041. [DOI] [PubMed] [Google Scholar]
- Lincoln GA. Appearance of antler pedicles in early foetal life in red deer. J Embryol Exp Morph. 1973;29:431–437. [PubMed] [Google Scholar]
- Lincoln GA, Tyler NJ. Role of oestradiol in the regulation of the seasonal antler cycle in female reindeer, Rangifer tarandus. J Reprod Fertil. 1999;115:167–174. doi: 10.1530/jrf.0.1150167. [DOI] [PubMed] [Google Scholar]
- Lord E, Clark DE, Martin SK, et al. Profiling genes expressed in the regenerating tip of red deer (Cervus elaphus) antler. In: Suttie JM, editor. Antler Science and Product Technology. 2004. [Google Scholar]
- Matich J, Basford Nicholson LF, Barling PM. Mitotic activity in the growing red deer antler. Cell Biol Int. 2003;27:625–632. doi: 10.1016/s1065-6995(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Muir PD, Sykes AR, Barrell GK. Changes in blood content and histology during growth of antlers in red deer (Cervus elaphus) and their relationship plasma testosterone levels. J Anat. 1988;158:31–42. [PMC free article] [PubMed] [Google Scholar]
- Mundy G, Gutierrez G, Gallwitz W, et al. Antler-derived bone growth factors and their potential for use in osteoporosis. In: Sim JS, Sunwoo HH, Hudson RJ, Jeon BT, editors. Antler Science and Product Technology. Canada: Antler Science and Production Technology. Research Centre; 2001. pp. 171–187. [Google Scholar]
- Nye HL, Cameron JA, Chernoff EA, Stocum DL. Regeneration of the urodele limb: a review. Dev Dyn. 2003;226:280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- Oliva A, Della Ragione F, Fratta M, Marrone G, Palumbo R, Zappia V. Effect of retinoic acid on osteocalcin gene expression in human osteoblasts. Biochem Biophys Res Commun. 1993;191:908–914. doi: 10.1006/bbrc.1993.1303. [DOI] [PubMed] [Google Scholar]
- Park C-K, Ishinmi Y, Ohmura M, Yamaguchi M, Ikegami S. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J Nutr Sci Vit. 1997;43:281–296. doi: 10.3177/jnsv.43.281. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee do H, Park SG, et al. Proteome analysis of red deer antlers. Proteomics. 2004;4:3642–3653. doi: 10.1002/pmic.200401027. [DOI] [PubMed] [Google Scholar]
- Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dyn. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- Price JS, Oyajobi BO, Oreffo RO, Russell RG. Cells cultured from the growing tip of red deer antler express alkaline phosphatase and proliferate in response to insulin-like growth factor-I. J Endocrinol. 1994;143:R9–R16. doi: 10.1677/joe.0.143r009. [DOI] [PubMed] [Google Scholar]
- Price JS, Oyajobi BO, Nalin AM, Frazer A, Russell RG, Sandell LJ. Chondrogenesis in the regenerating antler tip in red deer: expression of collagen types I, IIA, IIB, and X demonstrated by in situ nucleic acid hybridization and immunocytochemistry. Dev Dyn. 1996;205:332–347. doi: 10.1002/(SICI)1097-0177(199603)205:3<332::AID-AJA12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Price J, Faucheux C. Exploring the molecular mechanisms of antler regeneration. In: Sim JS, Sunwoo HH, Hudson RJ, Jeon BT, editors. Antler Science and Product Technology. Canada: Antler Science and Production Technology. Research Centre; 2001. pp. 53–67. [Google Scholar]
- Price J, Allen S. Exploring the mechanisms regulating regeneration of deer antlers. Philos Trans R Soc Lond B Biol Sci. 2004;359:809–822. doi: 10.1098/rstb.2004.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Faucheux C, Allen S. Deer antlers as a model of mammalian regeneration. Curr Topics Devel Biol. 2005;67:1–48. doi: 10.1016/S0070-2153(05)67001-9. [DOI] [PubMed] [Google Scholar]
- Riggs B, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Sadighi M, Haines SR, Skottner A, Harris AJ, Suttie JM. Effects of insulin-like growth factor-I (IGF-I) and IGF-II on the growth of antler cells in vitro. J Endocrinol. 1994;143:461–469. doi: 10.1677/joe.0.1430461. [DOI] [PubMed] [Google Scholar]
- Sadighi M, Li C, Littlejohn RP, Suttie JM. Effects of testosterone either alone or with IGF-I on growth of cells derived from the proliferation zone of regenerating antlers in vitro. Growth Hormone IGF Res. 2001;11:240–246. doi: 10.1054/ghir.2001.0232. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A. The freshwater planarian Schmidtea mediterranea: embryogenesis, stem cells and regeneration. Curr Opin Genet Dev. 2003;13:438–444. doi: 10.1016/s0959-437x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Regeneration and the need for simpler model organisms. Philos Trans R Soc Lond B Biol Sci. 2004;359:759–763. doi: 10.1098/rstb.2004.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L, Sugai JV, Trippel SB. Expression of collagens I, II, X, and XI and aggrecan mRNAs by bovine growth plate chondrocytes in situ. J Orthop Res. 1994;12:1–14. doi: 10.1002/jor.1100120102. [DOI] [PubMed] [Google Scholar]
- Sempere AJ, Boissin J, Dutourne B, Lacroix A, Blanc MR, Ravault JP. Variations in the plasma concentration of prolactin, LH and FSH and in testicular activity during the first year of life in the roe deer (Capreolus capreolus L.) Gen Comp Endocrinol. 1983;52:247–254. doi: 10.1016/0016-6480(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Sempere AJ, Grimberg R, Silve C, Tau C, Garabedian M. Evidence for extrarenal production of 1,25-dihydroxyvitamin during physiological bone growth: in vivo and in vitro production by deer antler cells. Endocrinology. 1989;125:2312–2319. doi: 10.1210/endo-125-5-2312. [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Barrell GK. Thyroid hormones are required for the expression of seasonal changes in red deer (Cervus elaphus) stags. Reprod Fertil Dev. 1994;6:187–192. doi: 10.1071/rd9940187. [DOI] [PubMed] [Google Scholar]
- Slack JM, Beck CW, Gargioli C, Christen B. Cellular and molecular mechanisms of regeneration in Xenopus. Philos Trans R Soc Lond B Biol Sci. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slootweg MC, Salles JP, Ohlsson C, De Vries CP, Engelbregt CP, Netelenbos JC. Growth hormone binds to a single high affinity receptor site on mouse osteoblasts: modulation by retinoic acid and cell differentiation. J Endocrinol. 1996;150:465–472. doi: 10.1677/joe.0.1500465. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie JM, Lincoln GA, Kay RN. Endocrine control of antler growth in red deer stags. J Reprod Fertil. 1984;71:7–15. doi: 10.1530/jrf.0.0710007. [DOI] [PubMed] [Google Scholar]
- Suttie JM, Gluckman PD, Butler JH, Fennessy PF, Corson ID, Laas FJ. Insulin-like growth factor 1 (IGF-1) antler-stimulating hormone? Endocrinology. 1985;116:846–848. doi: 10.1210/endo-116-2-846. [DOI] [PubMed] [Google Scholar]
- Suttie JM, Fennessy PF, Gluckman PD, Corson ID. Elevated plasma IGF 1 levels in stags prevented from growing antlers. Endocrinology. 1988;122:3005–3007. doi: 10.1210/endo-122-6-3005. [DOI] [PubMed] [Google Scholar]
- Suttie JM, White RG, Breier BH, Gluckman PD. Photoperiod associated changes in insulin-like growth factor-I in reindeer. Endocrinology. 1991;129:679–682. doi: 10.1210/endo-129-2-679. [DOI] [PubMed] [Google Scholar]
- Suttie JM, Fennessy PF, Lapwood KR, Corson ID. Role of steroids in antler growth of red deer stags. J Exp Zool. 1995;271:120–130. doi: 10.1002/jez.1402710207. [DOI] [PubMed] [Google Scholar]
- Szuwart T, Kierdorf H, Kierdorf U, Clemen G. Histochemical and ultrastructural studies of cartilage resorption and acid phosphatase activity during antler growth in fallow deer (Dama dama) Anat Rec. 2002;268:66–72. doi: 10.1002/ar.10135. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Effects of carcinogens on regenerating and non-regenerating limbs in amphibia (review) Anticancer Res. 1983;3:195–202. [PubMed] [Google Scholar]
- Van der Eems KL, Brown RD, Gundberg CM. Circulating levels of 1,25 dihydroxyvitamin D, alkaline phosphatase, hydroxyproline, and osteocalcin associated with antler growth in white-tailed deer. Acta Endocrinol (Copenh) 1988;118:407–414. doi: 10.1530/acta.0.1180407. [DOI] [PubMed] [Google Scholar]
- West NO, Nordan HC. Hormonal regulation of reproduction and the antler cycle in the male Columbian black-tailed deer (Odocoileus hemionus columbianus). Part I. Seasonal changes in the histology of the reproductive organs, serum testosterone, sperm production, and the antler cycle. Can J Zool. 1976;54:1617–1636. doi: 10.1139/z76-189. [DOI] [PubMed] [Google Scholar]
- Wislocki G. Essays in Biology. Berkeley, CA: University of California Press; 1943. Studies on growth of deer antlers; pp. 631–653. In. [Google Scholar]