Abstract
The potential for the N-hydroxyguanidine compound PR5 (N-(3,4-dimethoxy-2-chlorobenzylideneamino)-N′-hydroxyguanidine) as a cardioprotective agent in heart ischaemia and reperfusion injury was investigated using rat models.
Administration of 1–10 mg kg−1 of PR5 5 min before 10 min of left coronary artery occlusion, followed by 20 min reperfusion, strongly inhibited reperfusion burst of arrhythmias and markedly improved the survival of the animals (e.g. ventricular fibrillation incidence 93 vs 43% (P<0.05); mortality 47 vs 0% (P<0.05), for controls and for 3 mg kg−1 of PR5, respectively).
Administration of 3 mg kg−1 of PR5 1 min before reperfusion to rats subjected to 10 min occlusion, 20 min reperfusion was most effective in reducing arrhythmias and decreasing mortality (43 vs 0%, P<0.05), but effects were also seen when PR5 was administered 0, 1 and 5 min after start of reperfusion.
Coronary occlusion/reperfusion (10–20 min) increased malondialdehyde (MDA) of rat hearts (0.88±0.13 for sham vs 1.45±0.12 nmol mg−1 protein for ischaemic; P<0.05). In rats where 3 mg kg−1 PR5 were administered 1 min before reperfusion the increase was attenuated (MDA being 1.04±0.12; P<0.05 vs ischaemic).
PR5 caused a substantial reduction of the infarction size in rats subjected to 180 min left coronary artery occlusion, followed by 120 min of reperfusion; the necrotic zone being 326±32 mg for controls vs 137±21 mg for animals treated with 3×3 mg kg−1 of PR5 (P<0.01).
PR5 reduced the elevation of the ST-segment of the ECGs, as well as caused pronounced attenuation of the rapid blood pressure drop seen at the start of reperfusion following coronary artery occlusion.
We conclude that the N-hydroxyguanidine PR5 provides remarkable protection against ischaemia and reperfusion induced myocardial necrosis and life-threatening arrhythmias. These effects of PR5 are discussed in relation to a recently discovered ability of N-hydroxyguanidines to function as electron acceptors at the xanthine oxidase enzyme.
Keywords: N-Hydroxyguanidine, ischaemia, reperfusion injury, antiarrhythmic effect, rat heart
Introduction
Ischaemia followed by reoxygenation is associated with oxygen free radical generation, which is one of the major factors in the induction of arrhythmias and myocardial damage in the ischaemia and reperfusion syndrome. Several mechanisms are involved in the generation of oxygen free radicals (Ar'Rajab et al., 1996). Among others, the reduction of molecular oxygen by the xanthine oxidase enzyme (EC 1.1.3.22) during oxidation of hypoxanthine and xanthine is of crucial importance (McCord, 1985; Reilly et al., 1991). It is proposed that the free radical generation is dependent on the ischaemia-induced conversion of xanthine dehydrogenase to xanthine oxidase by partial proteolytic cleavage and sulphydryl oxidation (Amaya et al., 1990; Poss et al., 1996). As a result, the enzyme is unable to reduce its natural electron accepting substrate NAD+ and instead prefers molecular oxygen, which leads to the generation of oxygen centred radicals (Nishino et al., 1994; Saugstad, 1996).
Various methods have been tried to prevent the formation of oxygen reactive species during reperfusion. Thus, according to the xanthine oxidase hypothesis inhibitors of the enzyme, such as allopurinol and oxypurinol, were reported to decrease radical generation and heart injury (Castelli et al., 1995; Thompson-Gorman & Zweier, 1990). Also an inhibitor of adenosine deaminase was shown to reduce the formation of oxygen radicals and to improve the contractile performance of hearts in vitro, an effect presumed to be due to the inhibition of the formation of the reducing substrates for xanthine oxidase (Xia et al., 1996). Still another approach is to use radical scavenging compounds (Opie, 1989; Flaherty & Weisfeldt, 1988). Lipid peroxidation inhibitory aminosteroids (lazaroids) have been shown to protect effectively against heart injury in infarction models in vivo (Campo et al., 1996; 1997). Earlier much attention was paid to superoxide dismutase for quenching superoxide, but its use as a protector against ischaemia and reperfusion injuries has generally led to disappointment (Vanhaecke et al., 1991; Watanabe et al., 1993). Recently, however, it was found that overexpression of superoxide dismutase in transgenic mice led to reduction of superoxide, improvement of heart contractility and reduction of infarction size in reperfused hearts after ischaemia (Wang et al., 1998).
In the present study we have evaluated a new possibility to protect the heart in ischaemia and reperfusion. The approach originates from our recent finding that an N-hydroxyguanidine, guanoxabenz (1-(2,6-dichlorobenzylideneamino)-3-hydroxyguanidine), an α2-adrenoceptor active antihypertensive drug (Ledoux et al., 1981), could also act as an alternative electron acceptor in xanthine oxidase catalyzed oxidation of xanthine (Dambrova et al., 1998). In the continuation of these studies we synthesized a novel series of N-hydroxyguanidines and evaluated their capacity to function as electron acceptors in bovine milk xanthine oxidase catalyzed oxidation of xanthine. We then found N-(3,4-dimethoxy-2-chlorobenzylideneamino)-N′-hydroxyguanidine (PR5), which shows higher activity than guanoxabenz on the xanthine oxidase and seems to be essentially devoid of α2-adrenoceptor activity (Dambrova et al. manuscript in preparation).
Methods
Animals
Male Wistar rats (Latvian State Pharmaceutical Company ‘Grindex', Latvia) weighing 330–420 g were housed under standard conditions (21–23°C, 12 h light-dark cycle) with unlimited access to food and water. All experimental procedures were performed in accordance with the regulations of the Animal Ethical Committee of BaltLASA.
Ischaemia and reperfusion induced heart failure
The experimental procedure was a modification of the method by Kane et al. (1984). Rats were anaesthetized with sodium pentobarbital (60 mg kg−1 i.p.). The femoral vein was cannulated to allow administration of drugs. Systemic blood pressure was registered from the left carotid artery by a pressure transducer P23 DB (Gould Statham, U.S.A.) and ECG from a II standard lead on a physiograph DMP-4B (Narco Bio-Systems, U.S.A.). Rats were intubated through a tracheotomy and ventilated with room air by a V5kG respirator (Narco Bio-Systems, U.S.A.) at a pressure of 15 cm H2O and a rate of 55 strokes min−1. The chest was opened and a sling (6/0 silk Ethicon) was placed around the left coronary artery. After 10 min the coronary artery was occluded by applying tension to the plastic tube-silk string arrangement. The successful occlusion was confirmed by a decrease in arterial pressure and ischaemia-induced alterations in the ECG. Reperfusion was initiated by removing the clamp.
Experimental protocols
Three protocols with separate control groups were carried out as follows:
I Dose effect evaluation of PR5 on rats subjected to 10 min ischaemia and 20 min reperfusion
The rats were randomly divided into four groups: (1) (n=15) received saline vehicle (2 ml kg−1); (2) (n=14), (3) (n=14) and (4) (n=14) received 1, 3 and 10 mg kg−1 PR5, respectively. Saline and PR5 were administered by a single intravenous injection (i.v.) 5 min prior to the ischaemia.
II Evaluation of the importance of the dosing schedule for the effects of PR5 on rats subjected to 10 min ischaemia and 20 min reperfusion
The rats were randomly divided into five groups: (1) (n=14) received saline (2 ml kg−1 i.v.) at the ninth min of the occlusion; (2) (n=14) 3 mg kg−1 i.v. of PR5 at the ninth min of occlusion; (3) (n=12) 3 mg kg−1 i.v. of PR5 at the onset of reperfusion; (4) (n=10) 3 mg kg−1 i.v. of PR5 at the first min of reperfusion and (5) (n=10) 3 mg kg−1 i.v. of PR5 at the fifth min of reperfusion.
III The influence of PR5 on functional status and morphology of the heart in rats subjected to 180 min ischaemia and 120 min reperfusion
The rats were randomly divided into five groups: (1) (n=15) received saline (2 ml kg−1 i.v.) 5 min prior to occlusion; (2) (n=12) 3 mg kg−1 i.v. of PR5 5 min prior to occlusion; (3) (n=13) rats 3 mg kg−1 i.v. of PR5 170 min after occlusion; (4) (n=14) 3 mg kg−1 i.v. of PR5 at the fifth min of reperfusion and (5) (n=13) 3 mg kg−1 i.v. of PR5 at three times; namely: 5 min prior to occlusion, 170 min after occlusion and at the fifth min of reperfusion.
Exclusion criteria
Experiments were terminated and data were excluded from the final analysis if arrhythmias occurred prior to the coronary artery occlusion; if the mean arterial pressure was less than 60 mmHg prior to occlusion (drug or saline administration), or if severe arrhythmias or atrioventricular block occurred during the first 5 min of ischaemia.
Definition of arrhythmias
Definition of arrhythmias were based on criteria described in the Lambeth Conventions (Walker et al., 1988). Ectopic activity was categorized as a single ventricular premature beat (VPB), ventricular tachycardia (VT) or as ventricular fibrillation (VF). In all experiments the incidence of VT, VF and mortality (due to terminal ventricular fibrillation sustained for at least 3 min) was noted.
Quantification of myocardial morphological parameters
At the end of reperfusion of the animals subjected to experimental protocol III the ligature was re-tied around the coronary artery and Evans blue (5 mg ml−1) was quickly infused into the left atrium. The heart was then isolated, weighed and the left ventricle was dissected free from other structures and weighed. The non-stained myocardium was isolated, weighed and cut into 1 mm sections. The sections were incubated in a 0.5% solution of triphenyltetrazolium chloride (10 min at 37°C). After that the necrotic tissues were isolated and weighed.
Determination of malondialdehyde (MDA)
The rats were randomly divided into three groups: One ischaemia and reperfusion control group (i.e. group (1) in experimental protocol II, n=9); one PR5-treated group (i.e. group (2) in experimental protocol II, n=9) and one sham operated group (n=8), subjected to the same surgical procedures as experimental protocol II, except that the suture passed around the left coronary artery was not tied. The rats were then subjected to 10 min occlusion/20 min reperfusion, whereafter the chests were reopened, the hearts excised and the ventricles immediately frozen using previously cooled clamps. The MDA levels were determined according to the method of Ohkawa et al. (1979).
Statistics
The results are presented as the mean±s.e.mean. Statistical analysis was performed using ANOVA followed by Dunnett's t-test; the number of incidence of arrhythmia and mortality were analysed using Chi-square tests. Results were considered as significant when P<0.05. The number of incidences of arrhythmia and lethality were calculated in all animals, whereas only the data obtained for the surviving animals were used in the evaluation of the blood pressure, heart rate, duration of arrhythmia and morphological data.
Results
The dose effect relations of PR5 on rats subjected to 10 min ischaemia and 20 min reperfusion
The experimental protocol I procedures caused disturbances of the heart rhythm in all control rats, with seven out of 15 rats dying due to irreversible ventricular fibrillation (Figure 1). As can be seen from Figure 1, PR5 in a dose dependent fashion reduced the incidence of VT, VF and mortality, the effect being significant at 3 and 10 mg kg−1 for mortality and fibrillation, and at 10 mg kg−1 for tachycardia.
Figure 1.
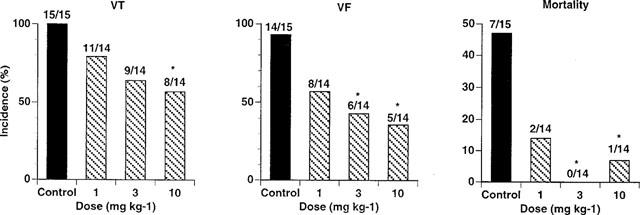
Effects of PR5 on the incidence of ventricular tachycardia (VT) and fibrillation (VF) during a 20 min reperfusion period following a 10 min left coronary occlusion in rats. PR5 (1, 3 or 10 mg kg−1) or saline control injections were given i.v. 5 min prior to the coronary occlusion. (*Indicates P<0.05).
Figure 2A shows the effect on the blood pressure. In controls the coronary artery occlusion caused an initial drop in the blood pressure of 20–25 mmHg, with the blood pressure thereafter slightly recovering. In the saline treated group the start of reperfusion caused a drastic decrease in the blood pressure of 45–50 mmHg during the first minutes of reperfusion, which was then rapidly followed by a rebound increase in the pressure (Figure 2A). The administration of 1, 3 and 10 mg kg−1 of PR5 caused a dose dependent and significant inhibition in reperfusion induced drop in blood pressure, as well as inhibition of the rebound increase in pressure. As seen from the Figure 2A, PR5 did not affect the initial ischaemia-induced blood pressure drop.
Figure 2.
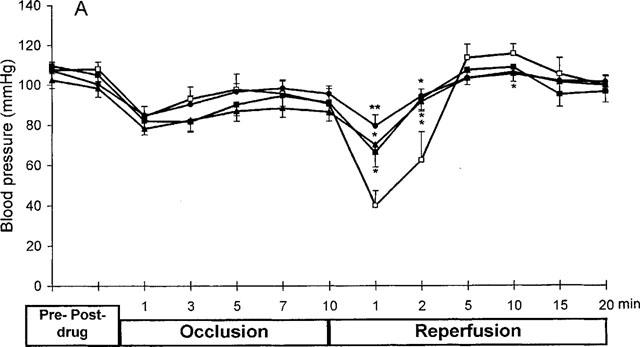
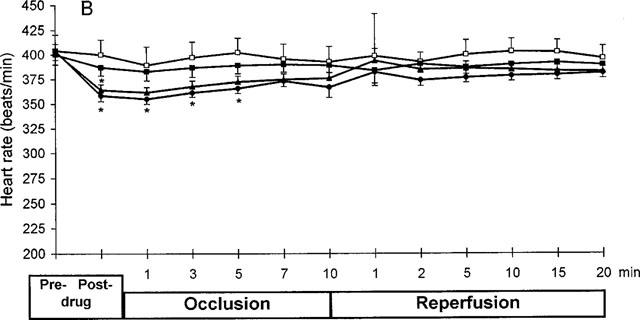
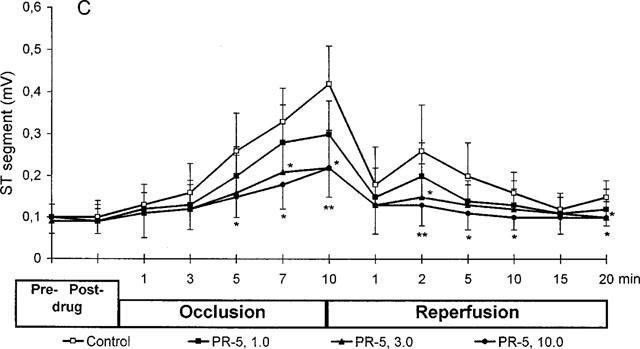
Effect of PR5 on blood pressure (A), heart rate (B) and ST-segment (C) in rats subjected to 10 min left coronary artery occlusion followed by 20 min reperfusion. PR5 (1, 3 or 10 mg kg−1) or saline control injections were given i.v. 5 min prior to the coronary occlusion. (*Indicates P<0.05; **Indicates P<0.01).
Figure 2B shows the effect of PR5 on heart rate. The two higher doses of PR5 appeared to cause some drop in heart rate, which then returned to essentially normal during the course of the experiment.
Figure 2C shows the effects of PR5 on the ST-segment elevation. As expected, the ischaemia caused a progressive increase in the ST-segment, which then almost normalized during reperfusion. The PR5 caused a dose-dependent inhibition in the ST-segment elevation during the ischaemia and improved the normalization of ST-segment during reperfusion; the effects being significant at 3 and 10 mg kg−1 of PR5.
The dosing schedule for the effect of PR5 on arrhythmia and mortality in rats subjected to 10 min ischaemia and 20 min reperfusion
The next set of experiments was designed to evaluate the importance for the time point of administration of PR5. Based on the studies above, we selected a PR5 dose of 3 mg kg−1 i.v. The same ischaemia and reperfusion protocol as above was used, but PR5 was administered at different times (protocol II).
Also in these tests reperfusion induced VT and VF, as well as death of many of the control animals. Thus, tachycardia was observed in all animals, fibrillation in 100% (14/14), and almost half (6/14) of the animals died (Figure 3). However, PR5 had a marked effect on these parameters (Figure 3), the largest effect being seen when PR5 was administered 1 min prior to reperfusion (Group 2); the incidence of tachycardia and fibrillation being reduced to 43% (6/14) and 21% (3/14), respectively, and all (0/14) of the animals surviving (P<0.05). Similar beneficial effect was seen when PR5 was administered simultaneously with the start of reperfusion (Group 3).
Figure 3.
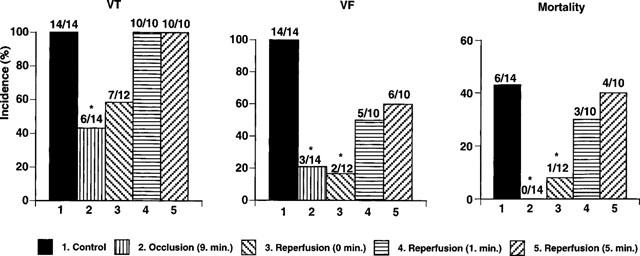
Effect of 3 mg kg−1 of PR5 injected at different time points during 10 min left coronary artery occlusion followed by 20 min reperfusion on the incidence of ventricular tachycardia (VT), fibrillation (VF) and mortality during reperfusion. Shown are saline injected animals (1 Control), animals injected with PR5 on the ninth min of occlusion (2 occlusion 9 min), animals injected with PR5 at the onset of reperfusion (3 reperfusion 0 min), animals injected with PR5 1 min after start of reperfusion (3 reperfusion 1 min), and animals injected with PR5 5 min after the start of reperfusion (5 reperfusion 5 min). (*Indicates P<0.05).
Figure 4 further amplifies the time course in individual animals for the occurrence of arrhythmias. Figure 4A shows data for the controls where VT and VF increased markedly at the start of reperfusion, and then gradually declined. For the animals receiving PR5 1 min prior to reperfusion (Figure 4B) life threatening arrhythmias became markedly decreased during the first minutes, and only few occasions of arrhythmias were registered thereafter.
Figure 4.
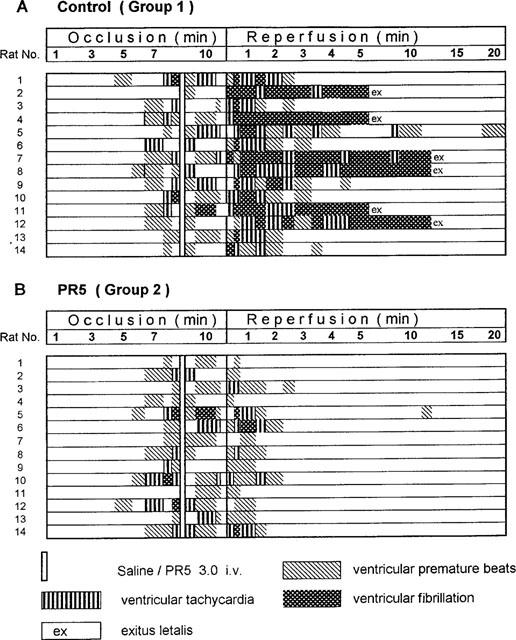
Occurrence of arrhythmia and death (exitus letalis) in individual animals during the course of 10 min ischaemia followed by 20 min reperfusion. Shown are data for control animals (A) and data for animals treated with 3 mg kg−1 of PR5 at the ninth min of occlusion (B).
Effect of PR5 on MDA
Figure 5 shows MDA levels in hearts excised after 10 min ischaemia and 20 min reperfusion. In the coronary occlusion/reperfusion group MDA was significantly elevated compared to sham-operated animals. For the animals treated with 3 mg kg−1 of PR5 1 min prior to reperfusion there was a statistically significant inhibition of the MDA formation compared to the non-treated ischaemia and reperfusion animals (P<0.05).
Figure 5.
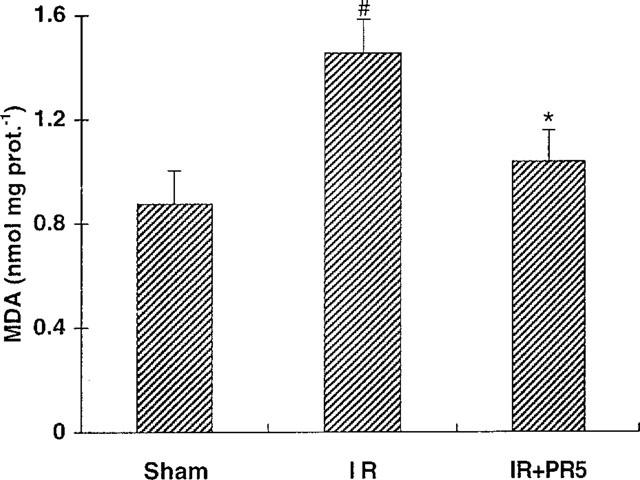
Effect of PR5 on malondialdehyde (MDA) in rat hearts subjected to ischaemia reperfusion. Sham=sham operated, IR= ischaemia reperfusion control, IR+PR5=PR5 treated (3 mg kg−1) ischaemia reperfusion animals. For further details see text. (*Indicates P<0.05 vs IR; #Indicates P<0.05 vs sham).
Influence of PR5 on functional status and morphology of rat hearts during 180 min ischaemia and 120 min reperfusion
In these tests the rats were treated according to the experimental protocol III. Results are presented in Table 1 and Figure 6. ANOVA showed that body weights, heart and left ventricle weights were essentially the same for all the five different groups (Table 1), indicating that the groups were well matched. Also the weight of the ischaemic zones caused by the coronary artery occlusion were of almost the same size (Table 1). However, the weight of the necrotic tissue was markedly reduced by the treatment with PR5; the effect being highly significant (P<0.01) for the Group 2, as well as Groups 3 and 4. Figure 6 shows the data normalized against ventricle and ischaemic zone weights. The largest reduction of infarction size is seen for Group 2, followed by Groups 3 and 4.
Table 1.
Body weight and weight of hearts, left ventricle, ischaemic zone and necrotic zone in rats subjected to 180 min left coronary artery occlusion followed by 120 min reperfusion
Figure 6.
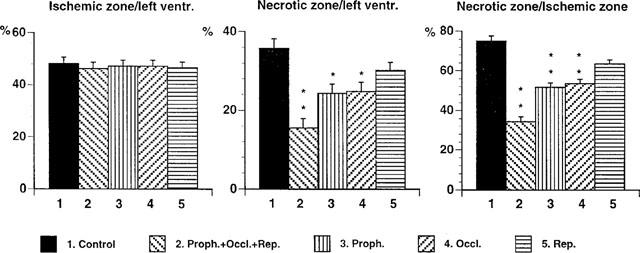
Effects of PR5 on ischaemic zone/left ventricular size index, necrotic zone/left ventricular size index and necrotic zone/ischaemic zone size index in rats subjected to 180 min left coronary artery occlusion followed by 120 min reperfusion. Shown are data for control animals (1), animals receiving 3×3 mg kg−1 of PR5, respectively 5 min before occlusion, 170 min after occlusion and 5 min after start of reperfusion (2), animals receiving 3 mg kg−1 of PR5 5 min before occlusion (3), animals receiving 3 mg kg−1 of PR5 170 min after occlusion (4), and animals receiving 3 mg kg−1 of PR5 5 min after start of reperfusion (5) ANOVA showed significant effects for necrotic zone/left ventricle F(1,49)=2.18; P<0.01 and necrotic zone/ischaemic zone F(1,49)=6.03; P<0.0001. (*Indicates P<0.05; **P<0.01).
Figure 7 shows the time course for the deviation of the ST-segment from the baseline throughout the ischaemia and reperfusion periods. The ischaemia caused an increase in the ST-segment, from about 0.1 mV to about 0.5 mV, as expected. During reperfusion the ST-segment normalized slightly, but it never returned to the baseline. Administration of PR5 gave a highly significant attenuation in the increase of the ST-segment during almost the whole ischaemic period, that extended also into reperfusion period (Groups 3 and 4). When PR5 was given 3×3 mg kg−1 (Group 2) the normalizing effect on the ST-segment increase was pronounced and highly significant throughout the whole ischaemia and reperfusion periods (P<0.0001) (Figure 7).
Figure 7.
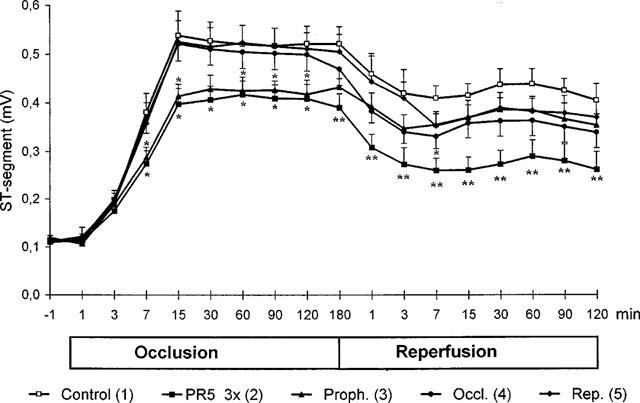
Effects of PR5 on ST-segment in rats during 180 min left coronary artery occlusion followed by 120 min reperfusion. Shown are data for control rats as well as data for animals receiving 3×3 mg kg−1 of PR5, respectively 5 min before occlusion, 170 min after occlusion and 5 min after start of reperfusion (2), animals receiving 3 mg kg−1 of PR5 5 min before occlusion (3), animals receiving 3 mg kg−1 of PR5 170 min after occlusion (4), and animals receiving 3 mg kg−1 of PR5 5 min after start of reperfusion (5). (*Indicates P<0.05, **P<0.01 vs the control).
Discussion
We have here shown that the hydroxyguanidine compound PR5 possesses a remarkable ability to protect the rat heart during reperfusion. Thus, PR5 strongly inhibited the burst of arrhythmias associated with reperfusion of ischaemic hearts, and it markedly improved the survival of the animals. Most notably, PR5 caused a substantial reduction (i.e. more than 2 fold at the best dosing schedule) of the infarction size, after 180 min of ischaemia followed by 120 min of reperfusion. These effects were associated with a reduction of the ischaemia induced elevation of the ST-segment of the ECGs, as well as a pronounced attenuation of the rapid blood pressure drop seen at the start of reperfusion, results which further indicate a strong protective effect by PR5 on the hearts haemodynamic function and/or its antiarrhythmic effect.
In a previous study we showed that the hydroxyguanidine guanoxabenz was capable of supporting the oxidation of xanthine to uric acid both during anaerobic and aerobic conditions (Dambrova et al., 1998). In subsequent studies we synthesized a novel series of hydroxyguanidines and found that PR5 showed a higher capacity than guanoxabenz to support the anaerobic oxidation of xanthine by xanthine oxidase (Dambrova et al. manuscript in preparation). From these results we hypothesized that PR5 could be useful in protecting the heart during ischaemia and reperfusion. It is well known that the ischaemia induces a rapid accumulation of hypoxanthine and xanthine due to the breakdown of the cellular ATP-pools due to insufficient oxidative phosphorylation (Xia et al., 1996; Saugstad, 1996). At the same time the release of intracellular proteolytic enzymes may cause a conversion of the xanthine dehydrogenase enzyme to its oxidase form. Upon the eventual reoxygenation of the tissue a rapid oxidation of hypoxanthine and xanthine will proceed, followed by the concomitant massive formation of toxic superoxide radicals (McCord, 1985). The thus formed free radicals may be responsible for the various injuries, which include life-threatening arrhythmias and cell death (Flaherty & Weisfeldt, 1988). However, additional mechanisms may also prevail that contribute to the damage (Bolli, 1988; Piper et al., 1998).
Thus, if PR5 is administered prior to deprivation of the blood supply to an organ it might be capable of supporting the oxidation of the hypoxanthine and xanthine during the ischaemia. Upon a subsequent reoxygenation there would then be present less oxidizable substrate (e.g. xanthine and hypoxanthine) for the xanthine oxidase, which accordingly would prevent formation of oxyradicals. It is also possible that in the presence of oxygen the hydroxyguanidine could accept the electrons generated during the hypoxanthine/xanthine oxidation, thus preventing the formation of oxyradicals.
Although at present the above described mechanism of action for PR5 is only hypothetical, the data of the present study support the hypothesis. In the present paper we have observed that the antiarrhythmic effect of PR5 is observed only during reperfusion, whereas no significant effect is seen during coronary artery occlusion. It is well accepted that the arrhythmias seen at the onset of reperfusion are caused by the massive formation of oxyradicals (Opie, 1989), thus indicating that the PR5 effect is linked to the radicals. It is interesting to note that PR5 has effect on the ST-segment also during the occlusion period (Figure 2C and 7). It is possible that this effect is due to the anaerobic oxidation of the xanthine oxidase substrates. Moreover, the results for MDA support an anti-oxidant mechanism for PR5. Still, of course, further studies will be necessary to prove the hypothesis for the mode of action of PR5.
Previous attempts to prevent toxic effects due to oxygen radical formation associated with ischaemia and reperfusion have been mainly focused on removing the superoxide or hydroxyradicals after their production. Thus, for example, compounds like lazaroids and a vitamin E analogue (raxofelast) are shown to prevent lipid peroxidation, possess antioxidant properties and have beneficial effects in models of myocardial damage (Campo et al., 1997; 1998). Another direction was to use xanthine oxidase inhibitors to prevent the oxidation of hypoxanthine/xanthine and oxyradical generation. Thus, allopurinol was reported to improve cardiac function in coronary artery bypass surgery (Castelli et al., 1995). Also an inhibitor of adenosine deaminase was shown to reduce the formation of oxygen radicals, an effect assumed to be due to the inhibition of the accumulation of the oxidizing substrates for xanthine oxidase (Xia et al., 1996). It should be also mentioned, that methylene blue, which is known to inhibit the generation of oxygen radicals by competing with molecular oxygen for the reduction of the latter by xanthine oxidase (Salaris et al., 1991), was recently found to prevent pulmonary injury after intestinal ischaemia and reperfusion (Galili et al., 1998), but its eventual effect in the heart has not yet been evaluated. Moreover, the mechanism of action of methylene blue is quite uncertain. The compound is also a soluble guanylate cyclase inhibitor, and it has been found to increase the incidence of reperfusion-induced ventricular fibrillation (Pabla et al., 1995).
The N-hydroxyguanidine guanoxabenz have been used as centrally active antihypertensive drug (Ledoux et al., 1981), but any possible antiischaemic effect of the guanoxabenz has not been reported. In the present study we have shown that the N-hydroxyguanidine PR5 provides remarkable protection against ischaemia and reperfusion induced myocardial necrosis and life-threatening arrhythmias. Since PR5 can act as an alternative electron acceptor for xanthine oxidase enzyme in vitro, we have hypothesized that xanthine oxidase-related mechanism of action may be a reason for its cardioprotective action.
In conclusion, we have shown that the N-hydroxyguanidine PR5 provides marked protection against ischaemia and reperfusion induced myocardial necrosis and life-threatening arrhythmias. Moreover, we have provided evidence that the rat heart lipid peroxidation damage (estimated as MDA formation) is inhibited by PR5. These effects of PR5 might be related to the recently discovered ability of N-hydroxyguanidines to function as electron acceptors at the xanthine oxidase enzyme.
Acknowledgments
We are grateful for technical assistance by Romans Sjomins, Latvian Institute of Organic Synthesis, Riga, Latvia. PR5 was synthesized by Dr Viktors Andrianovs at the Department of Medicinal Chemistry, Latvian Institute of Organic Synthesis, Riga, Latvia. This study was supported by the Swedish MRC (04X-05957) and the Åke Wiberg foundation. Dr Maris Veveris was supported by a stipend from the Swedish Institute.
Abbreviations
- ECG
electrocardiogram
- MDA
malondialdehyde
- PR5
N-(3,4-Dimethoxy-2-chlorobenzylideneamino)-N′-hydroxyguanidine
- VT
ventricular tachycardia
- VF
ventricular fibrillation
References
- AMAYA Y., YAMAZAKI K., SATO M., NODA K., NISHINO T., NISHINO T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. J. Biol. Chem. 1990;265:14170–14175. [PubMed] [Google Scholar]
- AR'RAJAB A., DAWIDSON I., FABIA R. Reperfusion injury. New Horizons. 1996;4:224–234. [PubMed] [Google Scholar]
- BOLLI R. Basic and clinical aspects of myocardial stunning. Progr. Cardiovasc. Dis. 1998;40:477–516. doi: 10.1016/s0033-0620(98)80001-7. [DOI] [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., ALTAVILLA D., SQUADRITO G., AVENOSO A., CANALE P., LOCULANO M., SPERANDEO A., CAPUTI A.P. Protection of ischemic and reperfused rat myocardium by the nonglucocorticoid 21-aminosteroid U-74389G, a new inhibitor of lipid peroxidation. J. Pharmacol. Exp. Ther. 1996;277:333–340. [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., CAMPO S., ALTAVILLA D., AVENOSO A., FERLITO M., SQUADRITO G., CAPUTI A.P. Antioxidant activity of U-83836E, a second generation lazaroid, during myocardial ischaemia/reperfusion injury. Free Radic. Res. 1997;27:577–590. doi: 10.3109/10715769709097861. [DOI] [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., CAMPO S., ALTAVILLA D., QUARTARONE C., CECCARELLI S., FERLITO M., AVENOSO A., SQUADRITO G., SAITTA A., CAPUTI A.P. Beneficial effect of raxofelast, an hydrophilic vitamin E analogue, in the rat heart after ischaemia and reperfusion injury. J. Mol. Cell Cardiol. 1998;30:1493–1503. doi: 10.1006/jmcc.1998.0713. [DOI] [PubMed] [Google Scholar]
- CASTELLI P., CONDEMI A.M., BRAMBILLASCA C., FUNDARO P., BOTTA M., LEMMA M., VANELLI P., SANTOLI C., GATTI S., RIVA E. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J. Cardiovasc. Pharmacol. 1995;25:119–125. doi: 10.1097/00005344-199501000-00019. [DOI] [PubMed] [Google Scholar]
- DAMBROVA M., UHLÉN S., WELCH C.J., WIKBERG J.E.S. Identification of an N-hydroxyguanidine reducing activity of xanthine oxidase. Eur. J. Biochem. 1998;257:178–184. doi: 10.1046/j.1432-1327.1998.2570178.x. [DOI] [PubMed] [Google Scholar]
- FLAHERTY J.T., WEISFELDT M.L. Reperfusion injury. Free Radic. Biol. Med. 1988;5:409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- GALILI Y., BEN-ABRAHAM R., WEINBROUM A., MARMUR S., IAINA A., VOLMAN Y., PEER G., SZOLD O., SOFFER D., KLAUSNER J., RABAU M., KLUGER Y. Methylene blue prevents pulmonary injury after intestinal ischaemia-reperfusion. J. Trauma. 1998;45:222–226. doi: 10.1097/00005373-199808000-00004. [DOI] [PubMed] [Google Scholar]
- KANE K.A., PARRATT J.R., WILLIAMS F.M. An investigation into the characteristics of reperfusion-induced arrhythmias in the anaesthetised rat and their susceptibility to antiarrhythmic agents. Br. J. Pharmacol. 1984;82:349–357. doi: 10.1111/j.1476-5381.1984.tb10769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDOUX F., WELSC M., STEIMER C., SCHWARTZ J. A clinical trial of guanoxabenz: a hypotensive agent with central and hypertensive action. Therapie. 1981;36:187–191. [PubMed] [Google Scholar]
- MCCORD J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Eng. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- NISHINO T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J. Biochem. 1994;116:1–6. doi: 10.1093/oxfordjournals.jbchem.a124480. [DOI] [PubMed] [Google Scholar]
- OHKAWA O., OHISHI N., YAGI K. Assay for peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- OPIE L.H. Reperfusion injury and its pharmacologic modification. Circulation. 1989;80:1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- PABLA R., BLAND-WARD P., MOORE P.K., CURTIS M.J. An endogenous protectant effect of cardiac cyclic GMP against reperfusion-induced ventricular fibrillation in the rat heart. Br. J. Pharmacol. 1995;116:2923–2930. doi: 10.1111/j.1476-5381.1995.tb15946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPER H.M., GARCIA-DORADO D., OVIZE M. A fresh look at reperfusion injury. Cardiovasc. Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- POSS W.B., HUECKSTEADT T.P., PANUS P.C., FREEMAN B.A., HOIDAL F.J. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am. J. Physiol. 1996;270:L941–L946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- REILLY P.M., SCHILLER H.J., BULKLEY G.B. Pharmacological approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am. J. Surg. 1991;161:488–503. doi: 10.1016/0002-9610(91)91120-8. [DOI] [PubMed] [Google Scholar]
- SALARIS S.C., BABBS C.F., VOORHEESIIIW D. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. Biochem. Pharmacol. 1991;42:499. doi: 10.1016/0006-2952(91)90311-r. [DOI] [PubMed] [Google Scholar]
- SAUGSTAD O.D. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98:103–107. [PubMed] [Google Scholar]
- THOMPSON-GORMAN S.L., ZWEIER J.L. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- VANHAECKE J., VAN DE WERF F., RONASZEKI A., FLAMENG W., LESAFFRE E., DE GEEST H. Effect of superoxide dismutase on infarct size and postischemic recovery of myocardial contractility and metabolism in dogs. J. Am. Coll. Cardiol. 1991;18:224–230. doi: 10.1016/s0735-1097(10)80243-8. [DOI] [PubMed] [Google Scholar]
- WALKER M.J.A., CURTIS M.J., HEARSE D.J., CAMPBELL R.W.F., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W.G., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSELL D.C., SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction and reperfusion. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WANG P., CHEN H., QIN H., SANKARAPANDI S., BECHER M.W., WONG P.C., ZWEIER J.L. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE B.I., PREMARATNE S., LIMM W., MUGIISHI M.M., MCNAMARA J.J. High- and low-dose superoxide dismutase plus catalase does not reduce myocardial infarct size in a subhuman primate model. Am. Heart J. 1993;126:840–846. doi: 10.1016/0002-8703(93)90697-8. [DOI] [PubMed] [Google Scholar]
- XIA Y., KHATCHIKIAN G., ZWEIER J.L. Adenosine deaminase inhibition prevents free radical-mediated injury in the postsichemic heart. J. Biol. Chem. 1996;271:10096–10102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]


