Abstract
Superoxide and superoxide-derived oxidants have been hypothesized to be important mediators of postischemic injury. Whereas copper,zinc-superoxide dismutase, SOD1, efficiently dismutates superoxide, there has been controversy regarding whether increasing intracellular SOD1 expression would protect against or potentiate cellular injury. To determine whether increased SOD1 protects the heart from ischemia and reperfusion, studies were performed in a newly developed transgenic mouse model in which direct measurement of superoxide, contractile function, bioenergetics, and cell death could be performed. Transgenic mice with overexpression of human SOD1 were studied along with matched nontransgenic controls. Immunoblotting and immunohistology demonstrated that total SOD1 expression was increased 10-fold in hearts from transgenic mice compared with nontransgenic controls, with increased expression in both myocytes and endothelial cells. In nontransgenic hearts following 30 min of global ischemia a reperfusion-associated burst of superoxide generation was demonstrated by electron paramagnetic resonance spin trapping. However, in the transgenic hearts with overexpression of SOD1 the burst of superoxide generation was almost totally quenched, and this was accompanied by a 2-fold increase in the recovery of contractile function, a 2.2-fold decrease in infarct size, and a greatly improved recovery of high energy phosphates compared with that in nontransgenic controls. These results demonstrate that superoxide is an important mediator of postischemic injury and that increasing intracellular SOD1 dramatically protects the heart from this injury. Thus, increasing intracellular SOD1 expression may be a highly effective approach to decrease the cellular injury that occurs following reperfusion of ischemic tissues.
Keywords: transgenic model, superoxide radical, myocardial infarction, electron paramagnetic resonance, nuclear magnetic resonance
With the advent of thrombolytic and other revascularization therapies reperfusion of ischemic tissues has become a common event in the treatment of important human diseases such as heart attack and stroke (1, 2). Although reperfusion limits ischemic damage, it leads to further cellular injury. There is evidence that the superoxide radical, O2⨪, formed in postischemic tissues is an important mediator of this reperfusion injury (3–5). However, with administration of exogenous copper,zinc-superoxide dismutase, SOD1, variable protective effects have been observed (6, 7), leading to the hypothesis that the efficacy of SOD1 treatment may be limited by the inability of exogenous enzyme to enter cells. It was also hypothesized that dose-dependent toxicity may occur (8). Furthermore, it has been proposed that native or mutant SOD1 may exert important deleterious effects. Increased peroxidase activity has been advanced as a possible cause of the familial form of the neurodegenerative disease amyotrophic lateral sclerosis, FALS (9–15). It has also been suggested that Down’s syndrome, trisomy 21, is caused by a 50% increase in SOD1 expression (16). Thus, fundamental questions have been raised regarding the effects of altered SOD1 expression on cellular function and development.
In view of the common occurrence of ischemia and reperfusion in the pathogenesis and treatment of clinical disease, there has been a great need to develop approaches suitable to prevent ischemic and reperfusion injury in man. With the advent of genetic engineering methods to modulate protein expression and the development of approaches to implement this in patients, much effort is now being directed at identification of molecular therapies to prevent postischemic injury.
Because of the recent controversy regarding the functional effects of SOD1, it is particularly important to determine whether overexpression of SOD1 prevents or potentiates cellular injury in tissues under pathophysiological processes of oxidant stress such as those that occur during myocardial ischemia and reperfusion. To demonstrate whether increased intracellular SOD1 expression can prevent postischemic injury and to further determine the importance of intracellular O2⨪ generation in the pathogenesis of this injury, it is necessary to modulate intracellular SOD expression. With the use of transgenic (TG) models increased intracellular expression of a given enzyme can be achieved.
To determine whether increased SOD1 protects the heart from ischemia and reperfusion, studies were performed in a newly developed TG mouse heart model in which direct measurement of superoxide, contractile function, bioenergetics, and cell death was performed. These studies demonstrate that SOD1 overexpression abolishes the reperfusion-associated burst of superoxide generation and greatly decreases cellular injury.
MATERIALS AND METHODS
TG Mice.
TG mice were constructed by using a previously characterized plasmid, pHGSOD-Svneo (17), encoding human SOD1 (hSOD1). This gene is contained within a 12-kb genomic DNA fragment that was excised by EcoRI and BamHI, gel-purified, and microinjected into hybrid (C57L/6J × C3H/HeJ) F2 mouse embryos. TG founder mice were identified by Southern blotting of DNA isolated from mouse tail with hSOD1 cDNA as a probe (15). Lines were established from four founders. The TG mice used had heterozygous expression of hSOD1, and the nontransgenic (NT) controls were littermates not expressing the gene.
Heart Perfusion.
Isolated perfused mouse hearts were studied to enable measurement of myocardial free radical generation, contractile function, energetic state, and cell death (infarction). TG and NT mice (7–8 weeks) were heparinized and anesthetized with intraperitoneal pentobarbital. Their hearts were rapidly excised and placed in ice-cold perfusate. The ascending aorta was rapidly cannulated under stereomicroscopy and perfused at 37°C and constant pressure of 70 mmHg with a modified Krebs bicarbonate perfusate (in mM: 16.7 glucose, 120.0 NaCl, 25.0 NaHCO3, 2.5 CaCl2, 0.5 EDTA, 5.9 KCl, and 1.2 MgCl2), which was bubbled with 95% O2/5% CO2 gas. To assess contractile function, a specially developed tiny fluid-filled balloon was inserted into the left ventricle through the mitral valve and secured with a ligature around the left atrium and connected to a pressure transducer via a hydraulic line. The balloon was initially inflated to an end-diastolic pressure of 4–8 mmHg, and all subsequent measurements of left ventricular pressures were made at this same end-diastolic volume.
EPR Spectroscopy and Spin Trapping.
Spin-trapping measurements of oxygen radical generation from TG and NT hearts were performed. Hearts were perfused in the presence of 50 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO), and effluent was sampled both before ischemia and during the first 2 min of reflow. EPR spectra were recorded in a quartz flat cell at room temperature with a Bruker ER 300 spectrometer operating at X-band with a 100-kHz modulation frequency and a TM110 cavity, as described (18, 19). The microwave frequency and magnetic field were precisely measured with an EIP 575 frequency counter and Bruker ER 035 NMR gaussmeter. Relative quantitation of the free radical signals was performed by double integration.
NMR Spectroscopy.
Pulsed Fourier transform 31P NMR spectra were obtained on isolated perfused mouse hearts placed in 10-mm NMR tubes with all the requisite lines secured to an attached support rod. Spectra were recorded on a Bruker MSL 500 at a 31P resonance frequency of 202.46 MHz by using a 10-mm broadband probe with proton decoupling (20, 21). Ten-minute spectral acquisitions were performed from 600 summed free induction decays with 1-s interpulse delay.
Infarct Size Measurement.
Myocardial infarction was measured by triphenyltetrazolium chloride (TTC) staining of heart sections after either 45 or 120 min of reperfusion. Hearts were sliced into 1.5-mm thick sections and stained with a 2% solution of TTC, which stains viable myocardium brick red and infarcted myocardium white (22). Quantitative analysis was performed by computerized planimetry of each section. Percent infarction was determined from the mass weighted average of the ratio of infarct area to the total cross-sectional area of the ventricle from each slice.
Immunoblotting and Immunohistology.
Expression of SOD1 in TG and NT hearts was measured by immunoblotting. Mouse heart tissue was homogenized in buffer containing 25 mM sodium phosphate (pH 7.2), 5 mM EGTA, 1% SDS, and 1 mM phenylmethylsulfonyl fluoride. Total protein (20 μg) was loaded onto a 15% polyacrylamide gel, electrophoresed, and transferred onto nitrocellulose filters. Endogenous mouse SOD1 (mSOD1) and wild-type SOD1 were detected by using a polyclonal antibody, pAB-m/h-SOD1, recognizing a common epitope of both mouse and hSOD1 (23). Signals were quantified with a Molecular Dynamics PhosphorImager.
Immunohistochemical localization of SOD1 in cardiac tissue was performed on 10-μm deparaffinized sections, which were processed for immunocytochemistry (peroxidase-antiperoxidase method) by using the primary antibody to SOD1. The immune reaction was visualized by diaminobenzidine, which results in characteristic brown staining (15).
SOD1 Activity Gels.
Mouse hearts were homogenized in buffer containing 20 mM Tris⋅Cl, pH 7.2, 1 mM EDTA, and 1% Triton X-100. After centrifugation at 10,000 × g for 5 min the supernatant was electrophoresed on a 7.5% polyacrylamide gel, and SOD1 activities were determined as previously reported by the method of Beauchamp and Fridovich (24). In this method the gels are soaked in 2.5 mM nitro-blue tetrazolium for 20 min followed by immersion for 15 min in phosphate buffer, pH 7.8, containing 28 μM riboflavin. After illumination the gels become uniformly blue except at the positions containing SOD. By using a dilution series of known amounts of purified hSOD1 as standards, SOD activity gels were quantitated with a Stratagene Eagle Eye II to digitize the images.
Statistical Analysis.
Results are expressed as mean ± SEM. Student’s unpaired t test was used to determine the statistical significance of difference between the means, and a P value of <0.05 was considered significant.
RESULTS
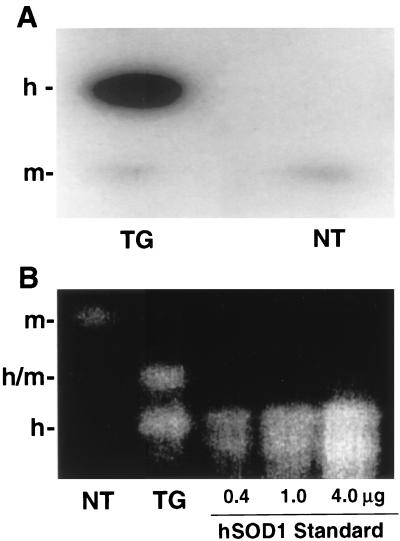
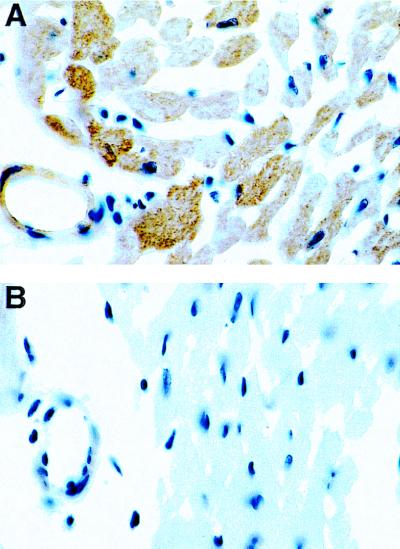
Protein immunoblotting was performed on heart extracts from NT and TG mice by using an antibody that recognizes a common epitope found on both mouse and hSOD1. In the TGs a very dense band of the human isoform of SOD1, hSOD1, was seen along with a much weaker band of the mouse isoform, mSOD1, whereas in the NTs only the weak band of mSOD1 was seen (Fig. 1A). The total SOD1 expression was increased 10-fold in TG hearts compared with NT controls. To determine whether the activity of SOD1 increased in parallel with the increase in the expression of the enzyme, activity gel measurements were performed. In the TGs strong bands were seen because of the hSOD1 dimer and the hSOD1/mSOD1 heterodimer, whereas in the NTs only a weak band of the mSOD1 dimer was seen (Fig. 1B). It was observed that the activity of SOD1 was increased approximately 10-fold in the hearts of the TG animals compared with NT controls. Immunohistology was performed to visualize the cellular expression of the enzyme, and it was observed that in the TG animals SOD1 expression was markedly increased both in cardiac myocytes and endothelial cells (Fig. 2).
Figure 1.
(A) Expression of SOD1 in TG and NT hearts measured by immunoblotting. Mouse heart tissue was homogenized in buffer containing 25 mM sodium phosphate (pH 7.2), 5 mM EGTA, 1% SDS, and 1 mM phenylmethylsulfonyl fluoride. Total protein (20 μg) was loaded onto a 15% polyacrylamide gel, electrophoresed, and transferred onto nitrocellulose filters. Endogenous mSOD1 and hSOD1 were detected with a polyclonal antibody, pAB-m/h-SOD1, recognizing a common epitope of both mouse and hSOD1. The position of the hSOD1 band is shown by h-, and the position of mSOD1 is shown by m-. (B) SOD1 activity gel of heart tissue extracts (20 μg of protein). The position of the homodimeric mSOD1 is shown by m-, that of homodimeric hSOD1 by h-, and that of the heterodimer by h/m. Both SOD1 protein and activity were increased by approximately 10-fold in the TG hearts compared with that in the NT controls.
Figure 2.
Immunohistochemical localization of SOD1 in cardiac tissue. Sections were processed for immunocytochemistry (peroxidase-antiperoxidase method) by using the primary antibody to SOD1. The immune reaction was visualized by diaminobenzidine, which results in characteristic brown staining. In the TGs, strong brown staining for SOD1 was seen in both myocytes and endothelial cells, whereas only very weak staining was seen in NT control hearts.
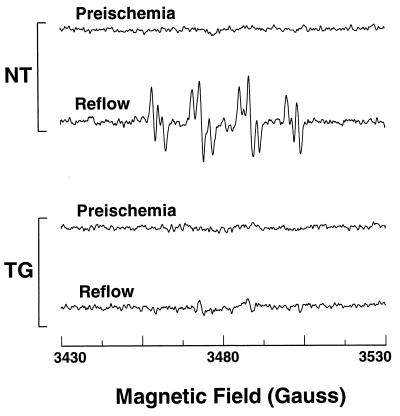
To determine the effect of increased SOD1 expression on the magnitude of O2⨪ generation in the postischemic heart, EPR and spin-trapping studies using the spin trap DMPO were performed to measure O2⨪ generation (18). Before ischemia no significant signal was seen from either NT or TG hearts. However, following 30 min of ischemia prominent signals were observed from the NT hearts during the first 5 min of reflow. A characteristic spectrum of DMPO–OOH derived from the direct trapping of O2⨪ was seen with hyperfine splittings, aN = 14.2 G, aH = 11.3 G, aHγ = 1.3 G, as well as a small DMPO–OH signal, aH = aN = 14.9 G, which can arise from breakdown of DMPO–OOH or direct trapping of ⋅OH (Fig. 3). In TG hearts, however, these radical signals were largely quenched demonstrating that increased SOD1 expression abolished the burst of radical generation that occurs on postischemic reperfusion (Fig. 3).
Figure 3.
EPR measurement of free radical generation from TG and NT hearts. Hearts were perfused in the presence of 50 mM DMPO, and effluent was sampled both before ischemia and during the first 2 min of reflow. Measurements were performed at 9.77 GHz with a Bruker ER 300 spectrometer using a TM110 cavity and flat cell with microwave power of 20 mW, and a modulation amplitude of 0.5 G. After reperfusion prominent spectra of DMPO–OOH and DMPO–OH radical adducts were seen in NT hearts but not in TG hearts.
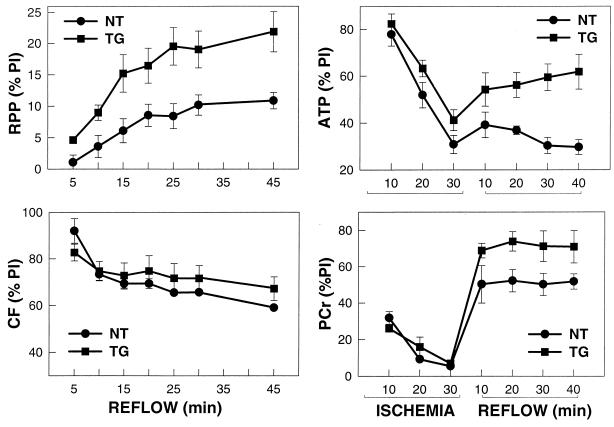
To determine the effects of increased SOD1 expression on functional recovery, a series of TG and NT hearts were subjected to 30 min of ischemia followed by 45 min of reperfusion with continuous measurement of contractile function. Although before ischemia no significant differences in function were seen, after ischemia the TG animals had a much higher recovery of contractile function than NT controls (Fig. 4). Throughout the time course of reflow TG hearts exhibited a much higher percent recovery of rate pressure product (RPP) with values of 22 ± 3% versus 10.8 ± 0.3% in NT controls after 45 min of reflow (P < 0.01) (Fig. 4). In contrast, similar recovery of coronary flow was seen in both groups, indicating that the increased recovery of contractile function in TG hearts was not because of differences in flow and truly reflects decreased myocyte injury. Thus, elevated SOD1 expression decreased cellular injury and increased functional recovery.
Figure 4.
Recovery of contractile function, coronary flow, and high energy phosphates in NT and TG hearts. The percent recovery of preischemic (PI) values of RPP, a measure of cardiac work, is shown on the upper left panel, and recovery of coronary flow (CF) is shown on the lower left panel. Percent recovery of ATP and PCr appear on the right panels. RPP was determined from the product of left ventricular developed pressure and heart rate. Values shown are mean ± SEM from seven hearts in each group. ATP was measured from the intensity of the β-ATP resonance of 31P NMR spectra obtained from a separate series of hearts. PCr was also measured from its corresponding resonance. Spectra were recorded at a 31P resonance frequency of 202.46 MHz by using a 10-mm broadband probe with proton decoupling. Ten-minute spectral acquisitions were performed from 600 summed free induction decays with a 1-s interpulse delay.
To determine the effects of increased SOD1 expression on the metabolic and energetic state of the heart, studies were performed to measure the alterations in intracellular high energy phosphates by using 31P NMR (20, 21). Whereas similar decreases in ATP and phosphocreatine (PCr) occurred during the 30-min period of ischemia, the TG hearts exhibited improved recovery throughout the time course of reperfusion with 2.1-fold higher ATP levels and 1.3-fold higher PCr after 45 min (P < 0.01 and P < 0.05, respectively) (Fig. 4). Thus, increased SOD1 expression resulted in an improved myocardial energetic state.
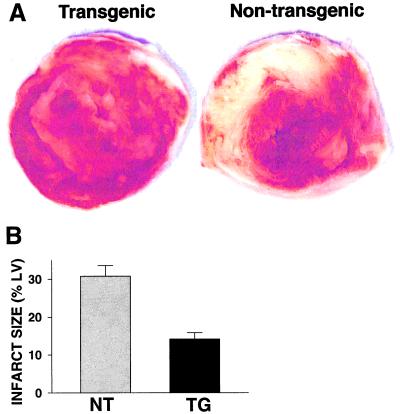
Myocardial infarct size was measured at the end of the 45-min reperfusion period by TTC staining. In NT hearts large areas of infarction were seen with mean values of 30.8 ± 2.8% of the left ventricle, whereas in the TGs infarct size was drastically decreased to values of 14.2 ± 1.7% (P < 0.01) (Fig. 5). Thus, SOD1 overexpression enhanced functional and metabolic recovery and greatly decreased myocardial infarction.
Figure 5.
Infarct size measurement in NT and TG hearts. Hearts were sliced into 1.5-mm thick sections and stained with a 2% solution of TTC, which stains viable myocardium brick red and infarcted myocardium white. A shows typical slices from NT and TG hearts. Quantitative analysis was performed by computerized planimetry of each section. Percent infarction was determined from the mass weighted average of the ratio of infarct area to total left ventricle area from each slice. Measurements were performed on seven hearts in each group with the mean values ± SEM shown in B.
To further determine whether this SOD1-mediated protection was sustained after more prolonged periods of reperfusion additional TG and NT hearts were subjected to 30 min of ischemia followed by reperfusion for 120 min. With this longer reperfusion time contractile function further decreased in NT hearts with mean RPP values of 7.2 ± 3.1% of baseline, but in the TG hearts much higher recovery was seen with values of 27.5 ± 3.8% (P < 0.01). After 120 min of reperfusion infarct size was 36.1 ± 3.1% of the left ventricle in NT hearts but only 14.1 ± 3.3% in the TGs (P < 0.01). Thus, with longer reperfusion time the marked protection seen with increased SOD1 expression was sustained.
DISCUSSION
SOD1 is a dimeric 32-kDa protein that efficiently dismutates O2⨪ forming hydrogen peroxide and oxygen (25, 26). It is a cytosolic enzyme that serves the critical function of limiting intracellular O2⨪ concentrations during oxidant stress such as occurs during ischemia and reperfusion where a variety of cellular oxidases are activated (5, 27). Superoxide or its protonated form, ⋅OOH, can react with and denature important cellular enzymes including aconitase and fumarase (28–31). Furthermore, O2⨪ can further react via the metal-catalyzed Haber Weiss reaction to form the highly reactive hydroxyl radical (6, 18). It also rapidly reacts with nitric oxide to form the potent oxidant peroxynitrite, which in turn causes protein nitration and cell death (32, 33). Therefore, O2⨪ can cause cellular injury either because of direct reactions with proteins or secondary formation of strong oxidants. In the postischemic heart there is evidence that each of these O2⨪-dependent reactions causes cellular injury (6, 34).
Although numerous studies have been performed with administration of exogenous SOD1 this 32-kDa protein is not readily internalized within cells, and in the setting of myocardial reperfusion injury variable protective effects were observed (6, 35–38). There have been reports that increased SOD1 expression can have deleterious effects, and it has even been suggested that modest increases in expression may trigger the abnormalities seen in Down’s syndrome (16). With administration of exogenous SOD1 to ischemic tissues it has been reported that dose-dependent toxicity may also occur (39). Therefore, it was unclear whether increased intracellular SOD1 levels protect or potentiate cellular injury under conditions of oxidant stress.
We observe that overexpression of SOD1 in myocardial cells exerts dramatic protection against the oxidant-mediated injury that occurs during ischemia and reperfusion. In the myocardium of TG mice engineered to overexpress SOD1, it was demonstrated that a 10-fold increase in SOD1 expression was achieved with a parallel increase in enzyme activity. Immunohistology demonstrated that SOD1 expression was increased in both endothelial cells and myocytes. This increased SOD1 expression was sufficient to largely quench the burst of O2⨪ generation that occurs on postischemic reperfusion. Although in NT control hearts EPR studies using the spin trap DMPO demonstrated prominent signals from O2⨪-derived DMPO–OOH or DMPO–OH adducts during the early minutes of reflow, these signals were largely abolished in the TGs. Hearts from TG animals with increased SOD1 expression exhibited much higher recovery of contractile function, with more than a 2-fold increase in the recovery of RPP. An improved bioenergetic state was also seen in TGs with a 2-fold higher recovery of myocardial ATP levels and 30% higher PCr levels compared with those seen in NT hearts. A marked reduction in myocardial cell death was also seen with more than a 2-fold decrease in infarct size. Thus, increased expression of SOD1 in the cells of the heart resulted in impressive protection against postischemic injury.
These results provide strong evidence for the role of intracellular superoxide generation in the pathogenesis of postischemic injury and show that modulation of intracellular SOD1 expression can prevent myocardial ischemia/reperfusion injury. With the recent development of cell-permeant SOD mimetic drugs as well as vectors to modulate intracellular SOD1 expression, it will be of great importance to determine whether increasing intracellular SOD activity is also effective at preventing postischemic injury in patients.
Acknowledgments
We thank Dr. V. P. Chacko for expert assistance and advice regarding the NMR studies performed. This work was supported by National Institutes of Health Grants HL-38324 and HL-52315 and by an American Heart Association grant-in-aid.
ABBREVIATIONS
- SOD
superoxide dismutase
- TG
transgenic
- NT
nontransgenic
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- TTC
triphenyltetrazolium chloride
- hSOD
human SOD
- mSOD
mouse SOD
- RPP
rate pressure product
- PCr
phosphocreatine
References
- 1.Rosamond W D, Shahar E, McGovern P G, Sides T L, Luepker R V. Am J Cardiol. 1996;78:271–277. doi: 10.1016/s0002-9149(96)00276-7. [DOI] [PubMed] [Google Scholar]
- 2.Hund E, Grau A, Hacke W. Neurol Clin. 1995;13:511–527. [PubMed] [Google Scholar]
- 3.Werns S W, Shea M J, Mitsos S E, Dysko R C, Fantone J C, Schork M A, Abrams G D, Pitt B, Lucchesi B R. Circulation. 1986;73:518–524. doi: 10.1161/01.cir.73.3.518. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo C M, Kramer J H, Dickens B F, Weglicki W B. FEBS Lett. 1987;221:101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- 5.Zweier J L. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 6.Hess M L, Kukreja R C. Ann Thorac Surg. 1995;60:760–766. doi: 10.1016/0003-4975(95)00574-5. [DOI] [PubMed] [Google Scholar]
- 7.Kukreja R C, Janin Y J. Thromb Thrombolysis. 1997;4:7–10. doi: 10.1023/a:1017569611074. [DOI] [PubMed] [Google Scholar]
- 8.McCord J M. Clin Biochem. 1993;26:351–357. doi: 10.1016/0009-9120(93)90111-i. [DOI] [PubMed] [Google Scholar]
- 9.Yim M B, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1990;87:5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yim M B, Kang J H, Yim H S, Kwak H S, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H X, Hentati A, Tainer J A, Iqbal Z, Cayabyab A, Hung W Y, Getzoff E D, Hu P, Herzfeldt B, Roos R P. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 12.Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H X. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 13.Wiedau-Pazos M, Goto J J, Rabizadeh S, Gralla E B, Roe J A, Lee M K, Valentine J S, Bredesen D E. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 14.McCord J M. Science. 1994;266:1586–1587. [PubMed] [Google Scholar]
- 15.Wong P C, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 16.De La Torre R, Casado A, Lopez-Fernandez E, Carrascosa D, Ramirez V, Saez J. Experientia. 1996;52:871–873. doi: 10.1007/BF01938872. [DOI] [PubMed] [Google Scholar]
- 17.Epstein C J, Avraham K B, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Proc Natl Acad Sci USA. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier J L, Kuppusamy P, Williams R, Rayburn B K, Smith D, Weisfeldt M L, Flaherty J T. J Biol Chem. 1989;264:18890–18895. [PubMed] [Google Scholar]
- 19.Xia Y, Zweier J L. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 20.Zweier J L, Jacobus W E. J Biol Chem. 1987;262:8015–8021. [PubMed] [Google Scholar]
- 21.Zweier J L, Jacobus W E, Korecky B, Brandejs-Barry Y. J Biol Chem. 1991;266:20296–20304. [PubMed] [Google Scholar]
- 22.Fishbein M C, Meerbaum S, Rit J, Lando V, Kanmatsuse K, Mercier J, Corday E, Ganz W. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 23.Pardo C A, Xu Z, Borchelt D R, Price D L, Sisodia S S, Cleveland D W. Proc Natl Acad Sci USA. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauchamp C, Fridovich I. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 25.Fridovich I. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 26.Beyer W, Imlay J, Fridovich I. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 27.Crapo J D, Oury T, Rabouille C, Slot J W, Chang L Y. Proc Natl Acad Sci USA. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner P R, Fridovich I. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 29.Hausladen A, Fridovich I. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 30.Flint D H, Tuminello J F, Emptage M H. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- 31.Liochev S I, Fridovich I. Arch Biochem Biophys. 1993;301:379–384. doi: 10.1006/abbi.1993.1159. [DOI] [PubMed] [Google Scholar]
- 32.Beckman J S. Nature (London) 1990;345:27–28. doi: 10.1038/345027b0. [DOI] [PubMed] [Google Scholar]
- 33.Xia Y, Dawson V L, Dawson T M, Snyder S H, Zweier J L. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Zweier J L. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 35.Werns S W, Shea M J, Driscoll E M, Cohen C, Abrams G D, Pitt B, Lucchesi B R. Circ Res. 1985;56:895–898. doi: 10.1161/01.res.56.6.895. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosio G, Becker L C, Hutchins G M, Weisman H F, Weisfeldt M L. Circulation. 1986;74:1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher K P, Buda A J, Pace D, Gerren R A, Shlafer M. Circulation. 1986;73:1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- 38.Uriazee A, Reimer K A, Murry C E, Jennings R B. Circulation. 1987;75:1237–1248. doi: 10.1161/01.cir.75.6.1237. [DOI] [PubMed] [Google Scholar]
- 39.Omar B A, Gad N M, Jordan M C, Striplin S P, Russell W J, Downey J M, McCord J M. Free Radical Biol Med. 1990;9:465–471. doi: 10.1016/0891-5849(90)90123-z. [DOI] [PubMed] [Google Scholar]