Abstract
Mitochondrial proton F0F1-ATPase/ATP synthase synthesizes ATP during oxidative phosphorylation. In this study, we examined the effects of several groups of polyphenolic phytochemicals on the activity of the enzyme.
Resveratrol, a stilbene phytoalexin that is present in grapes and red wine, concentration-dependently inhibited the enzymatic activity of both rat brain and liver F0F1-ATPase/ATP synthase (IC50 of 12–28 μM).
Screening of other polyphenolic phytochemicals using rat brain F0F1-ATPase activity resulted in the following ranking potency (IC50 in parenthesis): piceatannol (8 μM)>resveratrol (19 μM)=(−)epigallocatechin gallate (17 μM)>(−)epicatechin gallate, curcumin (45 μM)>genistein=biochanin A=quercetin=kaempferol=morin (55–65 μM)>phloretin=apigenin=daidzein (approx. 100 μM). Genistin, quercitrin, phloridzin, (+)catechin, (+)epicatechin, (−)epicatechin and (−)epigallocatechin had little effect at similar concentrations. Tannic acid, theaflavins (tea extract) and grape seed proanthocyanidin extract (GSPE) had IC50 values of 5, 20 and 30 μg ml−1, respectively. Several monophenolic antioxidants and non-phenolic compounds were ineffective at concentrations of 210 μM or higher.
The inhibition of F0F1-ATPase by resveratrol and genistein was non-competitive in nature.
The effects of polyphenolic phytochemicals were additive.
Both resveratrol and genistein had little effect on the Na+/K+-ATPase activity of porcine cerebral cortex, whereas quercetin had similar inhibitory potency as for F0F1-ATPase.
In conclusion, the ATP synthase is a target for dietary phytochemicals. This pharmacological property of these phytochemicals should be included in the examination of their health benefits as well as potential cytotoxicity.
Keywords: Phytoestrogens, F0F1-ATPase/ATP synthase, Na+/K+-ATPase, flavonoids, mitochondria, resveratrol, genistein, catechins, tea
Introduction
F0F1-ATPase/ATP synthase (F-type ATPase, complex V) is present in the inner membrane of eukaryotic mitochondria and acts as the powerhouse of the cell by synthesizing ATP. It can also operate in the reverse direction, hydrolysing ATP and pumping protons under certain conditions. The enzyme can be separated into two major complexes: F1 and F0 (Pedersen & Amzel, 1993; Boyer, 1997). F1 is a water-soluble catalytic complex consisting of five subunits (α3β3γδε), with the catalytic site located on the β subunit. F0 is made up of several membrane proteins (a, b, c, d, e, F6, A6L) and oligomycin sensitivity-conferring protein (OSCP), which contributes to the stalk region between F0 and F1. In addition, a native peptide is also bound to the F1 under de-energized conditions that serves to inhibit the ATPase activity of the enzyme (called F1 inhibitor protein, IF1).
Recent studies suggest that both α and β subunits of the F1-ATPase may be present on the surface of human umbilical vein endothelial cells and are binding sites for angiostatin, a proteolytic fragment of plasminogen that is both a potent antagonist of angiogenesis and an inhibitor of tumour growth (Moser et al., 1999). Antibodies against α subunit labelled the cell surface and inhibited angiostatin's anti-proliferative effect on endothelial cells. Earlier, β subunit had been localized to the cell surface of three human tumour cell lines (erythroleukaemia K562 cells, lung adenocarcinoma A549 cells and Burkitt's lymphoma Raji cells), but not to that of normal human erythrocytes or lymphocytes (Das et al., 1994). The surface β subunit in tumour cells induces lymphocyte-mediated cell killing, whereas antibodies against the β subunit inhibit this cytotoxicity. These studies suggest the key involvement of cell surface F-ATPase subunits in tumour growth and inhibition, which could be potential therapeutic targets for cancers.
Several inhibitors of ATP synthase have been described, including efrapeptin, oligomycin, aurovertin B and azide (Linnett & Beechey, 1979). The binding pocket for the F1-targeting inhibitor efrapeptin is localized in α, β and γ subunits (Abrahams et al., 1996) while that for aurovertin B is localized mainly in the β subunit (van Raajj et al., 1996). Inhibition of F0F1-ATPase/ATP synthase by the F0-targeting inhibitor, oligomycin, has been shown to protect or postpone cell injury by preserving ATP during ischaemia (Vuorinen et al., 1995) and to induce apoptosis in tumour cells (Wolvetang et al., 1994). Oligomycin also inhibits the apoptosis induced by Bax, a pro-apoptotic protein localized on the mitochondrial outer membrane, and F0F1-ATPase is required for the killing of cells by Bax (Matsuyama et al., 1998).
We recently reported that rat brain F0F1-ATPase was retained by oestradiol affinity columns and that oestradiol and several other oestrogens inhibited its activity (Zheng & Ramirez, 1999a,1999b). Earlier, diethylstilbestrol, a synthetic oestrogen, was shown to inhibit rat liver F0F1-ATPase (McEnery & Pedersen, 1986; McEnery et al., 1989). Although these effects require pharmacological (micromolar) concentrations of these oestrogens, which may not be physiologically relevant, phytoestrogens and other structurally similar polyphenolic phytochemicals are present in abundance in human food (Dewick, 1997; Bravo, 1998). It is worth mentioning that one group of weak oestrogenic phytochemicals–flavones, such as quercetin–has been shown to inhibit bovine and porcine heart F0F1-ATPase (Lang & Racker, 1974; Di Pietro et al., 1975). Quercetin also inhibits a number of other ATPases, such as Na+/K+-ATPase, Ca2+-ATPase and more than a dozen other enzymes (Fewtrell & Gomperts, 1977; Ferrell et al., 1979; Hirano et al., 1989; McKenna et al., 1996).
In this study, we report on the effects of several groups of polyphenolic phytochemicals that have been shown to be important in human diets on the activity of mitochondrial F0F1-ATPase/ATP synthase, including stilbenes, isoflavones, flavones, catechins, chalcones and several others (Figure 1). In addition, we report the kinetic mechanisms of inhibition for selected compounds as well as their effect on Na+/K+-ATPase. Many of these phytochemicals exhibit diverse activities such as antioxidant, cardiovascular protective, anti-osteoporotic, cancer chemopreventative, anti-mitotic and oestrogenic actions (Frankel et al., 1993; Adlercreutz, 1995; Gehm et al., 1997; Jang et al., 1997; Hsieh et al., 1998; Dashwood, 1998; Setchell, 1998; Murkies et al., 1998; Tham et al., 1998; Hollman et al., 1999; Yang et al., 1999; Ye et al., 1999). Our study, therefore, could provide a potential mechanism for the actions of phytochemicals through targeting of the F0F1-ATPase/ATP synthase.
Figure 1.
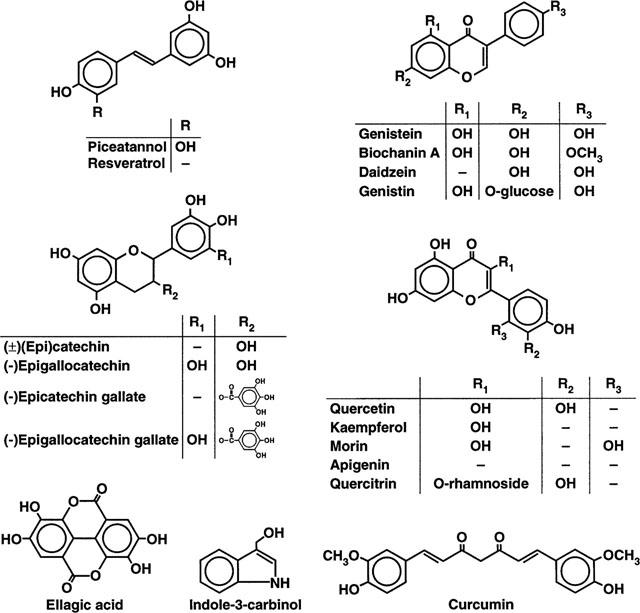
Structures of selected phytochemicals tested for their inhibitory effects on the rat mitochondrial F0F1-ATPase/ATP synthase activity. Many have been shown to be phytoestrogens, such as stilbenes, isoflavones and flavones.
Methods
Materials
4-(2-Aminoethyl)-benzenesulphonyl fluoride (AEBSF), an irreversible serine protease inhibitor, was from Boehringer Mannheim (Indianapolis, IN, U.S.A.). Oligomycin (mixture of A, B and C) was purchased from Aldrich (Milwaukee, WI, U.S.A.) and prepared in methanol as a stock solution (1 μg μl−1). Efrapeptin (efrastatin, A23871) was kindly provided by Dr J. Clemens (Eli Lilly & Co., Indianapolis, IN, U.S.A.) and dissolved in sterile distilled water at a concentration of 0.1 mM. IH636 Grape seed proanthocyanidin (GSPE) was kindly provided by Dr. D. Bagchi (InterHealth Nutraceuticals, Concord, CA, U.S.A.) as a mixture of proanthocyanidins (>54% dimeric, 13% trimeric, 6.8% tetrameric and small amount of monomeric and other high molecular weight oligomeric proanthocyanidin complexes [OPC]). All other chemicals and reagents, including porcine cortex Na+/K+-ATPase were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Ouabain (stock solution of 5 μg μl−1) was dissolved in sterile distilled water. Phytochemicals and diethylstilbestrol were usually prepared in 100% ethanol with a final concentration of 10 mM or above, except for daidzein, phloridzin and GSPE, which were prepared in 50% ethanol/50% dimethyl sulphoxide. The final solvent concentrations in the reaction solution were usually 0.7% and occasionally 1.4%. The amount of solvents had minimal effect on the responses (<5%). Nevertheless, in every experiment, control trials were performed with the same amount of vehicle included.
Animals
Adult female Sprague-Dawley rats (60–120 days old) were maintained on a 14:10 h light/dark cycle (lights on at 0700 h) with food and water available ad libitum. Animals were taken care of in accordance with federal and institutional guidelines and killed by rapid decapitation.
Preparation of mitochondria
Mitochondria fractions from whole brain and liver of adult Sprague-Dawley female rats (unknown oestrous cycle) were prepared in Tris buffer (mM: Tris-HCl 50, NaCl 120, KCl 5, MgSO4 1, CaCl2 1, 10% glycerol, AEBSF 0.5, bacitracin 0.1; pH 7.4 at 4°C), as described previously (Zheng & Ramirez, 1999a). Briefly, tissues were homogenized in Tris buffer (10 ml per g tissue) with a Teflon glass homogenizer (10 strokes in 1 min). Homogenates were centrifuged at 600×g for 10 min and the resulting supernatant was centrifuged again at 15,000×g for 5 min to precipitate the mitochondrial fraction. Samples were assayed for protein concentration by the method of Bradford (1976), using bovine serum albumin as the standard, and either kept at 4°C for use the same day or stored at −80°C.
Solubilization
Mitochondrial fractions were solubilized in Tris buffer containing 1% w v−1 digitonin (Zheng & Ramirez, 1999a) or 1% 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propane sulphonate (CHAPS) at 4°C as described (McEnery et al., 1984). Solubilization allows the exposure of F0F1-ATPase, which is not accessible to many reagents in a coupled assay (see below), in intact mitochondria.
Preparation of submitochondrial particles
Submitochondrial particles were prepared by sonication as described (Ragan et al., 1987). Briefly, freshly prepared brain mitochondrial fractions were sonicated for 6×10 s and centrifuged at 15,000×g for 5 min at 4°C. The supernatant was centrifuged again at 125,000×g for 60 min; the resulting pellet (submitochondrial particles) was resuspended in Tris buffer and used within 24 h.
Assay for ATPase activity
The mitochondrial F0F1-ATPase activity was measured spectrophotometrically at 340 nm by coupling the production of ADP to the oxidation of NADPH via the pyruvate kinase and lactate dehydrogenase reaction (coupled assay), as described (Zheng & Ramirez, 1999b). The reaction mixture (0.7 ml final volume) contained (in mM): Tris 100 (pH 8.0), Mg-ATP 4, MgCl2 2, KCl 50, EDTA 0.2, NADH 0.23, phosphoenol pyruvate 1, 1.4 unit pyruvate kinase, 1.4 unit lactate dehydrogenase and about 25–50 μg proteins, and was assayed at 30–31°C. The activity of the Na+/K+-ATPase from porcine cerebral cortex was measured similarly by the coupled assay as described above except that 100 mM NaCl was included. In a few cases, the F0F1-ATPase activity was also measured directly from the release of inorganic phosphate (Pi) from ATP as described (Harris, 1987). In this case the reaction solution (0.7 ml) contained (in mM): Tris 100, (pH 8.0), Mg-ATP 4, MgCl2 2, KCl 50, EDTA 0.2 and 25–50 μg proteins. The specific F0F1-ATPase activity in all cases was determined in the presence of the enzyme inhibitors, oligomycin or efrapeptin (Linnett & Beechey, 1979). Ouabain was used to determine the specific Na+/K+-ATPase activity.
To study possible effects of phytochemicals on the other enzymes used in the coupled assay of ATPase, i.e. pyruvate kinase and lactate dehydrogenase, ATP was omitted from the buffer and the reaction was started by adding 0.2 mM ADP.
Assay for ATP synthesis
ATP synthesis was measured by monitoring the increase in absorbance at 340 nm using an NADP+-linked, ADP-regenerating system (Cross & Kohlbrenner, 1978). The reaction mixture (0.7 ml final volume) contained (mM): HEPES 10 (pH 8.0), succinate 20, glucose 20, MgCl2 3, AMP 11, NADP+ 0.75, ADP 1, Pi 10, 4 u ml−1 hexokinase and 2 u ml−1 glucose-6-phosphate dehydrogenase. The reaction was assayed at 30–31°C and started by adding 20 μl of pre-incubated (5 min at 26°C) submitochondrial particles (about 50–61 μg protein) in 20 mM succinate. A high AMP concentration (11 mM) was used in this assay to inhibit the adenylyl kinase activity present in the submitochondrial particle preparations, which might interfere with measurement of respiration-dependent ATP synthesis by the ATP synthase. The preparations had ATP synthase activity of 0.057–0.083 μmol min−1 (mg protein)−1.
To exclude the possible effects of resveratrol on hexokinase and glucose-6-phosphate dehydrogenase used in the assay, the response induced by 140 μM ATP in the absence of submitochondrial particles were tested; these were not significantly affected.
Data analysis
To obtain reaction rates, the steady-state linear range of the absorbance change with respect to time was used for both ATPase and ATP synthase activity. A molar extinction coefficient of 6.22×103 M cm−1 was used for NAD(P)H to calculate the activity in μmol ATP hydrolysed or synthesized per min per mg protein. The responses were very stable as judged from control trials performed before, during and after the experiments (less than 4% variations). Data from three or more experiments were expressed as means (standard deviation (s.d.)). Statistical analysis was performed using Student's t-test to compare two groups and analysis of variance (ANOVA) with post-hoc Tukey test for comparison of three or more groups. P<0.05 was considered significant. For concentration-dependent effects of resveratrol with an experimental number of 3, IC50 values are quoted as geometric mean with 95% confidence interval (c.i., based on t distribution).
Results
Inhibition of rat F0F1-ATPase/ATP synthase by resveratrol
Mitochondrial fractions prepared from both female rat brains and livers were solubilized with 1% digitonin or 1% CHAPS to obtain functional solubilized mitochondrial fractions containing high F0F1-ATPase activity with little Na+/K+-ATPase activity in a coupled ATPase activity assay (Zheng & Ramirez, 1999b). Resveratrol, a phytoalexin present in high concentrations in red wine (usually 10–50 μM) and grapes, rapidly (within 1–2 min) inhibited the brain mitochondrial F0F1-ATPase activity in a concentration-dependent fashion in the range 0.7–70 μM, with IC50 values of 18.5 μM (geometric mean, 95% c.i. 16.7–20.6 μM; n=3) and 13.0 μM (8.80–19.3 μM; n=3) for digitonin-solubilized and CHAPS-solubilized preparations, respectively (Figure 2A,B). With 0.7 μM of resveratrol, the inhibition was already significant (5–7% inhibition, P<0.01). Similar results were obtained with both digitonin- and CHAPS-solubilized liver mitochondrial preparations (IC50 of 12 and 21 μM, respectively). The inhibitory effect of resveratrol appears to be specific to F0F1-ATPase given that this effect is blocked by oligomycin, an F0F1-ATPase/ATP synthase inhibitor (Linnett & Beechey, 1979). In the presence of 7 μg ml−1 oligomycin alone, the residual ATP hydrolysis activity in the digitonin-solubilized preparation was 16.1 (0.3)% (mean(s.d.); n=3) when the control activity was assumed to be 100%. In the presence of both 7 μg ml−1 oligomycin and 21 μM resveratrol, the residual activity was 15.8 (0.4)% (n=3). Efrapeptin, a specific inhibitor of the F0F1-ATPase/ATP synthase (Linnett & Beechey, 1979), completely inhibited the enzyme activity at 1–2 μM (Figure 2B). Therefore, the effect of resveratrol must be on the oligomycin and efrapeptin-sensitive ATPase (i.e. F0F1-ATPase) activity of this preparation, which corresponded to an average of 83.5 (2.2)% (n=7) of the total ATP hydrolysis activity. Resveratrol itself did not have any effect on the activity of the other enzymes, i.e. pyruvate kinase and lactate dehydrogenase, used in the ATPase activity assay because, in the absence of mitochondrial preparations, 21 μM resveratrol did not affect the ADP-induced NADH oxidation. In the presence of 21 μM resveratrol, the ADP-induced response was 100.2 (1.0)% (n=3) of control.
Figure 2.
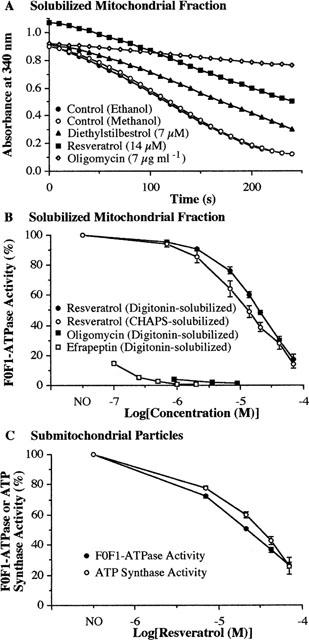
Effects of resveratrol on F0F1-ATPase/ATP synthase activity of rat brain mitochondrial preparations. (A) Typical examples of spectrophotometric read-out showing the absorbance change at 340 nm induced by 20 μl digitonin-solubilized brain mitochondrial preparation (51 μg protein) in the presence of ethanol vehicle, methanol vehicle, 7 μg ml−1 oligomycin in methanol, 7 μM diethylstilbestrol in ethanol and 14 μM resveratrol in ethanol. Resveratrol itself and, to a lesser degree, diethylstilbestrol and oligomycin, had extinction at 340 nm, so the starting point had higher absorbance. Diethylstilbestrol was included here as a comparison. Oligomycin and efrapeptin (see below and text) were included for determination of the F0F1-ATPase activity in the preparations. (B) The concentration-dependent effect of 0.7–70 μM resveratrol on digitonin- and CHAPS-solubilized rat brain mitochondrial preparations. Data are expressed as means (s.d.) from three experiments. Also shown is the concentration-dependent effect of oligomycin and efrapeptin. For studying the effect of efrapeptin on ATPase activity, efrapeptin was pre-incubated at 26°C with the solubilized preparation for 6 min to allow for binding. The F0F1-ATPase activity in 0.7% vehicle (this and following figures) was defined as 100%. (C) Effect of resveratrol on ATP synthesis and hydrolysis catalysed by rat brain submitochondrial preparations. F0F1-ATPase activity of rat brain submitochondrial preparations was determined similarly as solubilized mitochondrial preparation except that 2 μg ml−1 antimycin A was present. Data are expressed as means (s.d.) from three experiments. The F0F1-ATPase/ATP synthase activity in vehicle was defined as 100%.
To examine the effects of resveratrol on ATP synthesis by the F0F1-ATPase/ATP synthase we used a brain submitochondrial fraction. In the presence of 11 mM AMP the brain submitochondrial particles contained little adenylate kinase activity: more than 95% of the activity corresponded to ATP synthase as shown in the presence of 2 μM efrapeptin (data not shown). Resveratrol inhibited ATP synthesis with an IC50 of 27.7 μM (22.0–34.8 μM; Figure 2C) and had no effect on the other enzymes used in the assay. Resveratrol also inhibited the F0F1-ATPase activity of the submitochondrial particles with an IC50 of 21.6 μM (20.6–22.8 μM; Figure 2C). Therefore, it seems that resveratrol inhibited both ATP synthesis and hydrolysis by the enzyme, but the effect on the ATP synthase activity is slightly less than on the ATPase activity.
Direct measurement of Pi release from ATP catalysed by digitonin-solubilized brain mitochondrial preparation indicated that 70 μM resveratrol inhibited the oligomycin and efrapeptin-sensitive F0F1-ATPase activity by 84.6%, a value similar to that determined by the coupled assay.
Differential inhibition of F0F1-ATPase by polyphenolic phytochemicals
We further screened other phenolic phytochemicals for their effects on the activity of the ATPase in digitonin-solubilized rat brain mitochondrial preparation (Figure 3, Table 1). Three isoflavones, genistein, biochanin A and daidzein–phytoalexins with protein anti-tumour effects–inhibited the ATPase activity with IC50 values of 55–127 μM (Figure 3A, Table 1). However, the 7-glucose derivative of genistein (genistin) was essentially without effect at concentrations up to 140 μM. The isoflavone compounds had little effect on ADP-induced responses in the absence of the mitochondrial preparations (<10% effect at 70 μM) and, therefore, their actions are due to effects on the ATPase. Genistein and biochanin A, like resveratrol, did not significantly affect the oligomycin-insensitive ATP hydrolysis activity. In the presence of 7 μg ml−1 oligomycin, 15.3% residual ATP hydrolysis activity remained. When 70 μM genistein or biochanin A was added in the presence of 7 μg ml−1 oligomycin, the residual ATP hydrolysis activity was 13.4 and 12.9%, respectively. We also tested the effects of genistein on ATP synthase activity and found that at 70 μM it also inhibited the enzymatic activity by 62%. Therefore, like resveratrol, genistein inhibits both ATPase and ATP synthase activity of the enzyme.
Figure 3.
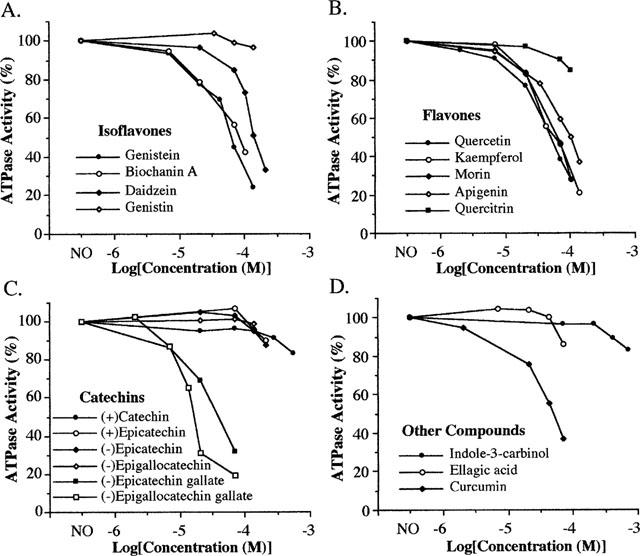
Concentration-dependent effects of selected phytochemicals on total ATPase activity of digitonin-solubilized rat brain mitochondrial preparations. These compounds include isoflavones (A), flavones (B), catechins (C) and several others (D). Experiments were conducted as in Figure 2. Control experiments indicated that (−)epicatechin-gallate, tannic acid and several flavonoids affected ADP-induced responses, i.e. pyruvate kinase or lactate dehydrogenase; therefore, the total ATPase activity was used here. The inhibition of ADP-induced responses was from 28 to 76% at 70 μM with kaempferol<apigenin<quercetin<(−)epicatechin gallate<morin. This inhibition, however, will not significantly affect the estimation of IC50 for the ATPase since ATP hydrolysis was the limiting step and the residual oligomycin-insensitive ATPase activity is low (13.4 (1.1)% of total activity). ‘NO' indicates the absence of phytochemicals.
Table 1.
Effect of phytochemicals on the mitochondrial F0F1-ATPase activity
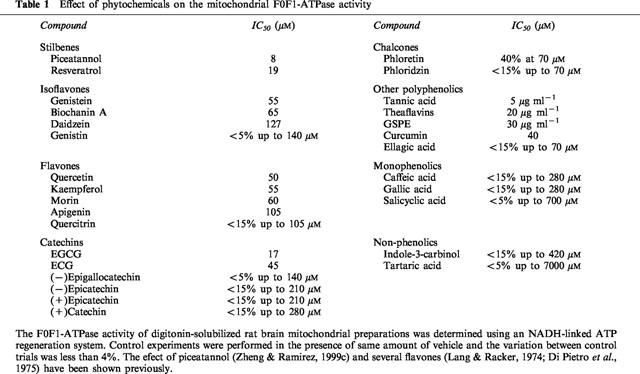
Consistent with earlier findings (Lang & Racker, 1974; Di Pietro et al., 1975), aglycone flavones also inhibited the brain F0F1-ATPase activity with IC50 values of 50–105 μM, while quercitrin had little effect (Figure 3B). When several catechins were tested it was found that diphenolic catechin, epicatechin and epigallocatechin had little effect on the F0F1-ATPase activity, while the gallate esters, including (−)epigallocatechin gallate (EGCG) and (−)epicatechin gallate (ECG) are potent inhibitors (Figure 3C). Other active inhibitors of F0F1-ATPase include curcumin (Figure 3D), phloretin, tea theaflavins, tannic acid and GSPE (Table 1). Ellagic acid had a modest inhibitory effect on F0F1-ATPase activity (14% at 70 μM) (Figure 3D). Monophenolic compounds, such as caffeic acid, gallic acid and salicylic acid, and nonphenolic compounds, such as indole-3-carbinol (I3C) and tartaric acid, had little effect at similar concentrations (Table 1).
Kinetic mechanism of inhibition by resveratrol and genistein
We further examined the effects of resveratrol and genistein on the ATP-dependence of the inhibition of F0F1-ATPase. As shown in Figure 4, the control Lineweaver-Burk plot revealed maximal specific activity (Vmax) for this preparation of 0.834 μmol ATP hydrolysed min−1 (mg protein)−1 with Michaelis constant (Km) of 0.344 mM. In the presence of resveratrol (7 μM), Vmax was drastically reduced to 0.370 μmol ATP hydrolysed min−1 (mg protein)−1. Km was also reduced by 20% (0.277 mM) in the presence of 7 μM resveratrol, suggesting that resveratrol results in mixed inhibition of F0F1-ATPase. Genistein (50 μM) also reduced the Vmax to 0.580 μmol min−1 (mg protein)−1, while Km was relatively unchanged (0.331 mM; 3.8% decrease), suggesting that genistein behaves like a classical non-competitive inhibitor.
Figure 4.
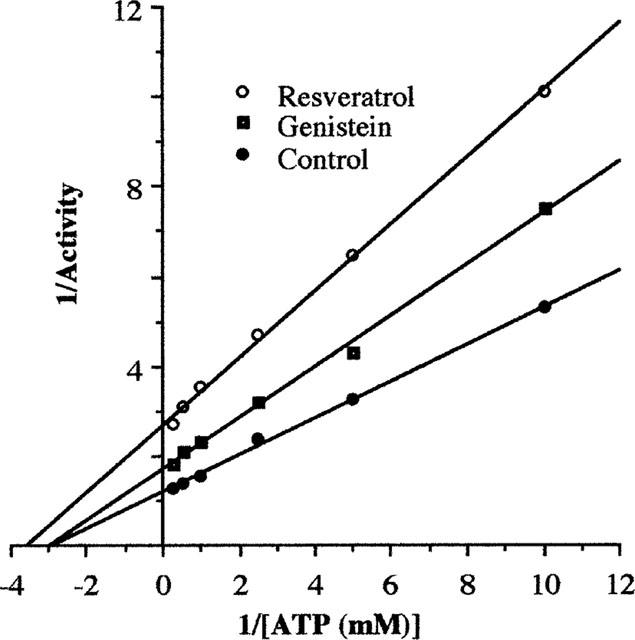
Inhibition of F0F1-ATPase of digitonin-solubilized brain mitochondrial preparation by resveratrol (7 μM) and genistein (50 μM) at several concentrations of 0.1–4 mM ATP as shown by Lineweaver–Burk plots. The linear correlation coefficients for all three groups are between 0.996 and 0.999. Note the different Vmax among the control, resveratrol and genistein, and the change in Km induced by resveratrol. The control F0F1-ATPase activity of the preparation at 4 mM ATP was 0.797 (0.007) μmol ATP hydrolysed min−1 (mg protein)−1 from three trials performed before, during and after the experimental trials.
Additive effect of polyphenolic compounds
To determine if resveratrol and other phytochemicals additively contribute to the inhibitory effect of dietary phytochemicals on F0F1-ATPase, we examined the effect of a combination of resveratrol with other active inhibitors at suboptimal concentrations. A combination of suboptimal concentrations of resveratrol, quercetin and kaempferol, which are present in red wine, had an additive inhibitory effect resulting in 56% inhibition as compared to single compounds alone (23, 19 and 17% inhibition for 7 μM resveratrol, 21 μM quercetin and 21 μM kaempferol, respectively) (P<0.001). Similar additive effects were found for resveratrol and genistein, two compounds that are usually not present in the same diet; the combination of 11 μM resveratrol and 35 μM genistein resulted in 58% inhibition as compared to 36% with resveratrol and 26% with genistein alone (P<0.001). Therefore, the presence of these or other active compounds in diet could potentially act together to alter the enzymatic activity of the F0F1-ATPase in vivo with considerably lower concentrations of individual compounds.
Lack of effect of resveratrol and genistein on Na+/K+-ATPase
To determine if resveratrol and genistein inhibit the activity of other ATPases, we examined their effect on Na+/K+-ATPase from porcine cerebral cortex. Resveratrol and genistein had little effect on this enzyme's activity (Figure 5). Therefore, the stilbene resveratrol and the isoflavone genistein are rather specific in their actions on mitochondrial ATPase/ATP synthase, unlike flavones such as quercetin, which were shown to affect many ATPases, including Na+/K+-ATPase (Figure 5) and several other enzymes such as 5′-nucleotidase, phosphorylase kinase and protein kinase C, while genistein did not (Akiyama et al., 1987).
Figure 5.
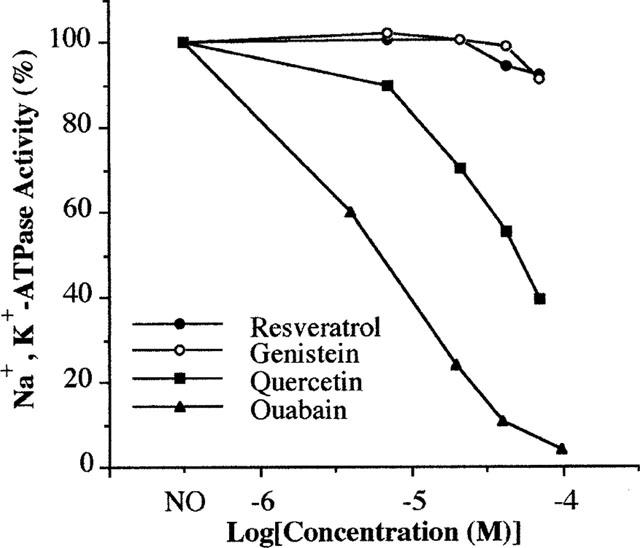
Effects of resveratrol, genistein and quercetin on porcine cerebral cortex Na+/K+-ATPase. Each point corresponds to a single trial except for control experiments, which were conducted at least three times with variation less than 3%.
Discussion
By using both rat brain and liver mitochondrial preparations, we have tested several groups of naturally occurring polyphenolic compounds on the activity of rat mitochondrial F0F1-ATPase/ATP synthase. Our studies demonstrate that F0F1-ATPase/ATP synthase is a common target site for resveratrol from red wine, aglycone isoflavones (genistein, biochanin A and daidzein) from soybean, gallate esters of catechins from many sources, and several other polyphenolic compounds at high nanomolar to low micromolar concentrations similar to those used in many biological studies (e.g. Barnes & Peterson, 1995; Jang et al., 1997; Yang et al., 1999). The effect of resveratrol is consistent with recent demonstration of its inhibitory effect on the ATPase activity of intact rat brain mitochondria (Zini et al,. 1999). Our recent studies indicate that resveratrol, like piceatannol and quercetin, inhibits the F0F1-ATPase activity by targeting F1, while genistein most likely targets F0 (Zheng & Ramirez, 1999c). Therefore, there are two potential binding sites in ATP synthase for polyphenolic phytochemicals. Interaction of phytochemicals with these binding sites could be a novel mechanism for the actions of these phytochemicals.
Comparison of several groups of polyphenolic phytochemicals indicate the importance of hydroxyl groups in particular positions. For example, three isoflavones–genistein, biochanin A and daidzein–inhibited the ATPase activity with IC50 values between 55 and 127 μM, while the 7-glucose derivative of genistein (genistin) was essentially without effect up to 140 μM. This, together with the fact that all three active isoflavones contain a 7-OH group, suggests that the 7-OH group is essential for the inhibitory effect of isoflavones. Omission of the 5-OH group in daidzein (IC50=127 μM) resulted in lower activity in comparison with genistein (IC50=55 μM). However, modification of the 4′-OH group to 4′-OCH3 in biochanin A (IC50=65 μM) seems to have relatively little effect. It seems that a meta-quinonic structure could be essential for the inhibitory activity. Similarly to genistin, the replacement of quercetin's R1-OH group by O-rhamnoside in quercitrin also resulted in much lower inhibitory activity. On the other hand, addition of a phenolic group in catechins converted inactive catechins to active catechins (the gallate esters, EGCG and ECG). The lack of inhibitory activity of monophenolic compounds tested suggests that the inhibition of F0F1-ATPase by phenolic phytochemicals requires two or more phenolic structures.
The levels of several phytochemicals in human body fluids have been determined. For instance, both genistein and daidzein (both free and conjugated forms) reached up to 7 μM in human plasma after soy meals (Xu et al., 1995; Watanabe et al., 1998; King & Bursill, 1998). Administration of decaffeinated green tea could result in maximal plasma concentrations of 0.7 and 1.8 μM in human volunteers for EGCG and EGC, respectively (Yang et al., 1999). However, the levels of these compounds in saliva could reach 10–48 and 38–143 μM, respectively (Yang et al., 1999). These data indicate that much higher concentrations of phytochemicals are present in the oral cavity, and likely in the oesophagus, and that they could be important for the application of tea in the prevention of oral and oesophageal cancers (Yang et al., 1999). Many of these polyphenolic phytochemicals are present in the same plant or food source or may be taken together. For example, red wine contains resveratrol, quercetin, kaempferol and catechins. Therefore, it is important to know that the effects of these compounds on ATPase are additive. This may explain why several brands of red wine and red grape juice, but not white wine, beer or ethanol, inhibit the ATPase activity with IC50 values of 0.7–1.4% v v−1 (unpublished data). The lack of effect of white wine on F0F1-ATPase activity is consistent with several earlier studies showing that red wine and grape juice, but not white wine, have cardioprotective and tumour-inhibiting actions in animal models (Demrow et al., 1995) and humans (Furhman et al., 1995; Serafini et al., 1998). Total polyphenolics, including resveratrol and proanthocyanidins, are several-fold lower in white wine than in red wine (Goldberg et al., 1995; Watkins, 1997) which may explain different effects of red and white wine.
Many of these polyphenolic phytochemicals are weak oestrogens (phytoestrogens). For example, resveratrol is a weaker oestrogen receptor α form agonist, having an effect only when its concentrations are above 3 μM (3–30 μM with IC50=10 μM) (Gehm et al., 1997). The aglycone isoflavones are more potent phytoestrogens than resveratrol, with their affinity for oestrogen receptor α and β forms in the following rank order: genistein>daidzein>biochanin A (Kuiper et al., 1998). While biochanin A had a similar inhibitory effect as genistein on F0F1-ATPase activity (Table 1) and tumour growth (Barnes & Peterson, 1995), it has 400 to 8700 fold lower affinity than genistein for oestrogen receptor α and β forms (Kuiper et al., 1998). Therefore, it is likely that oestrogen receptors are not involved in anti-growth actions of these phytochemicals on cultured cancer cells and that the structure-activity relationship for F0F1-ATPase/ATP synthase inhibition by these phytochemicals is different from that for affinity at oestrogen receptors.
Besides its oestrogen receptor-mediated actions, genistein also inhibits protein tyrosine kinases, while biochanin A and daidzein are at least 40 times less active than genistein (Akiyama et al., 1987). However, several earlier studies indicated that the tyrosine kinase was not required for the inhibitory effect of genistein on tumour growth (Barnes & Peterson, 1995; Stevens et al., 1994; Shao et al., 1998), nor for biochanin A and daidzein. Other mechanisms have been suggested, including the inhibition of cyclo-oxygenase-1 (Jang et al., 1997) and cytochrome P450 1A1 (Ciolino & Yeh, 1999; Chun et al., 1999) by resveratrol, and inhibition of 5α-reductase by genistein and biochanin A (Evans et al., 1995). However, the involvement of these and other mechanisms in both cardioprotection and tumour inhibition by diphenolic phytochemicals is unclear.
Several flavones have been shown to interact with other ATPases, such as Ca2+-ATPase (McKenna et al., 1996) and Na+/K+-ATPase (Hirano et al., 1989), in addition to their effects on F0F1-ATPase (Lang & Racker, 1974; Di Pietro et al., 1975). However, resveratrol and genistein had little effect on Na+/K+-ATPase, as shown in Figure 5. On the other hand, ellagic acid has been shown to increase the activity of Ca2+-ATPase and Ca2+ uptake in cardiac sarcoplasmic reticulum (EC50=10 μM) (Antipenko et al., 1999). However, ellagic acid has little effect on F0F1-ATPase of rat brain solubilized mitochondrial preparation at similar concentrations.
Our findings indicate that inhibition of mitochondrial F0F1-ATPase/ATP synthase could be a potential mechanism contributing to the many effects of dietary polyphenolics. It has been shown that the same ATP synthase is localized also on the plasma membranes of several tumour cell lines (Das et al., 1994) and human umbilical vein endothelial cells, and that it is a binding protein for angiostatin, a potent protein inhibitor of angiogenesis and cell proliferation (Moser et al., 1999). Therefore, phytoestrogens could also play a similar role by binding the ATP synthase on the plasma membrane. In fact, genistein has been shown to be a potent inhibitor of angiogenesis (Fotsis et al., 1993). Moreover, resveratrol and genistein also inhibit the bovine mitochondrial NADH:ubiquinone oxidoreductase and induce ornithine decarboxylase, a marker for cancer chemopreventive potency, with IC50=30 μM (Fang & Casida, 1998). The simultaneous effects of these phytochemicals on NADH:ubiquinone oxidoreductase and F0F1-ATPase/ATP synthase could significantly affect mitochondrial function and alter ATP level, mitochondrial transmembrane potential and generation of reactive oxygen species, which have been implicated in many cellular processes such as cellular protection, apoptosis, O2 sensing and ageing (Wallace, 1999). Further examinations of the effects of the phytochemicals on mitochondrial functions will, therefore, be necessary to identify precisely the mechanisms involved.
Acknowledgments
We should like to thank Dr K.E. Kwast of the Department of Molecular and Integrative Physiology and Dr W.G. Helferich of the Department of Food Science and Human Nutrition at the University of Illinois at Urbana-Champaign for critical reading and comments, Dr G. Dent of University of Southampton School of Medicine, U.K. for his advice and help in statistical analysis of data, Dr J. Clemens of Eli Lilly & Co., Indianapolis IN, for efrapeptin, and Dr D. Bagchi of InterHealth Nutraceuticals, Concord, CA, for GSPE. This work was supported by an NIH grant to V.D. Ramirez.
Abbreviations
- AEBSF
4-(2-aminoethyl)-benzenesulphonyl fluoride
- CHAPS
(3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulphonate
- c.i.
confidence interval
- ECG
(−)epicatechin gallate
- EGCG
(−)epigallocatechin gallate
- GSPE
grape seed proanthocyanidin extract
- I3C
indole-3-carbinol
- Km
Michaelis constant
- OPC
oligomeric proanthocyanidin complex
- OSCP
oligomycin sensitivity-conferring protein
- Pi
inorganic phosphate
- Vmax
maximal specific activity
References
- ABRAHAMS J.P., BUCHANAN S.K., VAN RAAJJ M.J., FEARNLEY I.M., LESLIE A.G.W., WALKER J.E. The structure of bovine F1-ATPase complexed with the peptide antibiotic efrapeptin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9420–9424. doi: 10.1073/pnas.93.18.9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLERCREUTZ H. Phytoestrogens: epidemiology and a possible role in cancer protection. Environ. Health Perspect. 1995;103:103–112. doi: 10.1289/ehp.95103s7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S.-I., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- ANTIPENKO A.Y., SPIELMAN A.I., KIRCHBERGER M.A. Interactions of 6-gingerol and ellagic acid with the cardiac sarcoplasmic reticulum Ca2+-ATPase. J. Pharmacol. Exp. Ther. 1999;290:227–234. [PubMed] [Google Scholar]
- BARNES S., PETERSON T.G. Biochemical targets of the isoflavone genistein in tumor cell lines. Proc. Soc. Exp. Biol. Med. 1995;208:103–108. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- BOYER P.D. The ATP synthase–a splendid molecular machine. Ann. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRAVO L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- CHUN Y.J., KIM M.Y., GUENGERICH F.P. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem. Biophys. Res. Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- CIOLINO H.P., YEH G.C. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol. Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- CROSS R.L., KOHLBRENNER W.E. The mode of inhibition of oxidative phosphorylation by efrapeptin (A23871) J. Biol. Chem. 1978;253:4865–4873. [PubMed] [Google Scholar]
- DAS B., MONDRAGON M.O.H., SADEGHIAN M., HATCHER V.B., NORIN A.J. A novel ligand in lymphocyte-mediated cytotoxicity: expression of the β subunit of H+ transporting ATP synthase on the surface of tumour cell lines. J. Exp. Med. 1994;180:273–281. doi: 10.1084/jem.180.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASHWOOD R.H. Indole-3-carbinol: anticarcinogen or tumor promoter in brassica vegetables. Chem. Biol. Interact. 1998;110:1–5. doi: 10.1016/s0009-2797(97)00115-4. [DOI] [PubMed] [Google Scholar]
- DEMROW H.S., SLANE P.R., FOLTS J.D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- DEWICK P.M. Medicinal Natural Products: a Biosynthetic Approach. John Wiley & Sons, Chichester; 1997. [Google Scholar]
- DI PIETRO A., GODINOT C., BOUILLANT M.-L., GAUTHERON D.C. Pig heart mitochondrial ATPase: properties of purified and membrane-bound enzyme. Biochimie. 1975;57:959–967. doi: 10.1016/s0300-9084(75)80218-5. [DOI] [PubMed] [Google Scholar]
- EVANS B.A., GRIFFITHS K., MORTON M.S. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary ligans and isoflavonoids. J. Endocrinol. 1995;147:295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- FANG N., CASIDA J.E. Anticancer action of cube insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3380–3384. doi: 10.1073/pnas.95.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRELL J.E., SING P.D.G.C., LOEW G., KING R., MANSOUR J.M., MANSOUR T.E. Structure/activity studies of flavonoids as inhibitors of cyclic AMP phosphodiesterase and relationship to quantum chemical indices. Mol. Pharmacol. 1979;16:556–568. [PubMed] [Google Scholar]
- FEWTRELL C.M.S., GOMPERTS B.D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature. 1977;265:635–636. doi: 10.1038/265635a0. [DOI] [PubMed] [Google Scholar]
- FOTSIS T., PEPPER M., ADLERCREUTZ H., FLEISCHMANN G., HASE T., MONTESANO R., SCHWEIGERER L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKEL E.N., WATERHOUSE A.L., KINSELLA J.E. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- FURHMAN B., LAVY A., AVIRAM M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995;61:549–554. doi: 10.1093/ajcn/61.3.549. [DOI] [PubMed] [Google Scholar]
- GEHM B.D., MCANDREWS J.M., CHIEN P.-Y., JAMESON J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG D.M., YAN J., NG E., DIAMANDIS E.P., KARUMANCHIRI A., SOLEAS G., WATERHOUSE A.L. A global survey of trans-resveratrol concentrations in commercial wines. Am. J. Enol. Vitic. 1995;46:159–165. [Google Scholar]
- HARRIS D.A. Spectrophotometric assays Spectrophotometry and Spectrofluorimetry: a Practical Approach 1987Oxford: IRL Press; 49–90.ed. Harris, D.A. & Bashford, C.L. pp [Google Scholar]
- HIRANO T., OKA K., AKIBA M. Effects of synthetic and naturally occurring flavonoids on Na+, K+-ATPase: aspects of the structure-activity relationship and action mechanism. Life Sci. 1989;45:1111–1117. doi: 10.1016/0024-3205(89)90168-9. [DOI] [PubMed] [Google Scholar]
- HOLLMAN P.C., FESKENS E.J.M., KATAN M.B. Tea flavonols in cardiovascular disease and cancer epidemiology. Proc. Soc. Exp. Biol. Med. 1999;220:198–202. doi: 10.1046/j.1525-1373.1999.d01-33.x. [DOI] [PubMed] [Google Scholar]
- HSIEH C.-Y., SANTELL R.C., HASLAM S.Z., HELFERICH W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- JANG M., CAI L., UDEANI G.O., SLOWING K.V., THOMAS C.F., BEECHER C.W., FONG H.H., FARNSWORTH N.R., KINGHORD A.D., MEHTA R.G., MOON R.C., PEZZUTO J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- KING R.A., BURSILL D.B. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy mean in humans. Am. J. Clin. Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- KUIPER G.G.J.M., LEMMEN J.G., CARLSSON B., CORTON J.C., SAFE S.H., VAN DER SAAG P.T., VAN DER BURG B., GUSTAFSSON J.-A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- LANG D.R., RACKER E. Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim. Biophys. Acta. 1974;333:180–186. doi: 10.1016/0005-2728(74)90002-4. [DOI] [PubMed] [Google Scholar]
- LINNETT P.E., BEECHEY R.B. Inhibitors of the ATP synthetase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- MATSUYAMA S., XU Q., VELOURS J., REED J.C. The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- MCENERY M.W., BUHLER E.L., Jr, AEBI U., PEDERSEN P.L. Proton ATPase of rat liver mitochondria: preparation and visualization of a functional complex using the novel zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propansulfonate. J. Biol. Chem. 1984;259:4642–4651. [PubMed] [Google Scholar]
- MCENERY M.W., HULLIHEN J., PEDERSEN P.L. F0 ‘proton channel' of rat liver mitochondria: rapid purification of a functional complex and a study of its interaction with the unique probe diethylstilbestrol. J. Biol. Chem. 1989;264:12029–12036. [PubMed] [Google Scholar]
- MCENERY M.W., PEDERSEN P.L. Diethylstilbestrol: a novel F0-directed probe of the mitochondrial proton ATPase. J. Biol. Chem. 1986;261:1745–1752. [PubMed] [Google Scholar]
- MCKENNA E., SMITH J.S., COLL K.E., MAZACK E.K., MAYER E.J., ANTANAVAGE J., WIEDMANN R.T., JOHNSON R.G. Dissociation of phospholamban regulation of cardiac sarcoplasmic reticulum Ca2+ ATPase by quercetin. J. Biol. Chem. 1996;271:24517–24525. doi: 10.1074/jbc.271.40.24517. [DOI] [PubMed] [Google Scholar]
- MOSER T.L., STACK M.S., ASPLIN I., ENGHILD J.J., HOJRUP P., EVERITT L., HUBCHAK S., SCHNAPER H.W., PIZZO S.V. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURKIES A.L., WILCOX G., DAVIS S.R. Clinical review 92: phytoestrogens. J. Clin. Endocrinol. Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- PEDERSEN P.L., AMZEL L.M. ATP synthase: structure, reaction center, mechanism, and regulation of one of nature's most unique machines. J. Biol. Chem. 1993;268:9937–9940. [PubMed] [Google Scholar]
- RAGAN C.I., WILSON M.T., DARLEY-USMAR V.M., LOWE P.N. Subfractionation of mitochondria and isolation of the proteins of oxidative phosphorylation Mitochondria: a Practical Approach 1987Oxford: IRL Press; 79–112.ed. Darley-Usmar, V.M., Rickwood, D. & Wilson, M.T. pp [Google Scholar]
- SERAFINI M., MAIANI G., FERRO-LUZZI A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J. Nutr. 1998;128:1003–1007. doi: 10.1093/jn/128.6.1003. [DOI] [PubMed] [Google Scholar]
- SETCHELL K.D.R. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- SHAO Z.-M., ALPAUGH M.L., FONTANA J.A., BARSKY S.H. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis. J. Cell. Biochem. 1998;69:44–54. [PubMed] [Google Scholar]
- STEVENS M.F., MCCALL C.J., LELIEVELD P., ALEXANDER P., RICHTER A., DAVIES D.E. Structural studies on bioactive compounds. 23. Synthesis of polyhydroxylated 2-phenylbenzothiazoles and a comparison of their cytotoxicities and pharmacological properties with genistein and quercetin. J. Med. Chem. 1994;37:1689–1695. doi: 10.1021/jm00037a020. [DOI] [PubMed] [Google Scholar]
- THAM D.M., GARDNER C.D., HASKELL W.L. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- VAN RAAJJ M.J., ABRAHAMS J.P., LESLIE A.G.W., WALKER J.E. The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6913–6917. doi: 10.1073/pnas.93.14.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VUORINEN K., YLITALO K., PEUHKURINEN K., RAATIKAINEN P., ALA-RAMI A., HASSINEN I.E. Mechanisms of ischemic preconditioning in rat myocardium: roles of adenosine, cellular energy state, and mitochondrial F1F0-ATPase. Circulation. 1995;91:2810–2818. doi: 10.1161/01.cir.91.11.2810. [DOI] [PubMed] [Google Scholar]
- WALLACE D.C. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- WATANABE S., YAMAGUCHI M., SOBUE T., TAKAHASHI T., MIURA T., ARAI Y., MAZUR W., WAHALA K., ADLERCREUTZ H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J. Nutr. 1998;128:1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- WATKINS T.R. Wine: Nutritional and Therapeutic Benefits. American Chemical Society, Washington, DC; 1997. [Google Scholar]
- WOLVETANG E.J., JOHNSON K.L., KRAUER K., RALPH S.J., LINNANE A.W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339:40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- XU X., HARRIS K.S., WANG H.J., MURPHY P.A., HENDRICH S. Bioavailability of soybean isoflavones depends upon gut microflora in women. Nutr. 1995;125:2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- YANG C.S., KIM S., YANG G.-Y., LEE M.-J., LIAO J., CHUNG J.Y., HO C.-T. Inhibition of carcinogenesis by tea: bioavailability of tea polyphenols and mechanisms of actions. Proc. Soc. Exp. Biol. Med. 1999;220:213–217. doi: 10.1046/j.1525-1373.1999.d01-36.x. [DOI] [PubMed] [Google Scholar]
- YE X., KROHN R.L., LIU W., JOSHI S.S., KUSZYNSKI C.A., MCGINN T.R., BAGCHI M., PREUSS H.G., STOHS S.J., BAGCHI D. The cytotoxic effects of a novel IH 636 grape seed proanthocyanidin extract on culture human cancer cells. Mol. Cell. Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- ZHENG J., RAMIREZ V.D. Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J. Steroid Biochem. Mol. Biol. 1999a;68:65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- ZHENG J., RAMIREZ V.D. Rapid inhibition of rat brain mitochondrial proton F0F1-ATPase activity by estrogens: comparison with Na+, K+-ATPase of porcine cortex. Eur. J. Pharmacol. 1999b;368:95–102. doi: 10.1016/s0014-2999(99)00012-6. [DOI] [PubMed] [Google Scholar]
- ZHENG J., RAMIREZ V.D. Piceatannol, a stilbene phytochemical, inhibits mitochondrial F0F1-ATPase activity by targeting the F1 complex. Biochem. Biophys. Res. Commun. 1999c;261:499–503. doi: 10.1006/bbrc.1999.1063. [DOI] [PubMed] [Google Scholar]
- ZINI R., MORIN C., BERTELLI A., BERTELLI A.A., TILLEMENT J.P. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp. Clin. Res. 1999;25:87–97. [PubMed] [Google Scholar]


