Abstract
Mice lacking the apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°) develop atherosclerosis and endothelial dysfunction. The aim of this study was to characterize the roles of L-arginine and tetrahydrobiopterin (BH4) for endothelium-dependent relaxation and the changes in the vasoconstrictor response to endothelin-1 (ET-1) in thoracic aortic rings of E°xLDLR° mice.
Histological examination revealed severe atherosclerosis of the thoracic aorta of E°xLDLR° mice. Relaxations induced by acetylcholine (Ach), but not that to sodium nitroprusside, were significantly impaired in E°xLDLR° mice compared to control mice indicating attenuated endothelium-dependent relaxations.
Preincubation with the nitric oxide (NO) substrate L-arginine did not affect, whereas the co-factor for NO synthase, BH4, slightly improved the relaxations induced by Ach. Combined preincubation with L-arginine and BH4 induced a pronounced enhancement of Ach-induced relaxations in E°xLDLR° mice. The relaxations induced by Ach in E°xLDLR° mice in the presence of L-arginine and BH4 were not different from those observed in control mice.
Preincubation with superoxide dismutase did not affect Ach-induced relaxations in aorta from E°xLDLR° mice.
The contractile response to ET-1 was enhanced in E°xLDLR° mouse aorta. The contractions were abolished by the ETA receptor antagonist LU 135252. The ETB receptor agonist sarafotoxin 6c did not induce contractions or relaxations.
It is concluded that endothelial dysfunction of E°xLDLR° mouse aorta is reversed by combined administration of L-arginine and BH4. In addition, the ETA receptor-mediated vasoconstriction by ET-1 is enhanced in E°xLDLR° mice.
Keywords: Apolipoprotein E, atherosclerosis, endothelin, endothelium, nitric oxide, tetrahydrobiopterin, L-arginine, mouse aorta
Introduction
The endothelium plays a crucial role in the regulation of vascular function. Endothelium-derived nitric oxide (NO) is an important factor involved in anti-atherogenic properties of the endothelium by modulating vascular tone and by inhibiting monocyte adhesion and smooth muscle cell proliferation (Moncada et al., 1991; Lüscher & Noll, 1995). It has been well documented that endothelium-dependent vasodilation is impaired in both animal models of atherosclerosis (Freiman et al., 1986; Kolodgie et al., 1990) and in atherosclerotic human coronary arteries (Bossaler et al., 1987; Förstermann et al., 1988; Zeiher et al., 1991). In addition, risk factors for the development of atherosclerosis such as hypercholesterolemia are associated with impaired endothelial function (Creager et al., 1992).
The biological activity of endothelial NO is a balance between the production and inactivation of NO. NO is constitutively produced in vascular endothelial cells from the amino acid L-arginine by the enzyme NO synthase (NOS III) through a five-electron oxidation (Palmer et al., 1988; Marletta et al., 1998). It has been suggested in several studies that NO synthesis is impaired in pathological states. Thus, administration of L-arginine has been shown to enhance endothelium-dependent vasodilation (Creager et al., 1992) and to prevent development of atherosclerosis in low density lipoprotein (LDL) receptor knockout mice (Aji et al., 1997). However, the role of L-arginine as a rate-limiting factor in NO synthesis is controversial based on the high concentration of intracellular L-arginine and the finding that vascular effects of L-arginine may be independent of NO synthesis (see Wever et al., 1998). Tetrahydrobiopterin (BH4) is a co-factor for NO synthase in the production of NO from L-arginine (Cosentino & Lüscher, 1998; Marletta et al., 1998; Werner et al., 1998). Administration of BH4 has been demonstrated to enhance NO production in prehypertensive rats (Cosentino & Lüscher, 1998) and to restore endothelium-dependent vasodilation in isolated coronary arteries following reperfusion injury (Tiefenbacher et al., 1996) and in patients with hypercolesterolemia (Stroes et al., 1997).
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide produced by the endothelium (Yanagisawa et al., 1988). ET-1 has been proposed to be involved in the pathogenesis of several cardiovascular disorders including atherosclerosis (Kowala, 1997). The expression of ET converting enzyme, ET-1 and the ETB receptor subtype is enhanced in human atherosclerotic plaques (Kowala, 1997; Iwasa et al., 1999). Experimental studies have demonstrated that the constrictor response to exogenous ET-1 is more pronounced in large arteries of atherosclerotic monkeys than in control animals (Kowala, 1997). In addition, an ETA receptor antagonist attenuated fatty streaks and foam cell accumulation in experimental atherosclerosis (Kowala, 1997). The vasoconstrictor properties together with the mitogenic action of ET-1 may contribute to the development and progression of atherosclerosis.
Mice lacking the apolipoprotein E and/or LDL receptor genes have elevated cholesterol and develop severe atherosclerotic lesions similar to those found in humans (Zhang et al., 1992; Plump et al., 1992 Nakashima et al., 1994). Therefore, the atherosclerotic mouse is a relevant animal model for studying cellular and functional consequences of atherosclerosis. The purpose of the present study was to investigate the changes in endothelial function in atherosclerotic mice lacking apolipoprotein E and LDL receptors (E°xLDLR° mice). In particular the roles of L-arginine and BH4 for endothelium-dependent relaxations and the vasoactive effects of ET-1 were characterized.
Methods
Mice and diets
Mice on the C57BL/6J background carrying targeted deletions of the apolipoprotein E and LDL receptor genes (Bomholt, Denmark) were put on an atherogenic diet containing 21% fat/0.15% cholesterol (R683, Analycen, Lidköping, Sweden) at 6 weeks of age. At 7–8 months of age the animals were used for experiments. Serum cholesterol levels at this stage have been shown to be 26 mmol l−1 (Caligiuri et al., 1999). The experiments were approved by the regional ethics committee.
Analysis of atherosclerosis
To evaluate whether atherosclerosis was present in tissue of the material employed for functional experiments, thoracic aortas were collected from four animals for sectioning and staining with the lipid marker Oil Red-O. The sampling site for histological evaluation corresponded to the segments that were used for the functional studies. Aortas were harvested and fixed in 4% formaldehyde for 24 h, thereafter kept on 10% sucrose in PBS until they were cryomounted in OCT. Consecutive 10 μm thick sections (n=20–30) from each animal were air-dried, dehydrated, and stained with Oil Red-O for 15 min. Counter-staining was performed with haematoxylin. After rinsing with water, the slides were mounted with Kaiser's glycerol gelatin (Merck, Darmstadt, Germany) under coverslips. The Oil Red-O stained cryosections were observed under microscope, and digitized images were obtained by a magnified video system (LEICA Qwin, Leica Imaging Systems Ltd, Cambridge, U.K.). The percentage of atherosclerosis was calculated semi-automatically by a computer program (Adobe PhotoShop 5.0, Adobe. CA, U.S.A.). The total area of the lumen was outlined and calculated using the internal elastic laminae as a lineation marker. Therafter the area of the atherosclerotic lesion was calculated, and the area of the lesion was divided by the total lumen to yield the percentage of the lumen occupied by the lesion.
Tissue preparation for functional experiments
The mice were anaesthetized with an intraperitoneal injection of sodium pentobarbitone (150 mg kg−1) together with sodium heparin (100 iu kg−1, i.p.) to prevent intravascular coagulation. The thoracic aorta was quickly removed and placed in Krebs Henseleit solution at room temperature (22–23°C). The Krebs Henseleit solution consisted of (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4(7H2O) 1.2, NaHCO3 25.2 and glucose 11.1. By means of a dissecting microscope, adhering perivascular tissue was carefully removed and the descending thoracic aorta was cut into 2-mm long rings. The vessels were mounted onto two thin stainless steel holders, one of which was connected to a force displacement transducer (Grass model FT03) and the other to a movable device that allowed the application of a passive tension of 650–700 mg, which was determined to be the optimal resting tension for obtaining the maximal active tension induced by 60 mM K+ solution. The high K+ solution (60 mM) was prepared by exchanging NaCl with an equimolar amount of KCl. The mounted rings were kept in 2-ml organ baths containing Krebs Henseleit solution, kept at 37°C and continuously bubbled with a gas mixture of 95% O2 and 5% CO2 to maintain a pH of 7.4. The isometric tension was recorded on a polygraph (Grass model 7).
Experimental protocol
After an equilibration period of 60 min the contractile function of the vessel was tested twice by replacing the Krebs Henseleit solution by 60 mM K+ solution. Following washout, the vessels were precontracted with 10 μM phenylephrine. Endothelium-dependent relaxations were determined by application of acetylcholine (Ach; 0.01–10 μM) to precontracted vessels. The relaxations induced by Ach in the aorta from E°xLDLR° and control mice are abolished or greatly reduced following endothelial removal or administration of NO synthase or cyclic GMP inhibitors (Jiang et al., 1999), demonstrating that Ach evokes relaxations via a NO and cyclic GMP-dependent pathway. After a washout period, similar concentration-response curves for sodium nitroprusside (SNP) were created to test endothelium-independent relaxation. The vessels were then preincubated for 30 min with one or a combination of substances to improve endothelium-dependent relaxations. These substances included vehicle (Krebs Henseleit buffer), L-arginine (1 mM), BH4 (100 μM) and superoxide dismutase (SOD; 200 U ml−1). Up to three aortic ring preparations were used from each mouse but only one substance or one combination of substances was tested on each ring preparation. Following the preincubation, the vessel was precontracted with phenylephrine and endothelium-dependent and -independent relaxations were determined again. The relaxations are expressed in per cent of the precontractile tone.
In separate experiments, the contractile effects of the endothelium-derived contracting factor ET-1 was investigated. The contractile effect is expressed in per cent of that evoked by the second application of 60 mM K+. The response to ET-1 was characterized by application of the ETA receptor antagonist LU 135252 either 10 min before administration of ET-1 or at maximal ET-1-induced contraction. The effect of the selective ETB receptor agonist sarafotoxin 6c on basal tension and after precontraction with phenylephrine were determined to analyse the contractile and relaxatory effects, respectively.
Drugs
Phenylephrine, Ach, SNP, BH4 (6-methyl-5,6,7,8-tetrahydrobiopterin), L-arginine and SOD (from bovine erythrocytes) (Sigma Chemical, St. Louis, MO, U.S.A.), ET-1 and sarafotoxin 6c (Alexis Corporation Laufelfingen, Switzerland) were dissolved in distilled water. LU 135252, a kind gift from Dr Manfred Raschack Knoll AG, Ludwigshafen Germany, was dissolved in 1 M NaOH and pH was then set to 7.5 by addition of 0.1 M HCl. All subsequent dilutions were made with Krebs Henseleit solution. All concentrations given are final molar concentrations in the organ chambers.
Statistics
Results are expressed as mean±s.e.mean. Since each pharmacological challenge was performed on only one aortic ring preparation from each mouse, the number of observations are equal to the number of mice in each group. Paired or unpaired Student's t-test was used to compare results in treated and untreated aortas from each strain and between control and E°xLDLR° mice, respectively. ANOVA was used for multiple comparisons. Differences were considered to be significant when P<0.05.
Results
Evaluation of atherosclerosis
Microscopic and macroscopic evaluation revealed that extensive atherosclerosis was present throughout the thoracic aorta of all animals investigated. Histological examination demonstrated that atherosclerotic lesions were observed in all sections investigated. Advanced fibrofatty lesions were observed in the vessel lumen, and a representative vessel is depicted in Figure 1. The percentage of the lumen occupied by the lesions was calculated to be 43±16%, while 88±8% of the luminal surface was covered by endothelium.
Figure 1.
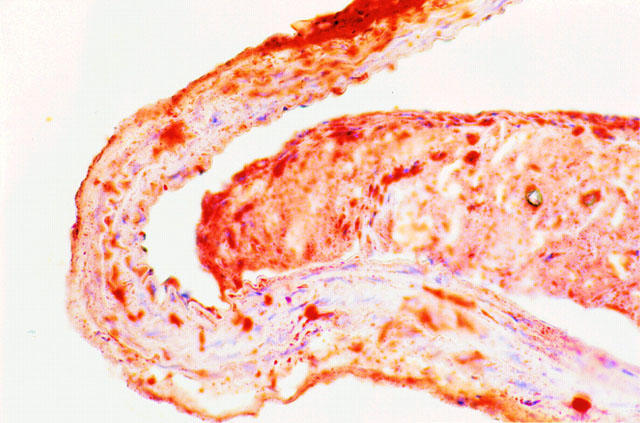
Transverse section of a representative thoracic aorta of an E°xLDLR° mouse after being fed a diet containing 21% fat and 0.15% cholesterol for 7 months, corresponding to the time of testing in vitro reactivity. The section is stained with Oil Red-O and counterstained with hematoxilin. Note the massive fibrofatty lesion obstructing a large part of the lumen. The plaque is partially detached from the vessel wall due to artefacts during sectioning and staining. Original magnification ×400.
Response to Ach and SNP
The precontractile tone induced by administration of phenylephrine was somewhat higher in the E°xLDLR° mice than in control mice (415±10 vs 334±7 mg; P<0.001) which is in agreement with a previous report (Jiang et al., 1999). Ach and SNP evoked concentration-dependent relaxation of aortic rings from both control mice and E°xLDLR° mice. The per cent relaxation induced by Ach was significantly impaired in E°xLDLR° mice (Figure 2a). The highest concentration of Ach (10 μM) relaxed the vessels by 45±2% in E°LDLR° mice and by 70±5% in control mice (P<0.001). The relaxation induced by SNP was similar in the two groups (Figure 2b).
Figure 2.
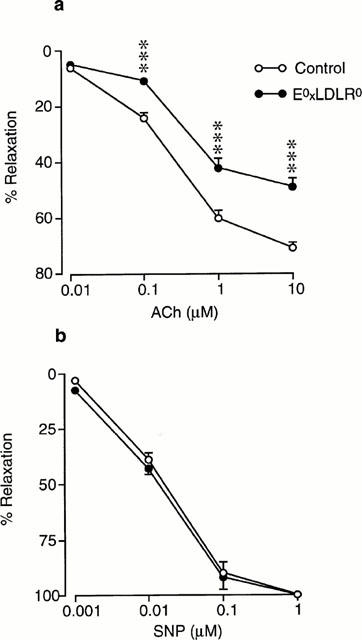
Concentration-response curves showing the relaxations induced by (a) acetylcholine (ACh) and (b) sodium nitroprusside (SNP) in aorta from control mice and mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°). The relaxations are expressed in per cent of the precontracted tone induced by 10 μM phenylephrine. Data are given as means and s.e.mean of 10–12 observations. Significant differences between the groups are shown; ***P<0.001.
Effect of L-arginine and BH4
Preincubation of aortic rings from E°xLDLR° mice with L-arginine (1 mM) did not affect the Ach-induced relaxation (Figure 3a). Administration of BH4 (100 μM) slightly improved the relaxation evoked by a low concentration (0.1 μM) Ach but did not affect the maximal relaxation (Figure 3b). Preincubation with the combination of BH4 and L-arginine resulted in a pronounced improvement in Ach-induced relaxations (Figure 3c). The Ach-induced relaxation of E°xLDLR° aorta following incubation with L-arginine and BH4 was not different from that observed in aortic rings from control mice. The relaxations induced by Ach were not affected by 30 min incubation with Krebs Henseleit solution only (48±6 vs 50±6% relaxation by 10 μM Ach, n=8). The response to Ach in control mice aorta were not affected by L-arginine and BH4. The relaxation induced by SNP in E°xLDLR° aorta was not changed following administration of L-arginine and BH4 (Figure 4).
Figure 3.
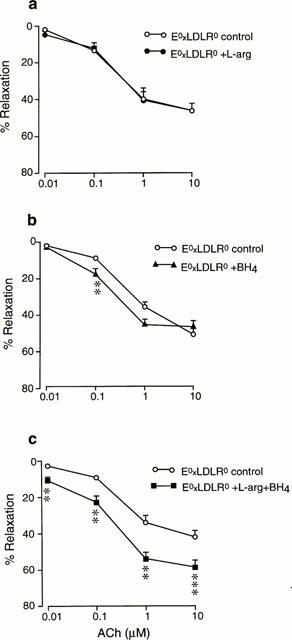
Concentration-response curves showing the relaxations induced by acetylcholine (ACh) in aorta from mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°) under control conditions and after preincubation with (a) L-arginine, (b) tetrahydrobiopterin (BH4) or (c) the combination of L-arginine and BH4. The relaxations are expressed in per cent of the precontracted tone induced by 10 μM phenylephrine. Data are given as means and s.e.mean of 6–9 observations. Significant differences from control conditions are shown; **P<0.01, ***P<0.001.
Figure 4.
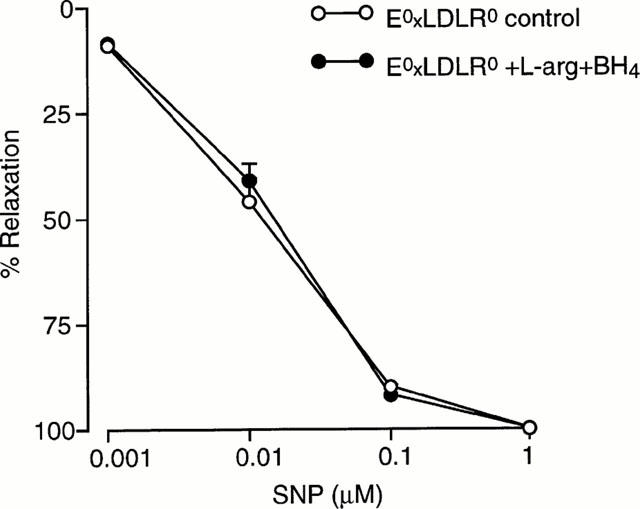
Concentration-response curves showing the relaxations induced by sodium nitroprusside (SNP) in aorta from mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°) under control conditions and after preincubation with the combination of L-arginine and tetrahydrobiopterin (BH4). The relaxations are expressed in per cent of the precontracted tone induced by 10 μM phenylephrine. Data are given as means and s.e.mean of seven observations.
Effect of SOD
Preincubation of E°xLDLR° aorta with SOD (200 U ml−1) did not affect the relaxation induced by Ach under control conditions or in the presence of L-arginine and BH4 (Figure 5).
Figure 5.
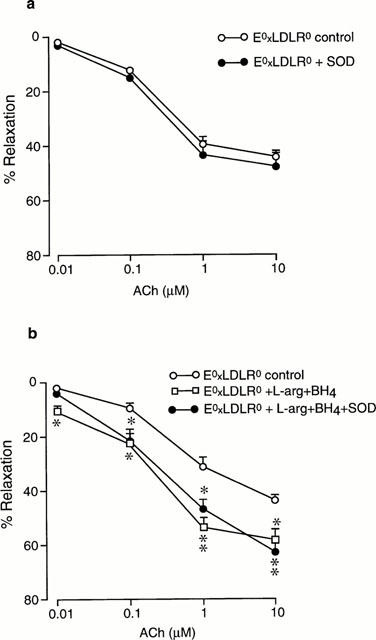
Concentration-response curves showing the relaxations induced by acetylcholine (ACh) in aorta from mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°) under control conditions and after preincubation with (a) superoxide dismutase (SOD) and (b) the combination of L-arginine and tetrahydrobiopterin (BH4), and L-arginine+BH4+SOD. The relaxations are expressed in per cent of the precontracted tone induced by 10 μM phenylephrine. Data are given as means and s.e.mean of six observations. Significant differences from control conditions are shown; *P<0.05, **P<0.01.
Effect of ET
ET-1 induced concentration-dependent contractions of the aortic rings from both strains of mice. The contractile effect was markedly enhanced in E°xLDLR° mice (Figures 6 and 7). Administration of the ETA receptor antagonist LU 135252 (10 μM) at maximal ET-1-induced contraction resulted in progressive relaxation in both control and E°xLDLR° mice by approximately 80% (Figure 7). Preincubation with LU 135252 completely abolished the contractile response to ET-1 (Figure 7). Administration of the ETB receptor agonist sarafotoxin 6c did not induce any reproducible contractile response in either mouse strain, nor did it cause relaxation in E°xLDLR° mice when administered after precontraction with phenylephrine (data not shown).
Figure 6.
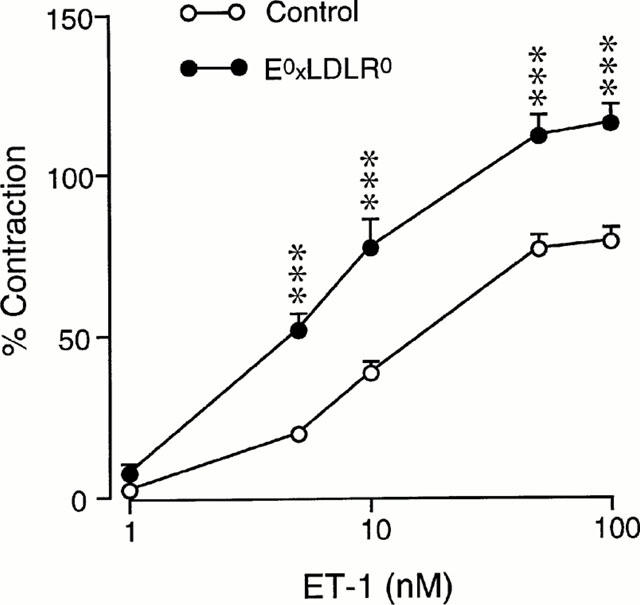
Concentration-response curves showing the contractions induced by endothelin-1 (ET-1) in aorta from control mice and mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°). The contractions are expressed in per cent of that evoked by 60 mM potassium. Data are given as means and s.e.mean of 11–14 observations. Significant differences between the groups are shown; ***P<0.001.
Figure 7.
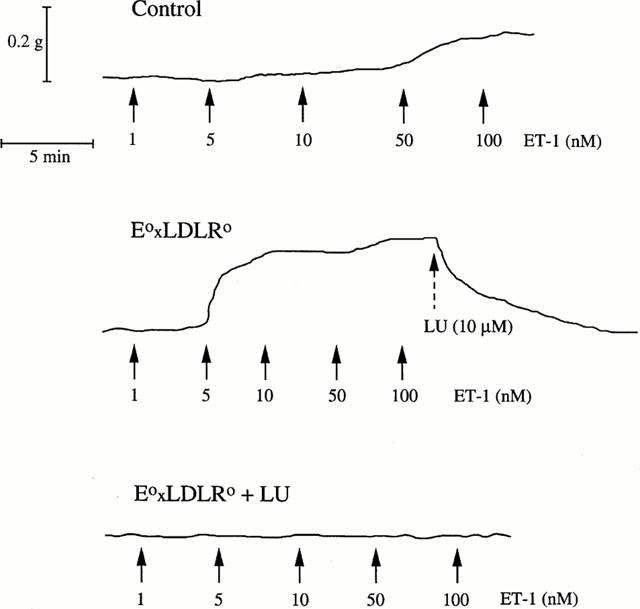
Original registrations showing the effect of endothelin-1 (ET-1) in aorta from a control mouse (upper panel) and two mice deficient of apolipoprotein E and low density lipoprotein receptor genes (E°xLDLR°) (lower two panels). The endothelin A receptor antagonist LU 135252 (LU) was administered at maximal contraction (middle panel) or 10 min before ET-1 (lower panel).
Discussion
The present study demonstrates that the aorta from atherosclerotic E°xLDLR° mice have impaired endothelium-dependent relaxations and that the endothelial function can be restored by the combined administration of L-arginine and BH4. We also demonstrate a marked enhancement of ET-1-mediated vasoconstriction mediated via the ETA receptor subtype.
The animals employed in the present study had developed severe atherosclerosis which was evident from the advanced fibrofatty lesions in all the transverse sections of the thoracic aorta, representing the vessel segments employed for in vitro reactivity. Only a small loss of endothelial cells were noted in the lesions. The relaxations induced by Ach but not that to the NO donor SNP were significantly reduced in E°xLDLR° mouse aorta. This finding indicates impaired endothelial function in agreement with previous findings in E°xLDLR° mice (Jiang et al., 1999) as well as in mice deficient in ApoE only (Barton et al., 1998; Deckert et al., 1999). The unchanged response to SNP demonstrates that the ability of the vascular smooth muscle cells to dilate when stimulated by NO is not affected in E°xLDLR° mice. Therefore, the impaired response to Ach reflects reduced bioavailability of endothelium-derived NO. It has been suggested that supplementation with the substrate L-arginine may enhance NO production and restore endothelial function in hypercholesterolemia and atherosclerosis (see Wever et al., 1998; Drexler, 1997). In the present study, preincubation with L-arginine (1 mM) did not affect the response to Ach, however, indicating that lack of substrate was not a primary cause of endothelial dysfunction. This is in accordance with the failure of L-arginine to improve endothelial function following exposure to LDL in rabbit aortic rings (Fontana et al., 1999). Administration of BH4, at a concentration which has been demonstrated to enhance production of NO and restore endothelial function (Cosentino & Katusic 1995; Tiefenbacher et al., 1996; Pieper, 1997), resulted in a slight enhancement but not normalization of endothelial function. This finding suggests that the dysfunction of the endothelium at least in part was due to lack of the co-factor BH4, which is in accordance with observations in patients with hypercholesterolemia (Stroes et al., 1997). When the vessels were preincubated with a combination of L-arginine and BH4, the endothelial function of E°xLDLR° mice was markedly enhanced and not different from that observed in vessels from control mice. The relaxations induced by SNP were unaffected by combined administration of L-arginine and BH4, indicating that the response of the vascular smooth muscle cells to NO was unchanged. Thus, in the presence of sufficient levels of the co-factor BH4 addition of the substrate L-arginine resulted in normalized endothelium-dependent relaxation.
The exact mechanism behind the effect of BH4 on endothelial NO synthase is not fully clarified. BH4 has been proposed to exert allosteric actions to stabilize the active dimeric state of the enzyme and to play a redox-active role in stimulating NO synthase (Werner et al., 1998, Marletta et al., 1998). Another important action of BH4 may be that it seems to co-operate with L-arginine. Thus, binding of L-arginine to NO synthase is enhanced by BH4 (Wever et al., 1998). Accordingly, an altered KM of NO synthase for L-arginine has been observed in mice with BH4 deficiency (Brand et al., 1995). It is therefore possible that administration of BH4 in the present experiments enhances binding of L-arginine to NO synthase which results in improved endothelial function. This would also indicate that E°xLDLR° mice are deficient in both BH4 and L-arginine. However, measurements of endogenous BH4 and L-arginine have not been performed in this mouse strain to confirm this hypothesis.
Reduced activity of endothelium-derived NO may also be caused by increased catabolism. NO reacts very rapidly with superoxide to produce peroxynitrite (Beckman & Koppenol, 1996). Since the generation of superoxide is increased in atherosclerotic lesions (see Wever et al., 1998) the catabolism of NO by its reaction with superoxide may be of importance in atherosclerosis. However, administration of SOD, at a concentration which has been demonstrated to improve endothelial function during stimulated superoxide production in rabbit aortic rings (Abrahamsson et al., 1992) and in aortic rings from spontaneously hypertensive rats (Cosentino et al., 1998), did not modify the Ach-induced relaxations in the present study. This suggests that enhanced inactivation of NO by superoxide did not to any major extent account for the reduced activity of NO. Moreover, SOD did not further modify the response to Ach in the presence of L-arginine and BH4. This finding indicates that BH4 did not undergo auto-oxidation with subsequent production of superoxide, which has been reported to occur in vitro (Wever et al., 1998).
The vasoconstrictor effect of ET-1 was markedly enhanced in the aorta from E°xLDLR° mice in comparison with control mice. The contractions were completely blocked by preincubation with the ETA receptor antagonist LU 135252. The antagonist also effectively reversed established contractions induced by ET-1. Furthermore the selective ETB receptor agonist sarafotoxin 6c failed to induce contractions per se or relaxations of precontracted vessels. Taken together these findings demonstrate that the vasoconstrictor activity of ET-1 is augmented in E°xLDLR° mice and that this vasoconstrictor effect is mediated via activation of the ETA receptor subtype. It is in this context interesting to note that E°xLDLR° mice subjected to hypoxic stress in vivo develops myocardial ischaemia and infarcts, an effect which is inhibited by ETA receptor blockade (Caligiuri et al., 1999). This implies that the enhanced ETA receptor-mediated vasoconstrictor activity in these atherosclerotic mice has functional consequences which may result in myocardial ischaemia and infarcts.
In conclusion, the present study demonstrates that atherosclerotic E°xLDLR° mice have impaired endothelial function. The endothelial function can be normalized by the combined administation of the substrate L-arginine and the co-factor BH4. In addition, the ETA receptor-mediated vasoconstriction of ET-1 is enhanced. These alterations in endothelial function which include reduced NO activity and enhanced ET-1 activity makes the vessel more susceptible to vasospasm which may have pathophysiological consequences.
Acknowledgments
The present study was supported by grants from the Swedish Medical Research Council (10857, 12665 and 4764), the Swedish Heart and Lung Foundation, Osterman Foundation, King Gustav V 80th Anniversary Fund and the Karolinska Institute.
Abbreviations
- Ach
acetylcholine
- BH4
tetrahydrobiopterin
- E°xLDLR°
apolipoprotein E and low density lipoprotein receptor knockout
- ET
endothelin
- NO
nitric oxide
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
References
- ABRAHAMSSON T., BRANST U., MARKLUND S.L., SJÖQVIST P.-O. Vascular bound recombinant extracellular superoxide dismutase type C protects against detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ. Res. 1992;70:264–271. doi: 10.1161/01.res.70.2.264. [DOI] [PubMed] [Google Scholar]
- AJI W., RAVALLI S., SZABOLCS M., JIANG X., SCIACCA R.R., MICHLER R.E., CANNON P.J. L-Arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation. 1997;95:430–437. doi: 10.1161/01.cir.95.2.430. [DOI] [PubMed] [Google Scholar]
- BARTON M., HAUDENSCHILD C., D'USCIO L., SHAW S., MÜNTER K., LÜSCHER T. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BOSSALER C., HABIB G.B., YAMOMOTO H., WILLIAMS C., WELLS S. Impaired muscarinic endothelial-dependent relaxation and cyclic guanosine 5-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAND M.P., HEALES S.J., LAND J.M., CLARK J.B. Tetrahydrobiopterin deficiency and brain nitric oxide synthase in the hph1 mouse. J. Inherit. Metab. Dis. 1995;18:33–39. doi: 10.1007/BF00711370. [DOI] [PubMed] [Google Scholar]
- CALIGIURI G., LEVY B., PERNOW J., THORÉN P., HANSSON G. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSENTINO F., KATUSIC Z. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., LÜSCHER T.F. Tetrahydrobiopterin and endothelial function. Eur. Heart J. 1998;19 Suppl. G:G3–G8. [PubMed] [Google Scholar]
- COSENTINO F., PATTON S., D'USCIO L.V., WERNER E.R., WERNER-FELMYER G., MOREAU P., MALINSKI T., LŸSCHER T.F. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J. Clin. Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREAGER M.A., GALLAGHER S.J., GIRERD X.J., COLEMAN S.M., DZAU V.J., COOKE J.P. L-Arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J. Clin. Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECKERT V., LIZARD G., DUVERGER N., ATHIAS A., PALLEAU V., EMMANUEL F., MOISANT M., GAMBERT P., LALLEMANT C., LAGROST L. Impairment of endothelium-dependent arterial relaxation by high-fat feeding in apoE-deficient mice. Toward normalization by human apoA-I expression. Circulation. 1999;100:1230–1235. doi: 10.1161/01.cir.100.11.1230. [DOI] [PubMed] [Google Scholar]
- DREXLER H. Endothelial dysfunction: clinical implications. Prog. Cardiovasc. Dis. 1997;39:287–324. doi: 10.1016/s0033-0620(97)80030-8. [DOI] [PubMed] [Google Scholar]
- FONTANA L., MCNEILL K.L., RITTER J.M., CHOWIENCZYK P.J. Effects of vitamin C and of a cell permeable superoxide dismutase mimietic on acute lipoprotein induced endothelial dysfunction in rabbit aortic rings. Br. J. Pharmacol. 1999;126:730–734. doi: 10.1038/sj.bjp.0702331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FÖRSTERMANN U., MÜGGE A., ACHEID U., HAVERICH A., FRÖLICH J.C. Selective attenuation of endothelial-mediated vasodilation atherosclerotic human coronary arteries. Circ. Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- FREIMAN P.C., MITCHELL G.C., HEISTAD D.D., ARMSTRONG M.L., HARRISON D.G. Atherosclerosis impairs endothelial-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ. Res. 1986;58:783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- IWASA S., FAN J., SHMOKAMA T., NAGATA M., WATANABE T. Increased immunoreactivity of endothelin-1 and endothelin B receptor in human atherosclerotic lesions. A possible role in atherogenesis. Atherosclerosis. 1999;146:93–100. doi: 10.1016/s0021-9150(99)00134-3. [DOI] [PubMed] [Google Scholar]
- JIANG J., THORÉN P., CALIGIURI G., HANSSON G.K., PERNOW J. Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: relation to Kv channels and nitric oxide. Br. J. Pharmacol. 1999;128:637–646. doi: 10.1038/sj.bjp.0702855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLODGIE F., VIRMANI R., RICE H.E., MERGNER W.J. Vascular reactivity during the progression of atherosclerotic plaque: a study in Watanabe heritable hyperlipidemic rabbits. Circ. Res. 1990;66:1112–1116. doi: 10.1161/01.res.66.4.1112. [DOI] [PubMed] [Google Scholar]
- KOWALA M.C. The role of endothelin in the pathogenesis of atherosclerosis. Adv. Pharmacol. 1997;37:299–318. doi: 10.1016/s1054-3589(08)60953-9. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., NOLL G. The pathogenesis of cardiovascular disease: role of the endothelium as target and mediator. Atherosclerosis. 1995;188 Suppl.:S81–S90. [PubMed] [Google Scholar]
- MARLETTA M.A., HURSHMAN A.R., RUSCHE K.M. Catalysis by nitric oxide synthase. Curr. Opin. Chem. Biol. 1998;2:656–663. doi: 10.1016/s1367-5931(98)80098-7. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NAKASHIMA Y., PLUMP A.S., RAINES E.W., BRESLOW J.L., ROSS R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J. Cardiovasc. Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- PLUMP A.S., SMITH J.D., HAYEK T., AALTO-SETÄLA K., WALSH A., VERSTUYFT J.G., RUBIN E.M., BRESLOW J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- STROES E., KASTELEIN J., COSENTINO F., ERKELENS W., WEVER R., KOOMANS H., LÜSCHER T., RABELINK T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J. Clin. Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIEFENBACHER C.P., CHILIAN W.M., MITCHELL M., DEFILY D.V. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- WERNER E.R., WERNER-FELMAYER G., MAYER B. Tetrahydrobiopterin, cytokines, and nitric oxide synthesis. Proc. Soc. Exp. Biol. Med. 1998;219:171–182. doi: 10.3181/00379727-219-44331. [DOI] [PubMed] [Google Scholar]
- WEVER R.M.F., LÜSCHER T.F., COSENTINO F., RABELINK T.J. Atherosclerosis and the two faces and endothelial nitric oxide synthase. Circulation. 1998;97:108–112. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- ZEIHER A.M., DREXLER H., WOLLSCHLÄGER H., JUST H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- ZHANG S.H., REDDICK R.L., PIEDRAHITA J.A., MAEDA N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]


