Abstract
The vasorelaxant activity of eicosapentaenoic acid (EPA, 20:5n-3), the omega-3 polyunsaturated fatty acid, was investigated in isolated Wistar Kyoto (WKY) rat aortae by measuring isometric tension.
Eicosapentaenoic acid (1–100 μM) relaxed rat aortae contracted with high K+ (80 mM) or noradrenaline (NA, 1 μM) in a concentration-dependent manner. Contractions induced by Bay K 8644 or increasing concentrations of calcium were unaffected by EPA.
The relaxant effect of EPA (3–100 μM) was significantly inhibited by indomethacin (10 μM), the cyclo-oxygenase inhibitor, but not by the nitric oxide (NO) synthesis inhibitor, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 μM). Removal of the endothelium did not alter EPA-induced relaxations.
In Ca2+-free, EGTA 2 mM solution, EPA (10–30 μM significantly inhibited NA-sustained contractions. Incubation with EPA (5, 10 μM) diminished both NA-induced (1 μM) phasic and sustained contractions.
The vasorelaxant effects of EPA (⩾30 μM) on NA-induced (1 μM) contractions were significantly inhibited by the K+ channel blocker, glibenclamide (10 μM), but not tetraethylammonium (1 mM). Moreover, indomethacin and glibenclamide combined significantly inhibited EPA-induced (1–100 μM) responses.
These results indicate EPA exerts its endothelium-independent vasorelaxant effects in WKY rat aortae through production of prostanoids which activate K+ATP channels. Inhibition of Ca2+ mobilization from intracellular pools and influx through the non-L-type, but not the L-type, Ca2+ channel are also possible mechanisms action of EPA's.
Keywords: Eicosapentaenoic; omega-3 fatty acid; relaxation; rat aorta; potassium channels; calcium; cyclo-oxygenase, prostanoids
Introduction
Eicosapentaenoic acid (EPA, 20:5n-3) is a long chain, omega-3 polyunsaturated fatty acid (PUFA) and is one of the major components found in seafood and fish oil supplements. There is increasing evidence that the omega-3 fatty acids, EPA and docosahexaenoic (DHA, 22:6n-3), may be cardiovascular protective nutrients (Chin & Dart, 1995; McLennan et al., 1996; de Deckere et al., 1998). In vessels from various animal models, EPA induces relaxations and antagonizes contractions by mechanism(s) which are endothelium-dependent (Shimokawa et al., 1987; Yanagisawa & Lefer, 1987), endothelium-independent (Juan et al., 1987; Yanagisawa & Lefer, 1987; Engler & Engler, 1996; Engler et al., 1994; 1999a,1999b), independent of trienoic cyclo-oxygenated products (Juan & Sametz, 1986; Yanagisawa & Lefer, 1987), and independent of increases in cyclic AMP and cyclic GMP levels (Juan et al., 1987; Engler, 1992a). To date, the possible role of nitric oxide (NO), prostanoid/cyclo-oxygenase products, K+ channels, or calcium ions in the direct relaxant effect of EPA has not been clearly defined.
Our laboratory has previously reported that the relaxant effect of EPA in Sprague-Dawley rat aortae was specific to α-adrenoceptor stimuli and related to intracellular calcium mechanisms (Engler, 1992b). Others have indicated that EPA acts as a non-selective antagonist of sympathetic transmitters in small peripheral vessels (Juan et al., 1988). In vitro studies have also shown that EPA alters intracellular free calcium concentration ([Ca2+]i) following incubation in cultured endothelial and vascular smooth muscle cells (Okuda et al., 1994; Locher et al., 1991; Engler et al., 1999a).
It is known that EPA can compete with arachidonic acid (20:4n-6) as a substrate in vascular and platelet production of eicosanoids (Boulanger et al., 1990). Although, there is a shift towards the production of 3-series eicosanoids that occurs with increased dietary intake of EPA; vasodilator prostacyclin (PGI2) is still formed from 20:4n-6 in endothelial cells by cyclooxygenase. These 3-series eicosanoids include the physiologically inactive eicosanoid, thromboxane A3, produced by platelets and PGI3, which has similar vasodilator and platelet anti-aggregatory properties to PGI2.
In addition to prostacyclin (PGI2), other endothelium-derived relaxing factors include endothelium-derived hyperpolarizing factor (EDHF) and nitric oxide (NO), which is formed following the conversion of L-arginine to citrulline by the enzyme NO synthase (Palmer et al., 1988). The mechanism of EDHF's relaxant effects is primarily due to hyperpolarization from activation of K+ channels (Cowan & Cohen, 1991; Nagao & Vanhoutte, 1992). The opening of K+ channels and resultant hyperpolarization can be prevented by K+ channel blockers or increased external K+ concentrations (Nagao & Vanhoutte, 1991; Garland et al., 1995).
To investigate the relaxant mechanism(s) of action of EPA in normotensive Wistar Kyoto (WKY) rat aortae, we examined the effects of EPA on high K+-induced and noradrenaline-induced contractions under normal calcium and calcium-free conditions. Bay K 8644, a L-type calcium channel agonist, was also used as a contractile agonist and calcium-response curves were generated in the presence and absence of EPA. Moreover, the specific properties of EPA in relation to the endothelium and production of NO, prostanoids, and opening of K+ channels were investigated. A preliminary report of some of these findings was presented at the American Heart Association's 70th Scientific Sessions in Orlando, FL, U.S.A., on November 9–12, 1997 (Engler & Engler, 1997).
Methods
Male Wistar-Kyoto (WKY) rats, (Harlan Sprague-Dawley, Inc., Indianapolis, IN, U.S.A., Taconic, Germantown, NY, U.S.A.) (ages 16–17 weeks, mean weight 355±11 g) were anaesthetized with a mixture of 70% oxygen, 30% nitrous oxide, and 5% halothane. Prior to anaesthesia, systolic blood pressure was 117±1 mm Hg, n=32 as measured by tail-cuff plethysmography. Thoracic aortae were excised, cleaned of all connective and fat tissue and cut into rings of 3 mm length. Each ring was mounted under optimal resting tension of 2 g in tissue baths (Radnoti Glass Technology Inc., Monrovia, CA, U.S.A.) containing 15 ml Krebs-Ringer bicarbonate solution (pH 7.4) of the following composition (mM); NaCl 118.3, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0, glucose 11.1. Calcium-free solution contained the same components except that ethylene glycol-bis (β-aminoethylether)-N,N′-tetra-acetic acid (EGTA, 2 mM) was substituted for CaCl2. The solutions were aerated with 95% O2/5% CO2 and maintained at 37°C.
Isometric tension was recorded with force displacement transducers (Grass FTO3, Grass Instrument Co., Quincy, MA, U.S.A.) coupled to an eight-channel chartwriter (model WR3701, Western Graphtec, Inc., Irvine, CA, U.S.A.). Data acquisition was performed by a PO-NE-MAH computerized system (Gould, Inc., Cleveland, OH, U.S.A.). Tension adjustments and bath washes were controlled by an automated system (STC 400, Buxco Electronics Inc., Troy, NY, U.S.A.). Tissues were allowed to equilibrate for 60–90 min before the start of the experiments. Tissue viability was determined with KCl 30 mM and the integrity of the endothelium was assessed by acetylcholine-induced (1 μM) relaxation in KCl-contracted rings. In some rings, the endothelium was removed with the tip of curved forceps. Removal of the endothelium was confirmed by lack of relaxation to acetylcholine. The guidelines of the Committee on Animal Research, University of California San Francisco were followed for all experimental procedures performed.
Experimental Procedure
Protocol 1
This series of experiments was performed to determine the relaxant responses of eicosapentaenoic acid in WKY rat aortae precontracted by noradrenaline (NA, 1 μM), potassium chloride (KCl, 80 mM), or Bay K 8644 (1 μM, in the presence of 15 mM KCl). Concentration response curves of relaxation were obtained by the addition of cumulative concentrations of EPA (1–100 μM) following the plateau of contraction to NA, KCl, or Bay K 8644. Concentrations of EPA (1–30 μM) were chosen since this range represents physiological-obtainable levels in normal human plasma (Engler 1992a); whereas, EPA at 100 μM is considered a high non-physiological level. Relaxations were expressed as a percentage of the maximum tension by contractile agonist addition. The endothelium was removed prior to noradrenaline-induced contraction and administration of EPA (1–100 μM) in some rings. KCl-contracted aortic rings were pretreated (20 min) with phentolamine (1 μM) to prevent the effect of endogenous catecholamines. This series of experiments also included a noradrenaline-contracted group pretreated (20 min) with indomethacin (10 μM) or Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 μM). Indomethacin, a cyclooxygenase enzyme inhibitor, and L-NAME, a nitric oxide (NO) synthesis inhibitor, were used to determine the possible role of prostanoids or endothelial nitric oxide involvement in the EPA-induced relaxation. The relaxant responses to EPA were then assessed as previously described. The possible role of ATP-sensitive potassium channels and potassium conductance on EPA-induced aortic relaxation was also examined in another set of experiments. Glibenclamide (10 μM), an inhibitor of ATP-sensitive potassium channels (KATP), or tetraethylammonium (TEA, 1 mM), a Ca2+-activated potassium channel (KCa) blocker, were preincubated for 20 min prior to the EPA-induced relaxation in noradrenaline-contracted rings. Combinations of indomethacin (10 μM) and glibenclamide (10 μM) were also preincubated for 20 min prior to EPA-induced relaxation in noradrenaline-contracted rings to determine the possible involvement of prostanoid-mediated opening of K+ channels.
Protocol 2
In this series of experiments, aortic rings were initially equilibrated in Krebs solution with 2.5 mM CaCl2 and were then washed four times at 4 min intervals for a total of 20 min in calcium-free solution containing 2.0 mM EGTA. The rings were then exposed to noradrenaline (1 μM) in the calcium-free solution, which produced an initial phasic contraction followed by a sustained contraction. At the plateau of the sustained noradrenaline contraction, cumulative concentration-response curves to EPA (1–30 μM) were generated. The possible inhibitory effect of EPA on the sustained noradrenaline contraction in calcium-free solution was determined.
Protocol 3
Experiments in this series examined the possible influence of EPA (10, 30 μM) incubation (20 min) on cumulative calcium (Ca2+)-concentration (0.05–10 mM) response curves. Aortic rings were exposed to EPA in Ca2+-free buffer without EGTA as described previously for 20 min. Noradrenaline (1 μM) was then administered and at the plateau NA response, calcium chloride was added in a cumulative manner.
Protocol 4
In this protocol, the possible inhibitory effect of EPA on both the initial phasic and sustained noradrenaline contractile response in calcium-free solution containing EGTA (2 mM) were examined. EPA (5, 10 μM) was incubated for 20 min prior to noradrenaline (1 μM) administration. Both the initial phasic and sustained contractions to noradrenaline in the calcium-free solution are expressed as the percentage of the NA-induced (1 μM) contraction in Krebs (calcium-containing) solution.
Chemicals
Acetylcholine chloride, norepinephrine (−)-arterenol bitartrate salt, tetraethylammonium chloride, glibenclamide, L-NAME, EGTA, eicosapentaenoic acid (sodium salt, >99% purity), phentolamine, and indomethacin were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Bay K 8644 was obtained from Research Biochemical International, Natick, MA, U.S.A. EPA was dissolved in nitrogen saturated methanol and stored at −70°C under nitrogen with preparation as previously described (Engler & Engler, 1996). The fatty acid vehicle (0.9% NaCl) was used in control vessels. Noradrenaline was preserved in 0.2% ascorbic acid stock solution. Glibenclamide and Bay K 8644 were dissolved in dimethyl sulphoxide (DMSO) at a final concentration of DMSO of less than 0.1%. Indomethacin was dissolved in sodium carbonate solution (0.1 M).
Data analysis
Data are presented as mean±s.e.mean of the percentage or absolute tension maximal responses. Statistical significance was determined by applying the Student's t-test for unpaired observations and analysis of variance (ANOVA) for multiple comparisons. The post-hoc Scheffe procedure was used following ANOVA for statistically significant F values. When the assumption of equal variances required for the ANOVA was not satisfied, the Kruskal-Wallis test was used for group comparisons. Repeated measures ANOVA was also used for comparisons of concentration response curves. The Greenhouse-Geisser adjustment for multi-sample asphericity was used to avoid excessive Type I error (Ludbrook, 1994). Significance criteria was set at 0.05 and when multiple comparisons were performed at each concentration, the test of simple main effects with Bonferroni correction was used, e.g., 0.05/5=0.01. n refers to the number of animals with an average of 2–3 ring responses per permutation.
Results
Vasorelaxant response to EPA: effects of indomethacin/L-NAME and potassium channel blockers
As seen in Figure 1, EPA (1–100 μM) induced similar relaxant responses in noradrenaline (1 μM)-(EPA) and KCl (80 mM) (KCl-EPA)-contracted rings (n.s.). Control responses for noradrenaline- and KCl-contracted rings ranged from 1±0.7% to 3±3% and 0.1±1% to 0.4±3%, respectively (n=3–6). Significantly greater relaxations to EPA⩾30 μM, (P⩽0.01) were seen in noradrenaline-contracted rings as compared to control responses. In KCl-contracted rings, EPA evoked greater relaxations (P⩽0.05–P⩽0.01) at lower concentrations (1–30 μM) when compared to control responses.
Figure 1.
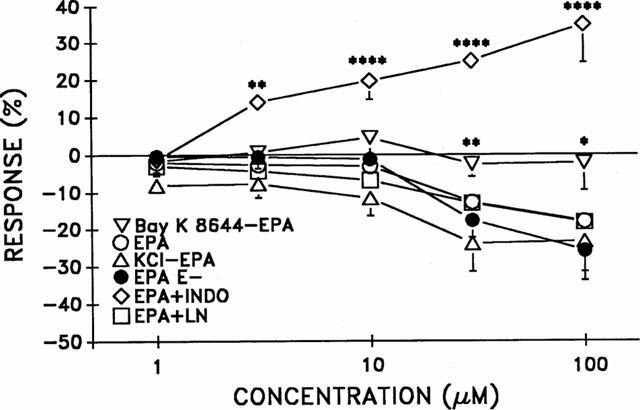
The concentration-dependent effect of eicosapentaenoic acid (EPA) in aortic rings (intact and de-endothelialized, E-) contracted with noradrenaline (NA, 1 μM) +indicates incubation (20 min) with indomethacin (INDO, 10 μM) or L-NAME (LN, 100 μM) in NA-contracted rings. The responses to EPA in Bay K 8644 (1 μM)- and potassium chloride-(KCl, 80 mM) contracted rings are also depicted as Bay K 8644-EPA and KCl-EPA, respectively. n=3–15 per group. *Indicates significance at P<0.05, **P⩽0.01, ****P⩽0.0001 as compared to EPA group.
The maximal contractile response to KCl (80 mM) in the presence of phentolamine (1 μM) and noradrenaline (1 μM) was 1610±128 mg (n=6) and 2939±171 mg (n=10), respectively. As seen in Figure 1, EPA responses following Bay K 8644 contractions were significantly less (P⩽0.05) than EPA responses (⩾30 μM) in noradrenaline-contracted rings (EPA). Control responses in Bay K 8644 contracted rings were similar to Bay K 8644-EPA responses at −0 to −2±4% (n.s., n=3). The maximal contractile response to Bay K 8644 was 1695±26 mg (n=4). Removal of the endothelium did not alter EPA-induced responses (EPA E-) as compared to the group with intact endothelium (EPA), n.s., (Figure 1).
The noradrenaline-contractile response in the de-endothelialized group was 2990±400 mg (n=5). The contractile response to noradrenaline following separate pretreatment with L-NAME (100 μM) or indomethacin (10 μM) were 2706±76 and 1075±49 mg, respectively. When comparing EPA relaxant responses in noradrenaline-contracted rings in the presence (EPA+LN) and absence (EPA) of L-NAME, there was no significant difference. However, a significant contractile effect was seen with EPA (3–100 μM) in the presence of indomethacin (Figure 1).
The effects of potassium channel inhibitors, glibenclamide (10 μM) or TEA (1 mM) on EPA-induced relaxations in noradrenaline-contracted rings are depicted in Figure 2. Glibenclamide but not TEA treatment significantly inhibited EPA-induced (⩾30 μM) relaxations (P⩽0.001). Combination of indomethacin and glibenclamide treatment prior to EPA (1–100 μM) evoked significant vasoconstriction (P⩽0.01) as seen in Figure 2.
Figure 2.
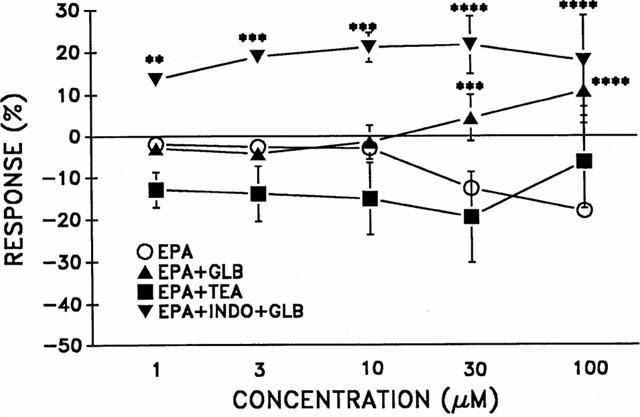
The effect of eicosapentaenoic acid (EPA) in intact aortic rings contracted with noradrenaline (NA, 1 μM) following pretreatment (20 min) with the K+-channel blockers, glibenclamide (+GLB, 10 μM) or TEA (1 mM). INDO+GLB reflects incubation (20 min) with indomethacin (10 μM) and glibenclamide (10 μM). n=3–10 per group. **Indicates significance at P⩽0.01, ***P⩽0.001, ****P⩽0.001 as compared to EPA group.
Inhibitory effect of EPA on the sustained noradrenaline-induced contraction in calcium-free solution containing EGTA (2 mM)
After 20 min in calcium-free solution, addition of noradrenaline (1 μM) produced a phasic contraction followed by a sustained one. EPA significantly inhibited noradrenaline sustained contractions at concentrations (10–30 μM) as compared to the control (vehicle) as seen in Figure 3. Relaxation responses to EPA (1–30 μM) ranged from −7±1% to −30±4%. After the above experimental procedure, aortic rings were washed with Krebs (calcium-containing) solution. The resultant spontaneous increase in aortic tension was not significantly different between the EPA-treated group (1825±40 mg, n=8) and the control group (1714±150 mg, n=6, n.s.).
Figure 3.
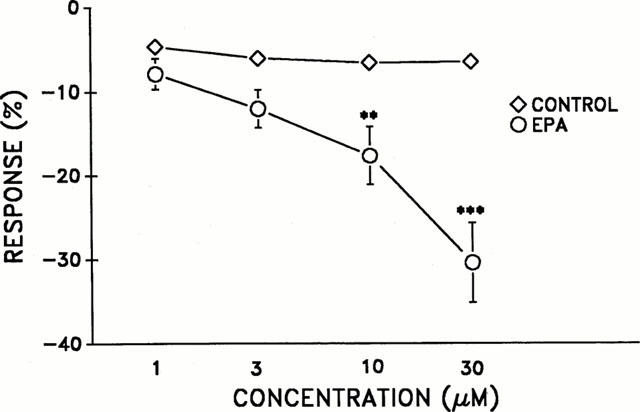
Inhibitory effect of eicosapentaenoic acid (EPA) on the sustained noradrenaline (1 μM) contraction in calcium-free solution containing EGTA (2 mM). n=6 per group. **P⩽0.01 and ***P⩽0.001 (statistical difference compared with parallel control data).
Influence of EPA incubation on concentration-response curves to calcium
As demonstrated in Figure 4, no significant difference in calcium-induced concentration curves (0.05–10 mM) were noted in the presence of EPA (10 μM or 30 μM) as compared to the control.
Figure 4.
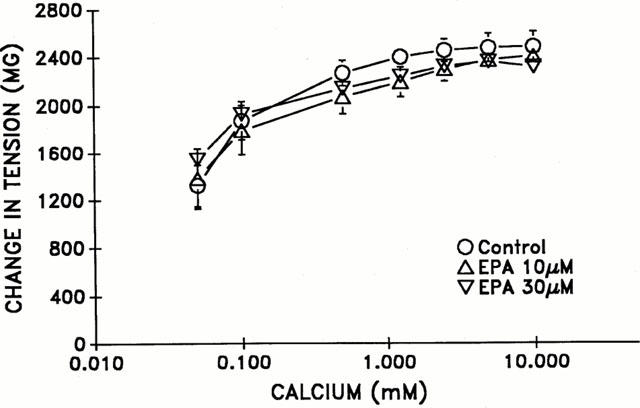
The effect of eicosapentaenoic acid (EPA) in intact aortic rings on cumulative concentration-response curves to calcium (0.05–10 mM). Aortic rings were initially exposed to calcium-free buffer without EGTA for a period of 20 min. followed by administration of noradrenaline (1 μM). n=3 per group.
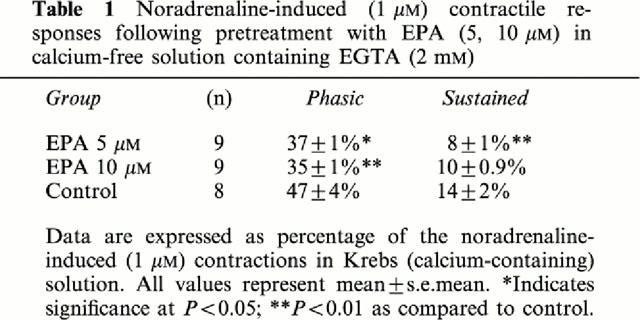
Effect of EPA on increases in tension induced by noradrenaline in calcium-free solution containing EGTA (2 mM)
Following incubation of EPA (5, 10 μM) for 20 min in calcium-free solution, noradrenaline (1 μM) induced phasic and sustained contractions, were significantly diminished (Table 1).
Table 1.
Noradrenaline-induced (1 μM) contractile responses following pretreatment with EPA (5, 10 μM) in calcium-free solution containing EGTA (2 mM)
Discussion
In the present study, we examined the mechanisms associated with the vasorelaxant properties of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in normotensive Wistar Kyoto rat aortae. The results indicate EPA induces an endothelium-independent relaxant effect primarily through the production of prostanoids which activate K+ATP channels. Prostanoid-mediated activation of ATP sensitive potassium channels has been previously documented in rat arteries (Bouchard et al., 1994). Specifically, prostacyclin (and its analogue iloprost) produce relaxation mediated by opening of K+ATP channels (Quast, 1993). Dietary intake of fish oils, rich in EPA, has been documented to increase production of total prostacyclin, PGI2 and PGI3 (Chin & Dart, 1995).
The inhibitory effects of indomethacin in EPA-induced relaxations (3–100 μM) suggests the involvement of vasodilator prostanoids, such as PGI2/3. Moreover, the contractile responses to EPA (1–100 μM) in the presence of both indomethacin and glibenclamide indicate that these vasodilator prostanoids activate KATP channels. Fatty acids may directly or indirectly through metabolic conversion affect ion channels on an associated cell membrane site (Ordway et al., 1991). Recent work by Asano et al. (1997) demonstrated EPA's (30 μM) induction of K+ currents in rat A7r5 smooth muscle cells. These findings support our current data.
Criteria for K+ channel openers has been established to include: (1) the induction of an outward K+ current and hyperpolarization of the cell membrane by K+ channel openers; (2) the vasorelaxant effect of K+ channel openers is abolished in media containing high concentrations of K+ (>50 mM); and (3) the vasorelaxant and K+ channel opening effects are inhibited by sulphonylureas, i.e., glibenclamide (Quast, 1993). The experimental evidence from the current study demonstrates that EPA meets criteria 3 as a K+ channel opener. Eicosapentaenoic acid under criteria 2 does not match the characteristics of a typical K+ channel opener since EPA inhibits high K+-induced contractions in the current study. In fact, this criteria is considered insufficient in itself to prove that membrane K+ opening is involved in the relaxing effect, since depolarization may effect many cellular activities.
It has been shown that pinacidil, a well known KATP opener vasodilator, inhibits contraction in rabbit mesenteric arteries and canine coronaries induced by high concentrations (90–128 mM) of extracellular K+ without decreasing [Ca2+]i (Itoh et al., 1991; Yanagisawa et al., 1990). We have also found pinacidil to inhibit contractions to high K+ (80 mM) in rat aortic rings. Interestingly, the inhibitory effect is similar to EPA. Many endogenous vasodilators act through membrane hyperpolarization caused by K+ channel activation in addition to other mechanisms independent of K+ channel activation (Nelson & Quayle, 1995). For example, calcitonin gene-related peptide (CGRP) induced relaxations in rabbit mesenteric arteries are inhibited only 57% by glibenclamide (Nelson et al., 1990).
The KATP opener vasodilators, pinacidil and levcromakalim, also cause vasorelaxation through mechanisms other than K+ channel opening (Itoh et al., 1991; Okada et al., 1993). Such mechanisms of pinacidil include a direct inhibitory action on the contractile apparatus in vascular smooth muscle (Itoh et al., 1991) and a decrease in [Ca2+]i by possible redistribution of Ca2+ to more peripheral intracellular sites. Levcromakalim's actions include a reduction in Ca2+ sensitivity of the contractile elements, which is antagonized by glibenclamide (Okada et al., 1993). It is apparent that EPA-induced relaxation may also be attributed to additional mechanisms aside from prostanoid-mediated K+ channel opening. It is evident that EPA-induced relaxation is not associated with opening of Ca2+-activated K+ channels since TEA had no effect.
The relaxant effect of EPA is further characterized as endothelium-independent and not dependent on the production/release of nitric oxide. This is evidenced by the negative findings resulting from pretreatment with L-NAME and physical removal of the endothelium. Although, activation of K+ channels is involved in EPA-induced relaxation, EDHF as a contributing factor is also not probable since removal of the endothelium and hence, EDHF, did not alter EPA's relaxant responses. The vasorelaxant properties of EPA due to plasmalemmal K+ channel opening and subsequent repolarization and/or hyperpolarization of the cell membrane are supported by the current results. Potassium channel openers have been shown to decrease the opening probability of voltage-dependent L- and T-type Ca2+ channels, reduce agonist-induced Ca2+ release from intracellular stores, and lower the efficiency of calcium as an activator of contractile proteins (Quast et al., 1994).
The lack of effect of EPA on Bay K 8644-induced contraction as well as calcium-induced contractile responses does suggest that EPA's action in rat aortae are not specifically directed at influx of Ca2+ through the L-type Ca2+ channel. Moreover, the spontaneous increase in aortic tension following refill with calcium-containing solution was not altered by EPA (30 μM). This indicates that EPA does not inhibit extracellular Ca2+ entry into the vascular smooth muscle cells nor refilling of intracellular Ca2+ stores. Although, previous studies have demonstrated the effects of omega-3 PUFAs in preventing ouabain toxicity-induced calcium overload and their modulation of calcium ion influx via L-type calcium channels in pretreated rat cardiac myocytes (Pepe et al., 1994; Hallaq et al., 1990), our results suggest a different mechanism of action.
Potassium channel openers also inhibit agonist induced contractions in calcium-free solution (Quast et al., 1994). In calcium-free solution, noradrenaline induces a biphasic contractile response (phasic, tonic) which is mediated by two different intracellular Ca2+ pools, i.e., SR, plasma membrane Ca2+ storage compartments in rat aortae (Heaslip & Rahwan, 1982). The initial phasic contraction and transient increase in [Ca2+]i with noradrenaline is believed to be due to release of Ca2+ from the sarcoplasmic reticulum (Karaki et al., 1997). In contrast, the sustained portion involves Ca2+ influx through both L-type and non-L-type Ca2+ channels. This suggests that EPA's actions not only involve intracellular Ca2+ pools, i.e., SR, but possibly Ca2+ influx through non-L-type Ca2+ channels. The current findings with EPA in Bay K 8644 seem to rule out the possible involvement of L-type Ca2+ channels in EPA-induced relaxation. The inhibitory effect of EPA on noradrenaline-induced phasic and sustained contractions in calcium-free medium may be the result of inhibition of intracellular Ca2+ release from the two dissimilar Ca2+ pools. Dietary investigations have also shown an altering effect by fish oil on calcium transport properties in rat myocytes (Karmazyn et al., 1987) and on modulation of calcium metabolism in WKY rat aortae (Smith et al., 1992).
In a previous study we also found EPA's vasorelaxant actions in Sprague-Dawley rat aortae to be possibly attributed to alterations in the intracellular Ca2+ pool (Engler, 1992b). The effects of EPA may involve interference with signal transduction systems, i.e., InsP3 formation. EPA reportedly has an inhibitory influence on phospholipase C and impairs synthesis of DAG and InsP3 in stimulated rat vascular smooth muscle (VSM) cells (Hui et al., 1992; Locher et al., 1989). Our recent studies with cultured VSM cells from spontaneously hypertensive rat (SHR) aortae also suggest an inhibitory effect of EPA on Ca2+ signalling (Engler et al., 1999a). EPA (30 μM) pretreatment in these cells attenuates angiotensin II-induced Ca2+ transients by 95%. Moreover, EPA per se produced an increase in [Ca2+]i lasting 20 min in cultured SHR VSM cells which was not altered by removal of extracellular Ca2+. We also found the level of InsP3 was unaffected in response to EPA. The slightly increased [Ca2+]i seen with EPA in SHR VSM cells may be important for subsequent signalling which underlie the vasorelaxant effects of EPA (Engler et al., 1999a).
Whether the relaxant effects of EPA and associated mechanism(s) of action in large conduit arteries (aortae) are similar in smaller resistance arteries is not known and requires further study. However, it is known that rat aortae and mesenteric arteries share the same extracellular Ca2+ influx and intracellular Ca2+ release mechanisms associated with noradrenaline-induced tension development (Godfraind et al., 1982). Given these similarities of calcium-mediated mechanisms in different sized vessels, the current calcium-related findings with EPA in aortae may have relevance in small resistance vessels. The use of isolated rat aortae as a bioassay system is in some respects ideal to study the direct effects of fatty acids or drugs on the vasculature. This is due to the lack of interference from nervous innervation and other central-mediated mechanisms that govern vascular responses.
Vascular structure and function shows some similarities between different species and thus careful extrapolation of animal studies to man is certainly warranted. For example, Ca2+ mobilization appears to be the key process in overall control of vascular smooth muscle responsiveness (Fleisch 1974). It remains to be elucidated whether the reduced vascular responsiveness seen in clinical trials with dietary fish oil is directly attributed to interference with Ca2+ mechanisms in VSM.
Several clinical trials have examined the in vivo vascular effects of dietary omega-3 fatty acid supplementation. Dietary fish oil reduced forearm vascular resistance responses to angiotensin II in normotensive men (Kenny et al., 1992) and improved endothelium-dependent flow-mediated dilation in hypercholesterolemic subjects (Goodfellow et al., 2000). Dietary intake of the omega-3 fatty acids has also been found to improve coronary vasomotion seen with sympathetic stimulation in patients with coronary artery disease (Yamamoto et al., 1992) and attenuated the reduction in forearm blood flow to noradrenaline in healthy subjects (Chin et al., 1993). This attenuation was abolished with indomethacin which supports the current in vitro findings with similar inhibitory effects by indomethacin in EPA-induced relaxation.
In conclusion, the primary mechanism of EPA-induced relaxation in WKY rat aortae appears to be mediated by prostanoids which activate K+ATP channels. Interestingly, EPA's effects are independent of nitric oxide and the endothelium. In WKY rat aortae, EPA's effects are concentration-dependent in inhibition of noradrenaline- and high K+-induced contractility. The vasorelaxant actions of EPA may also be attributed, in part, to specific inhibition of Ca2+ mobilization from the SR or other intracellular Ca2+ pools as well as non-L-type Ca2+ entry. L-type Ca2+ channels are less likely to be involved in EPA-induced relaxation in WKY aortae.
Acknowledgments
This work was supported by grant NR-02407 from the National Institute of Health, Bethesda, Maryland, USA. Special thanks to Scott Walz and Diane Heininger for typing of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- EDHF
endothelium-derived hyperpolarizing factor
- EPA
eicosapentaenoic acid
- K+ATP
ATP-sensitive K+ channel
- KCa
Ca2+-activated K+ channel
- KCl
potassium chloride
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- NA
noradrenaline
- NO
nitric oxide
- PGl2, PGl3
prostacyclin
- TEA
tetraethylammonium
- WKY
Wistar Kyoto
References
- ASANO M., NAKAJMA T., IWASAWA K., HAZAMA H., OMATO M., SOMA M., YAMASHITA K., OKUDA Y. Inhibitory effects of ω-3 polyunsaturated fatty acids on receptor-mediated non-selective cation currents in rat A7r5 vascular smooth muscle cells. Br. J. Pharmacol. 1997;120:1367–1375. doi: 10.1038/sj.bjp.0701047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHARD J.F., DUMONT E., LAMONTAGNE D. Evidence that prostaglandin I2, E2, D2 may activate ATP sensitive potassium channels in the isolated rat heart. Cardiovasc. Res. 1994;28:901–905. doi: 10.1093/cvr/28.6.901. [DOI] [PubMed] [Google Scholar]
- BOULANGER C., SHIMOKAWA H., SCHINI V.B., VANHOUTTE P.M.Vascular endothelium and ω-3 unsaturated fatty acids Endothelium-derived Contracting Factors 1990Basel: Karger; 169–177.ed. Rubanyi, G.M. & Vanhoutte, P.M. pp [Google Scholar]
- CHIN J.P.F., DART A.M. How do fish oils affect vascular function. Clin. Exp. Pharmacol. Physiol. 1995;22:71–81. doi: 10.1111/j.1440-1681.1995.tb01959.x. [DOI] [PubMed] [Google Scholar]
- CHIN J.P.F., GUST A.P., DART A.M. Indomethacin inhibits the effects of dietary supplementation with marine oils on vasoconstriction of human forearm resisitance vessels in vivo. J. Hypertens. 1993;11:1229–1234. [PubMed] [Google Scholar]
- COWAN C.L., COHEN R.A. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and NO-independent responses. Am. J. Physiol. 1991;261:H830–H835. doi: 10.1152/ajpheart.1991.261.3.H830. [DOI] [PubMed] [Google Scholar]
- DE DECKERE E.A.M., KORVER O., VERSCHUREN P.M., KATAN M.B. Health aspects of fish and n-3 polyunsaturated fatty acids from plant and marine origin. Eur. J. Clin. Nutr. 1998;52:749–753. doi: 10.1038/sj.ejcn.1600641. [DOI] [PubMed] [Google Scholar]
- ENGLER M.B. Vascular relaxation to omega-3 fatty acids: Comparison to sodium nitroprusside, nitroglycerin, papaverine, and D600. Cardiovasc. Drugs Ther. 1992a;6:605–610. doi: 10.1007/BF00052562. [DOI] [PubMed] [Google Scholar]
- ENGLER M.B. Effect of omega-3 fatty acids, docosahexaenoic and eicosapentaenoic, on norepinephrine-induced contractions. Can. J. Physio. Pharmacol. 1992b;70:675–679. doi: 10.1139/y92-086. [DOI] [PubMed] [Google Scholar]
- ENGLER M.B., ENGLER M.M. Influence of aging on the relaxant responses to ω-3 fatty acids in Fischer 344 rat aorta. Gerontology. 1996;42:25–35. doi: 10.1159/000213767. [DOI] [PubMed] [Google Scholar]
- ENGLER M.B., ENGLER M.M. Vasocactive properties of eicosapentaenoic acid in Wistar-Kyoto rat aorta. Circulation. 1997;96:1–132. [Google Scholar]
- ENGLER M.B., ENGLER M.M., MAYES M.M., URSELL P.C. Effects of the omega-3 fatty acids on vascular tone in hypercholesterolemia and balloon arterial injury. Asia Pacific Heart J. 1999b;8:27–35. [Google Scholar]
- ENGLER M.B., ENGLER M.M., URSELL P.C. Vasorelaxant properties of n-3 polyunsaturated fatty acids in aortas from spontaneously hypertensive and normotensive rats. J. Cardiovasc. Risk. 1994;1:75–80. [PubMed] [Google Scholar]
- ENGLER M.B., MA Y.H., ENGLER M.M. Calcium-mediated mechanisms of eicosapenatenoic acid-induced relaxation in hypertensive rat aorta. Am. J. Hypertens. 1999a;12:1225–1235. doi: 10.1016/s0895-7061(99)90060-2. [DOI] [PubMed] [Google Scholar]
- FLEISCH J.H. Pharmacoloy of the aorta. Blood Vessels. 1974;11:193–211. doi: 10.1159/000158014. [DOI] [PubMed] [Google Scholar]
- GARLAND C., PLANE F., KEMP B., COCKS T. Endothelium-dependent hyperpolarization: A role in the control of vascular tone. Trends Pharmacol. Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- GODFRAIND T., MILLER R., LIMA J.S. Selective α1- and α2-adrenoceptor agonist induced contractions and 45Ca fluxes in rat isolated aorta. Br. J. Pharmacol. 1982;77:597–604. doi: 10.1111/j.1476-5381.1982.tb09337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFELLOW J., BELLAMY M.F., RAMSEY M.W., JONES C.J.H., LEWIS M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000;35:265–270. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- HALLAQ H., SELLMAYER A., SMITH T.W., LEAF A. Protective effect of eicosapentaenoic acid on ouabain toxicity in neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7834–7838. doi: 10.1073/pnas.87.20.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEASLIP R.J., RAHWAN R.G. Evidence for the existence of two distinct pools of intracellular calcium in the rat aorta accessible to mobilization by norepinephrine. J. Pharmacol. Exp. Ther. 1982;221:7–13. [PubMed] [Google Scholar]
- HUI R., ROBILLARD M., FALARDEAU P. Inhibition of vasopressin-induced formation of diradylglycerols in vascular smooth muscle cells by incorporation of eicosapentaenoic acid in membrane phospholipids. J. Hypertens. 1992;10:1145–1153. doi: 10.1097/00004872-199210000-00006. [DOI] [PubMed] [Google Scholar]
- ITOH I., SUZUKI S., KURIYAMA H. Effects of pinacidil on contractile proteins in high K+-treated intact, and in β-escin-treated skinned smooth muscle of the rabbit mesenteric artery. Br. J. Pharmacol. 1991;103:1697–1702. doi: 10.1111/j.1476-5381.1991.tb09849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUAN H., SAMETZ W. Vasoconstriction induced by noradrenaline and angiotensin II is antagonized by eicosapentaenoic acid independent of formation of trienoic eicosanoids. Naunyn Schmiedebergs Arch. Pharmacol. 1986;332:288–292. doi: 10.1007/BF00504869. [DOI] [PubMed] [Google Scholar]
- JUAN H., SAMETZ W., SARIA A., PÖCH G. Eicosapentaenoic acid inhibits vasoconstrictor- and noradrenaline-potentiating effects of neuropeptide Y in the isolated rabbit ear. J. Auton. Nerv. Syst. 1988;22:237–242. doi: 10.1016/0165-1838(88)90112-9. [DOI] [PubMed] [Google Scholar]
- JUAN H., SUTTER D., SAMETZ W.Influence of eicosapentaenoic acid on noradrenaline and angiotensin-induced contractions of the rabbit aorta: Mode of action Prostaglandins in Clinical Research 1987New York: Alan R. Liss, Inc; 57–62.ed. Sinzinger, H. & Schrör, K. pp [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMATO S., NAZAKAWA H., WON K., SATO K. Calcium movements, distribution, and function in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KARMAZYN M., HORACKOVA M., MURPHY M. Effects of dietary cod liver oil on fatty-acid composition and calcium transport in isolated adult rat ventricular myocytes and on the response of isolated hearts to ischemia and reperfusion. Can. J. Physiol. Pharmacol. 1987;65:201–209. doi: 10.1139/y87-037. [DOI] [PubMed] [Google Scholar]
- KENNY D., WARLTIER D.C., PLEUSS J.A., HOFFMAN R.G., GOODFRIEND T.L., EGAN B.M. Effect of omega-3 fatty acids on the vascular response to angiotensin in normotensive men. Am. J. Cardiol. 1992;70:1347–1352. doi: 10.1016/0002-9149(92)90773-r. [DOI] [PubMed] [Google Scholar]
- LOCHER R., SACHINIDIS A., BRUNNER C., VETTER W. Intracellular free calcium concentration and thromboxane A2 formation of vascular smooth muscle cells are influenced by fish oil and n-3 eicosapentaenoic acid. Scand. J. Clin. Lab. Invest. 1991;51:541–547. doi: 10.3109/00365519109104563. [DOI] [PubMed] [Google Scholar]
- LOCHER R., SACHINIDIS A., STEINER A., VOGT E., VETTER W. Fish oil affects phosphoinositide turnover and thromboxane A metabolism in cultured vascular muscle cells. Biochim. Biophys. Acta. 1989;1012:279–283. doi: 10.1016/0167-4889(89)90109-2. [DOI] [PubMed] [Google Scholar]
- LUDBROOK J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc. Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- MCLENNAN P., HOWE P., ABEYWARDENA M., MUGGLI R., RAEDERSTORFF D., MANO M., RAYNER T., HEAD R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharmacol. 1996;300:83–89. doi: 10.1016/0014-2999(95)00861-6. [DOI] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Hyperpolarization contribute to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am. J. Physiol. 1991;261:H1034–H1037. doi: 10.1152/ajpheart.1991.261.4.H1034. [DOI] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Hyperpolarization as a mechanism for endothelium-dependent relaxations of the porcine coronary artery. J. Physiol. (Lond) 1992;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.T., HUANG Y., BRAYDEN J.E., HESCHELER J., STANDEN N.B. Activation of K+ channels is involved in arterial dilations to calcitonin gene-related peptide. Nature Lond. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M.Physiological roles and properties of potassium channels in arterial smooth muscle Am. J. Physiol. 1995268C799–C822.Cell Physiol., 37 [DOI] [PubMed] [Google Scholar]
- OKADA Y., YANAGISAWA T., TAIRA N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in canine coronary artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:438–444. doi: 10.1007/BF00165396. [DOI] [PubMed] [Google Scholar]
- OKUDA Y., ENZURE M., TSUKAHARA K., SAWADA T., MIZUTANI M., KATORI T., BANNAI C., YAMASHITA K. Eicosapentaenoic acid enhances intracellular free calcium in cultured human endothelial cells. Biochem. Med. Metab. Biol. 1994;51:166–168. doi: 10.1006/bmmb.1994.1021. [DOI] [PubMed] [Google Scholar]
- ORDWAY R.W., SINGER J.J., WALSH J.V. Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991;14:96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-Arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PEPE S., BOGDANOV K., HALLAQ H., SPURGEON H., LEAF A. ω-3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAST U. Do the K+ channel openers relax smooth muscle by opening K+ channels. Trends Pharmacol. Sci. 1993;14:332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- QUAST U., GUILLON J.M., CAVERO I. Cellular pharmacology of potassium channel openers in vascular smooth muscle. Cardiovasc. Res. 1994;28:805–810. doi: 10.1093/cvr/28.6.805. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., LAM J.Y.T., CHESEBRO J.H., BOWIE E.J.W., VANHOUTTE P.M. Effects of dietary supplementation with cod-liver oil on endothelium-dependent responses in porcine coronary arteries. Circulation. 1987;76:898–905. doi: 10.1161/01.cir.76.4.898. [DOI] [PubMed] [Google Scholar]
- SMITH J.M., PAULSON D.J., LABAK S. Effects of dietary fish oil on Rb+ efflux from aorta of stroke prone spontaneously hypertensive rats. Am. J. Hypertens. 1992;5:473–479. doi: 10.1093/ajh/5.7.473. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H., YOSHIMURA H., NOMA M., ORIGUCHI H., KUBOTA T., HARADA S., TAJIMI T., SUGIHARA M., KIKUCHI Y. Fish oil improves coronary vasomotion in response to sympathetic stimulation in patients with coronary artery disease. Circulation. 1992;86 Suppl I:I–835. [Google Scholar]
- YANAGISAWA A., LEFER A.M. Vasoactive effects of eicosapentaenoic acid on isolated vascular smooth muscle. Basic Res. Cardiol. 1987;82:186–196. doi: 10.1007/BF01907066. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA A., TESHIGAWARA T., TAIRA N. Cytoplasmic calcium and the relaxation of canine coronary arterial smooth muscle produced by cromakalim, pinacidil and nicorandil. Br. J. Pharmacol. 1990;101:157–165. doi: 10.1111/j.1476-5381.1990.tb12106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


