Abstract
Our previous finding that copper ions oxidize nitroxyl anion released from Angeli's salt to nitric oxide prompted us to examine if copper-containing enzymes shared this property.
The copper-containing enzyme, tyrosinase, which catalyses the hydroxylation of monophenols to diphenols and the subsequent oxidation of these to the respective unstable quinone, failed to generate nitric oxide from Angeli's salt by itself, but did so in the presence of tyrosine.
L-DOPA, the initial product of the reaction of tyrosinase with tyrosine, was not the active species, since it failed to generate nitric oxide from Angeli's salt. Nevertheless, L-DOPA and two other substrates, namely, catechol and tyramine did produce nitric oxide from Angeli's salt in the presence of tyrosinase, suggesting involvement of the respective unstable quinones. In support, we found that 1,4-benzoquinone produced a powerful nitric oxide signal from Angeli's salt.
Coenzyme Qo, an analogue of ubiquinone, failed to generate nitric oxide from Angeli's salt by itself, but produced a powerful signal in the presence of its mitochondrial complex III cofactor, ferricytochrome c.
Experiments conducted on rat aortic rings with the mitochondrial complex III inhibitor, myxothiazol, to determine if this pathway was responsible for the vascular conversion of nitroxyl to nitric oxide were equivocal: relaxation to Angeli's salt was inhibited but so too was that to unrelated relaxants.
Thus, certain quinones oxidize nitroxyl to nitric oxide. Further work is required to determine if endogenous quinones contribute to the relaxant actions of nitroxyl donors such as Angeli's salt.
Keywords: Angeli's salt, coenzyme Q, cytochrome c, mitochondria, nitric oxide, nitrovasodilator, nitroxyl anion, quinones, ubiquinone, vasodilatation
Introduction
Although nitric oxide radical (NO.) is generally accepted to be the most physiologically important form of NO, recent work suggest that its one-electron reduced species, i.e. nitroxyl anion (NO−), is produced during a number of biological reactions. These include production by nitric oxide synthase (Pufahl et al., 1995; Schmidt et al., 1996), although this has been challenged (Xia & Zweier, 1997), by the oxidation of azide by peroxidase (Tatarko & Bumpus, 1997), by the decomposition of S-nitrosothiols in the presence of thiol (Arnelle & Stamler, 1995), by the decomposition of peroxynitrite (Khan et al., 2000), and by the reduction of nitric oxide by ferrocytochrome c (Sharpe & Cooper, 1998) or cytochrome oxidase (Borutaite & Brown, 1996). Despite being unable to activate soluble guanylate cyclase (Dierks & Burstyn, 1996), nitroxyl anion generators such as Angeli's salt are powerful relaxants of smooth muscle. Moreover, their relaxant actions are associated with elevations of cyclic GMP content and blocked by the inhibitor of soluble guanylate cyclase, ODQ (Fukuto et al., 1992; Li et al., 1999). Consequently, it is now accepted that such relaxation takes place following tissue-dependent oxidation of nitroxyl to nitric oxide, and a number of potential pathways have been proposed, including oxidation by superoxide dismutase, flavin adenine dinucleotide, or methaemoglobin (Murphy & Sies, 1991; Fukuto et al., 1993; Schmidt et al., 1996).
We have recently reported that copper ions catalyse the one-electron oxidation of nitroxyl anion, released from Angeli's salt, to nitric oxide (Nelli et al., 2000). As copper ions are almost always bound in biological systems, we have been screening a number of copper-containing enzymes to determine their ability to promote this oxidation step. In this paper we describe our findings with one such copper-containing enzyme, tyrosinase, which catalyses two distinct reactions: the hydroxylation of monophenols to diphenols and the subsequent oxidation of these to the respective unstable quinone (Jiménez & García-Carmona, 2000). Tyrosinase has no effect by itself, but the quinones formed following its reaction with tyrosine and other substrates rapidly and efficiently promote the oxidation of nitroxyl to nitric oxide.
Methods
Measurement of nitric oxide generation
Nitric oxide generation was measured using ISO-NOP200 amperometric electrodes fitted to an ISO-NO Mark II nitric oxide meter (World Precision Instruments Ltd, U.K.), as previously described (Nelli et al., 2000). The signals generated were captured and displayed on a MacLab (8e Series, AD Instruments, U.K.). Electrodes were calibrated before use by generating nitric oxide from S-nitroso-N-acetyl-D,L-penicillamine (SNAP). Briefly, this was performed at room temperature (22°C) by inserting the electrode tip into a stirring solution of CuSO4 (0.1 M; 10 ml) prepared in distilled water taken to pH 4 with sulphuric acid, and allowing the background current to stabilise (usually 3 – 5 min). SNAP was then added in increasing concentrations (10 nM – 10 μM) and the maximum change in current (ΔpA) produced by each new addition recorded. The threshold concentration of SNAP found to generate a nitric oxide signal was ∼10 nM, and the response remained linear up to the highest concentration tested (10 μM).
Experiments involving the generation of nitric oxide from the nitroxyl anion liberated by Angeli's salt were conducted at 22°C with constant stirring in HEPES (N-[hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid])-buffered Krebs solution (pH 7.4) containing (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 2.4, D-glucose 11 and HEPES 5. Ethylenediaminetetraacetic acid (EDTA 30 μM) was added in all experiments to prevent the oxidation of nitroxyl to nitric oxide by contaminating copper ions (Nelli et al., 2000). Angeli's salt was added either as a single concentration (10 μM) or in increasing concentrations (10 nM – 10 μM) and the generation of nitric oxide was assessed as a function of the maximum change in current (ΔpA) produced upon each new addition. When the effects of agents were to be examined on the generation of nitric oxide, these were added 3 – 5 min before the addition of Angeli's salt.
Spectrophotometry
The reduction of ferricytochrome c (100 μM) to ferrocytochrome c by Angeli's salt (10 μM and 100 μM) and coenzyme Qo (1 mM) was assessed by the increase in absorbance measured at 550 nm in a Shimadzu dual beam spectrophotometer (model UV-2401 PC) at 22°C.
Vascular relaxation studies
Male Wistar rats (200 – 250 g) were killed by stunning and exsanguination. The thoracic aorta was then carefully removed, cleared of fat and connective tissue, and cut into transverse rings (2.5 mm wide). In all experiments except those involving relaxation to acetylcholine, the endothelium was removed by gentle abrasion of the intimal surface using a moist wooden stick (successful removal of the endothelium was confirmed later by the inability of acetylcholine 1 μM to induce relaxation). Aortic rings were then mounted under 1 g resting tension on stainless steel hooks within 10 ml tissue baths and maintained at 37°C in Krebs solution (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, glucose 11, gassed with 95% O2 and 5% CO2. Tension was recorded isometrically with Grass FTO3C transducers and displayed on a MacLab (E Series, AD Instruments) or a Grass polygraph model 7D. Tissues were allowed to equilibrate for 60 min before experiments were carried out, during which time the resting tension was re-adjusted to 1 g, as required.
All experiments involving relaxation were conducted on aortic rings following induction of 40 – 60% of maximal phenylephrine (PE)-induced tone: in control endothelium-denuded and endothelium-containing rings this level of tone was achieved with PE at 10 – 30 nM and 30 – 100 nM, respectively. In some experiments, the effects of myxothiazol (10 μM, 20 min), an inhibitor of mitochondrial complex III (Matsuno-Yagi & Hatefi, 1999), were examined on relaxation produced by Angeli's salt, sodium nitroprusside, 8-bromo-cGMP, acetylcholine, atrial natriuretic factor and papaverine. We found that myxothiazol depressed tone, but added higher concentrations of PE to ensure that the tone in each case was also 40 – 60% of the maximum. Data obtained from any tissue that fell outside this range were excluded.
Drugs
Acetylcholine chloride, L-ascorbic acid, atrial natriuretic factor (rat), 1,4-benzoquinone, 8-bromo-cGMP (8-bromoguanosine-3′:5′-cyclic monophosphate), catechol, coenzyme Qo (2,3-dimethoxy-5-methyl-1,4-benzoquinone), L-DOPA (L-3,4-dihydroxyphenylalanine), duroquinone (tetramethyl-1,4-benzoquinone), ferricytochrome c (horse heart), hydroquinone (1,4-dihydroxybenzene), menadione (vitamin K3; 2-methyl-1,4-naphthoquinone), myxothiazol, β-NADH (β-nicotinamide adenine dinucleotide, disodium salt), papaverine, phenylephrine hydrochloride, pyrogallol (1,2,3-trihydroxybenzene), S-nitroso-N-acetyl-D,L-penicillamine (SNAP), sodium nitroprusside, succinate (disodium salt, hexahydrate), superoxide dismutase (Cu-Zn-containing form from bovine erythrocytes), tyramine hydrochloride, L-tyrosine and tyrosinase (mushroom) were obtained from Sigma (Poole, U.K.). Angeli's salt (sodium trioxodinitrate) was obtained from Alexis (Nottingham, U.K.). All drugs were dissolved in saline (0.9%) except for: Angeli's salt (0.01 M), which was dissolved in 0.01 M NaOH; coenzyme Qo (0.1 M) and menadione (0.1 M), which were dissolved in acetone; duroquinone (0.03 M), myxothiazol (0.01 M) and papaverine (0.1 M), which were dissolved in dimethylsulphoxide; L-tyrosine (0.1 M) and catechol (0.1 M), which were dissolved in 0.1 M HCl; and SNAP (0.01 M) which was dissolved at pH 9 in distilled water containing EDTA (0.54 mM).
Analysis of data
Results are expressed as the mean±s.e.mean of n separate experiments. Relaxant responses are expressed as percentage (%) relaxation of PE-induced tone. Graphs were drawn and statistical comparisons were made by one-way analysis of variance followed by the Bonferroni post hoc test, or by Student's t-test, using a computer-based programme (GraphPad, Prism). A probability (P) of 0.05 or less was considered significant.
Results
Effects of tyrosinase and substrates on nitroxyl anion
When either the enzyme tyrosinase (250 u ml−1) or its substrate tyrosine (100 μM) was added alone to a solution of the nitroxyl anion generator, Angeli's salt (10 nM – 10 μM), in HEPES-buffered Krebs solution at 22°C no nitric oxide was produced. In contrast, if both enzyme and substrate were present together in solution with Angeli's salt, a powerful nitric oxide signal was detected (7419±380 pA, n=7; Figures 1 and 2). L-DOPA (100 μM), the primary product of the reaction between tyrosinase and tyrosine, also failed by itself to generate nitric oxide from Angeli's salt (10 μM), but together with tyrosinase, produced a powerful nitric oxide signal. Two other substrates for tyrosinase, i.e. catechol and tyramine (each at 100 μM), when present with the enzyme also produced powerful nitric oxide signals from Angeli's salt. Catechol also produced a small but significant signal from Angeli's salt in the absence of tyrosinase.
Figure 1.
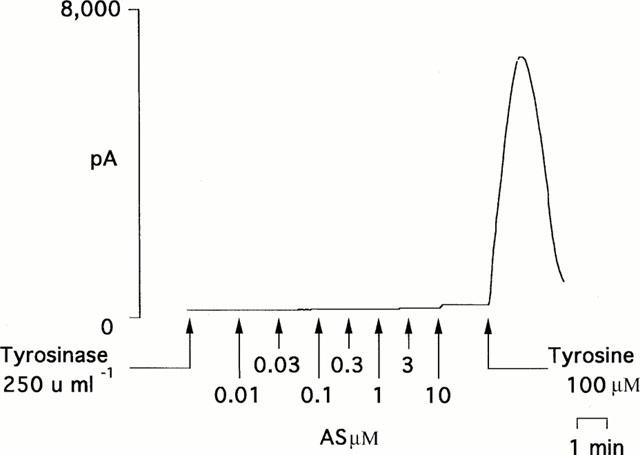
An individual experimental trace showing that tyrosinase (250 u ml−1) alone failed to promote the production of nitric oxide from the nitroxyl generator, Angeli's salt (AS; 0.01 – 10 μM). The additional presence of the substrate tyrosine (100 μM) led, however, to the generation of a powerful nitric oxide signal.
Figure 2.
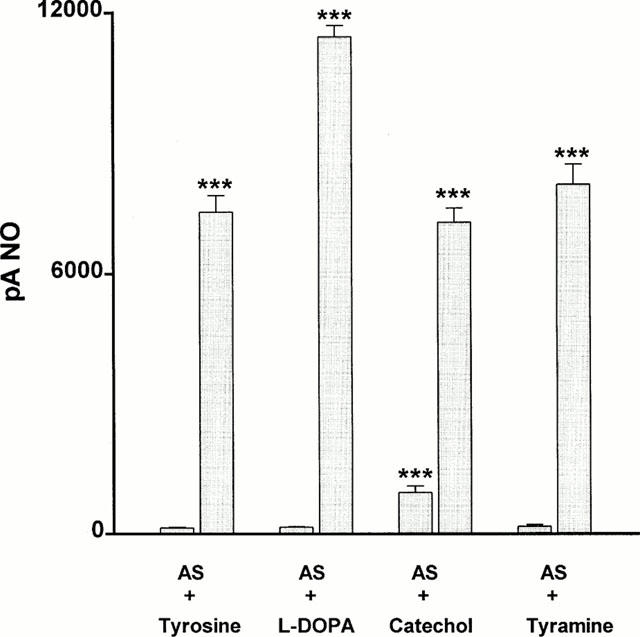
Bar graph showing the ability of tyrosine, L-DOPA, catechol and tyramine (each at 100 μM) either alone (first bar of pair) or in combination with tyrosinase (250 u ml−1; second bar of pair) to promote the production of nitric oxide from the nitroxyl generator, Angeli's salt (10 μM). Each value is the mean±s.e.mean of 5 – 7 observations. ***P<0.001 indicates a significant generation of nitric oxide.
Effects of quinones on nitroxyl anion
In addition to hydroxylating monophenols to diphenols, tyrosinase also catalyses the oxidation of these to the respective unstable quinone (Jiménez & García-Carmona, 2000). Consequently, we determined whether a number of quinone compounds had the potential to oxidize nitroxyl to nitric oxide in the absence of tyrosinase. 1,4-Benzoquinone (100 μM) produced a powerful nitric oxide signal (11,533±2213 pA, n=5) when reacted with Angeli's salt (10 μM; Figure 3). This generation of nitric oxide was unaffected following pretreatment with superoxide dismutase (250 u ml−1), but was abolished by ascorbate (100 μM). Three other quinones, i.e. coenzyme Qo, duroquinone and menadione (all at 100 μM) each failed to generate nitric oxide from Angeli's salt. Two hydroquinone compounds, i.e. hydroquinone itself and pyrogallol (each at 100 μM), also failed to generate nitric oxide from Angeli's salt.
Figure 3.
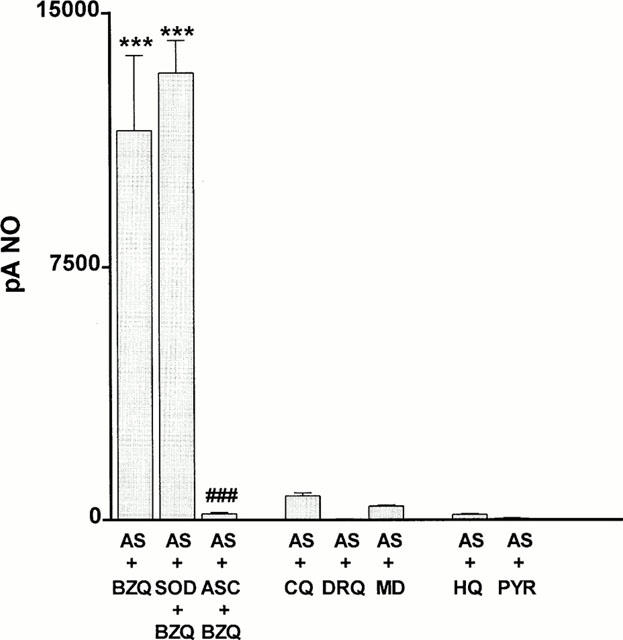
Bar graph showing the ability of benzoquinone (BZQ), coenzyme Qo (CQ), duroquinone (DRQ), menadione (MD), hydroquinone (HQ) and pyrogallol (PYR) (all at 100 μM) to promote the production of nitric oxide from the nitroxyl generator, Angeli's salt (10 μM). The ability of superoxide dismutase (SOD; 250 u ml−1) or ascorbate (ASC; 100 μM) to inhibit the generation of nitric oxide by benzoquinone is also shown. Each value is the mean±s.e.mean of 5 – 6 observations. ***P<0.001 indicates a significant generation of nitric oxide and ###P<0.001 indicates a significant inhibition by ascorbate.
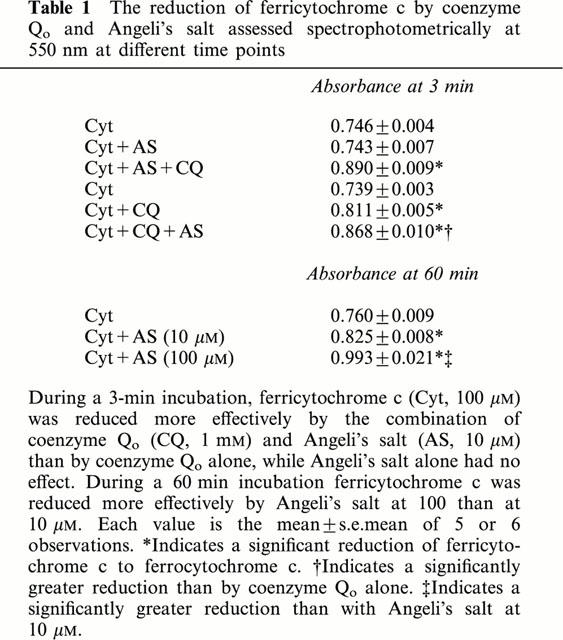
Since the major endogenous quinone, coenzyme Q participates in reactions in mitochondrial complex 1 (NADH – coenzyme Q reductase), complex II (succinate – coenzyme Q reductase) and complex III (coenzyme Q – cytochrome c reductase), we determined whether the additional presence of the respective cofactor had any effect on the actions of coenzyme Qo. Figure 4 shows that neither NADH (100 μM) nor succinate (100 μM) had any effect on the inability of coenzyme Qo (1 mM) to generate nitric oxide from Angeli's salt (10 μM). In contrast, in the presence of ferricytochrome c (100 μM), coenzyme Qo generated a powerful and immediate (maximal within 1 min) nitric oxide signal from Angeli's salt (Figures 4 and 5). For this reaction to take place, it was necessary to pre-react Angeli's salt with ferricytochrome c before the addition of coenzyme Qo. Moreover, pre-incubation with ascorbate (100 μM) prevented the formation of nitric oxide. Evidence of a concomitant redox reaction involving the reduction of ferricytochrome c was obtained by measuring absorbance at 550 nm: at 3 min Angeli's salt (10 μM) alone had no effect on ferricytochrome c (100 μM), coenzyme Qo alone produced a small reduction, but the two combined produced a greater reduction regardless of the order of addition (Table 1). Measurements taken at a later time point (60 min) showed evidence of a slowly developing direct reduction of ferricytochrome c by Angeli's salt (10 and 100 μM) in the absence of coenzyme Qo, but no concomitant generation of nitric oxide was detected (data not shown). The presence of ferricytochrome c had no effect on the inability of either duroquinone (100 μM) or menadione (100 μM) to generate nitric oxide from Angeli's salt (10 μM): maximum nitric oxide signals were 160±56 and 150±83 pA, respectively (n=5 for both).
Figure 4.
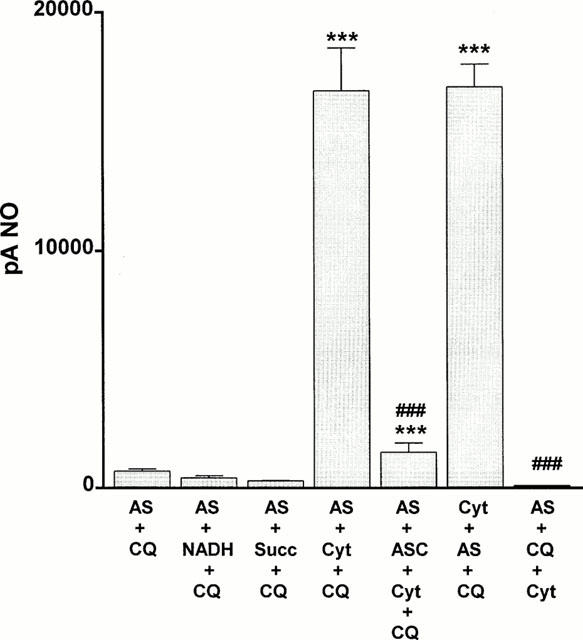
Bar graph showing the ability of coenzyme Qo (CQ; 1 mM) either alone or in combination with 100 μM of each of NADH, succinate (Succ) or ferricytochrome c (Cyt) to promote the production of nitric oxide from the nitroxyl generator, Angeli's salt (10 μM). Note that the order of addition is critical for nitric oxide formation, i.e. coenzyme Qo was effective only when added following pre-reaction of Angeli's salt with ferricytochrome c. The ability of ascorbate (ASC; 100 μM) to inhibit the production of nitric oxide by the combination of coenzyme Qo and ferricytochrome c is also shown. Each value is the mean±s.e.mean of five observations. ***P<0.001 indicates a significant generation of nitric oxide and ###P<0.001 indicates a significant reduction.
Figure 5.
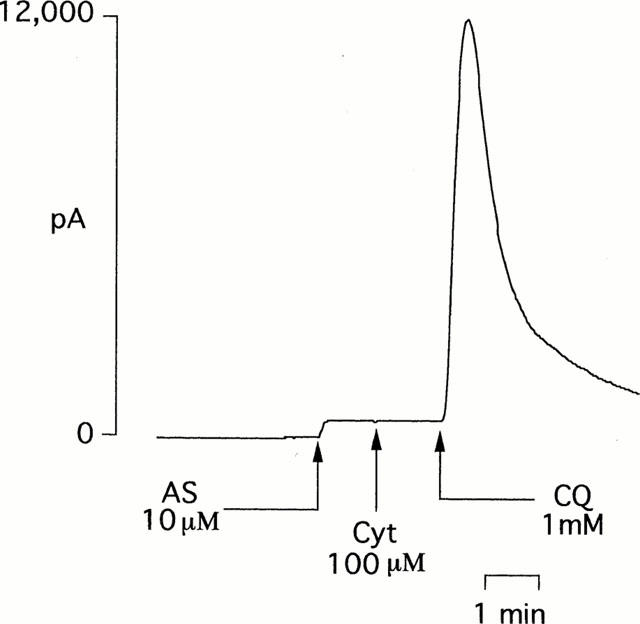
An individual experimental trace showing that in the presence of ferricytochrome c (Cyt; 100 μM), coenzyme Qo (1 mM) promotes the production of nitric oxide from the nitroxyl generator, Angeli's salt (10 μM).
Table 1.
The reduction of ferricytochrome c by coenzyme Qo and Angeli's salt assessed spectrophotometrically at 550 nm at different time points
Effects of myxothiazol on Angeli's salt-induced vasodilatation
The ability of coenzyme Qo to oxidize nitroxyl to nitric oxide in the presence of ferricytochrome c prompted us to determine if depleting the endogenous coenzyme by inhibiting mitochondrial complex III with myxothiazol (Matsuno-Yagi & Hatefi, 1999) affected the ability of Angeli's salt to relax endothelium-denuded rings of rat aorta. Treatment of aortic rings with myxothiazol (10 μM) for 20 min did indeed inhibit the relaxation induced by Angeli's salt (1 nM – 3 μM; Figure 6). Myxothiazol also inhibited relaxation to sodium nitroprusside (0.01 – 100 nM) and atrial natriuretic factor (0.1 – 10 nM), had no significant effect on relaxation to 8-bromo cGMP (1 – 300 μM), but enhanced slightly that to papaverine (0.1 – 100 μM). Myxothiazol also abolished relaxation to acetylcholine (0.01 – 10 μM) in endothelium-containing rings.
Figure 6.
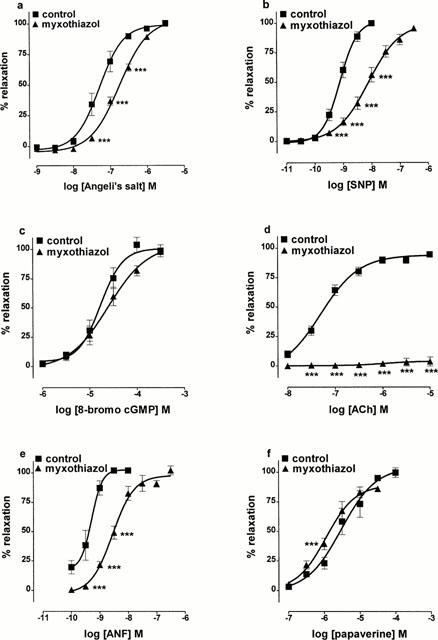
Concentration-response curves showing the effects of pretreating rat aortic rings with the mitochondrial complex III inhibitor, myxothiazol (10 μM; 20 min), on relaxation to (a) Angeli's salt, (b) sodium nitroprusside (SNP), (c) 8-bromo-cGMP, (d) acetylcholine (ACh), (e) atrial natriuretic factor (ANF), (f) papaverine. Each point is the mean±s.e.mean of 6 – 10 observations. ***P<0.001 indicates a significant difference from control.
Discussion
The possibility that endogenous copper might contribute at least in part to the vasodilator actions of the nitroxyl anion generator, Angeli's salt, is suggested by the ability of the copper chelator, diethyldithiocarbamate, to produce significant inhibition of relaxation to this agent in rat aorta (Pino & Feelisch, 1994) and by our recent report describing the oxidation of nitroxyl anion to nitric oxide by copper ions (Nelli et al., 2000). Since copper ions are almost always bound in biological systems, we began to screen copper-containing enzymes for their potential to account for the vasodilator actions of Angeli's salt arising from the one-electron oxidation of nitroxyl to nitric oxide. In keeping with previous reports (Murphy & Sies, 1991; Schmidt et al., 1996), we found that Cu-Zn superoxide dismutase could carry out this oxidation, but the amount of enzyme required to do so was far greater than exists in cells (Nelli et al., 2000). Ceruloplasmin was also effective, but we dismissed it for similar reasons, and ascorbate oxidase and tyrosinase were ineffective.
This study confirms that tyrosinase, which carries out the two-step process of converting monophenols to diphenols followed by their oxidation to the respective unstable quinone, and which is involved in melanization (Jiménez & García-Carmona, 2000), is itself unable to generate nitric oxide. Nevertheless, we found that when present together with its natural substrate, tyrosine, a large nitric oxide signal was generated from the nitroxyl released by Angeli's salt. L-DOPA, the primary product of the reaction of tyrosine with tyrosinase, was not the active agent, however, since it failed to promote oxidation of nitroxyl to nitric oxide in the absence of tyrosinase. Nevertheless, when present together with tyrosinase, L-DOPA produced a slightly larger nitric oxide signal than was seen with tyrosine as substrate. It was therefore possible that the oxidation of nitroxyl to nitric oxide took place in response to formation of the unstable secondary product of the enzymic reaction of tyrosine (or L-DOPA) with tyrosinase, i.e. DOPAquinone. Indeed, the intermediate formation of DOPAquinone was supported by the development of an intense pink colour, consistent with its rapid intramolecular cyclization to stable DOPAchrome (Jiménez & García-Carmona, 2000). Moreover, catechol and tyramine, two alternative diphenol substrates, which result in the formation of their respective aminoquinone, also produced large nitric oxide signals from Angeli's salt when present together with tyrosinase.
In view of the unstable nature of aminoquinones, we could not study their effects directly, but we were able to assess the effects of other more stable quinones. Indeed, we found that 1,4-benzoquinone produced a large nitric oxide signal from Angeli's salt in the absence of tyrosinase. This reaction was unaffected by superoxide dismutase so was unlikely to have proceeded via the ability of the quinone to generate superoxide anion. Moreover, the reduced species of 1,4-benzoquinone, i.e. hydroquinone (1,4-dihydroxybenzene) failed to generate nitric oxide from Angeli's salt, as did 1,4-benzoquinone in the presence of the reducing agent, ascorbate. Pyrogallol (1,2,3-trihydroxybenzene), a related hydroquinone species, also failed to generate nitric oxide from Angeli's salt. Taken together, these data suggest that it is the quinone form that participates in a direct redox reaction in which it is reduced, presumably to the hydroquinone, with concomitant oxidation of nitroxyl to nitric oxide. Not all quinones were active, however, since duroquinone and menadione (vitamin K3) each failed to generate nitric oxide from Angeli's salt.
Coenzyme Q (ubiquinone), which participates in mitochondrial electron transfer reactions, is one of the most abundant endogenous quinones, but its insolubility in an aqueous environment prevented us from examining directly its ability to oxidize nitroxyl to nitric oxide. Experiments with its more soluble analogue, coenzyme Qo, failed, however, to produce nitric oxide from Angeli's salt. Nevertheless, since coenzyme Q participates in reactions in mitochondrial complex I (NADH – coenzyme Q reductase), complex II (succinate – coenzyme Q reductase) and complex III (coenzyme Q – cytochrome c reductase), we investigated whether the additional presence of the respective cofactor had any effect on the actions of coenzyme Qo. Although coenzyme Qo failed to generate nitric oxide from Angeli's salt in the presence of NADH or succinate, it did produce a large signal in the presence of ferricytochrome C. Moreover, the order of addition was critical: nitric oxide was formed if Angeli's salt was permitted to pre-react with ferricytochrome c and coenzyme Qo was added subsequently, but none was formed if coenzyme Qo was added before ferricytochrome C. Nitroxyl released from Angeli's salt has previously been shown to react, albeit slowly, with ferricytochrome c leading to reduction to ferrocytochrome c and the predicted release of nitric oxide (Doyle et al., 1988). We confirmed the slow rate of this reduction of ferricytochrome c, i.e. none had taken place 3 min after the addition of Angeli's salt but measurable reduction was seen at 60 min. We, however, found no detectable formation of nitric oxide during this time. This outcome contrasts markedly with the immediate (maximal within 1 min) and powerful formation of nitric oxide seen when coenzyme Qo is added following the pre-reaction of ferricytochrome c with Angeli's salt. On this basis, it is therefore likely that coenzyme Qo acts by participating in a redox reaction with an intermediate formed from the reaction of ferricytochrome c and nitroxyl, presumably the nitrosylferricytochrome c complex, leading to the rapid formation of ferrocytochrome c and nitric oxide. Consistent with this, we found that ascorbate blocked the formation of nitric oxide by coenzyme Qo, presumably by blocking this redox reaction.
Our finding that coenzyme Qo leads to the production of nitric oxide from nitroxyl in the presence of ferricytochrome c, suggests that conditions suitable for this reaction may be present in mitochondrial complex III. Previous work suggests that nitric oxide/nitroxyl metabolism in mitochondria is highly complex. Specifically, nitric oxide synthase is present in the mitochondrion (Ghafourifar & Richter, 1997) and nitric oxide produced by this or by the other isoforms present in cells is believed to regulate respiration by two distinct mechanisms: a reversible inhibition at low concentrations involving reduction of nitric oxide to nitroxyl at the level of complex IV (Borutaite & Brown, 1996; Sharpe & Cooper, 1998) and an irreversible inhibition at high concentrations at the level of complex I involving the formation of peroxynitrite (Clementi et al., 1998; Orsi et al., 2000). Moreover, the stable breakdown product of nitric oxide, nitrite, can be recycled to nitric oxide in mitochondria under hypoxic conditions by a myxothiazol-sensitive process, suggesting reduction by complex III (Kozlov et al., 1999). Thus, different mitochondrial complexes have been implicated in the net removal or formation of nitric oxide. Consequently, we wished to test our additional suggestion that mitochondrial complex III might be involved in the formation of nitric oxide from nitroxyl by examining the effects of myxothiazol on the ability of Angeli's salt to induce relaxation of endothelium-denuded rings of rat aorta. Our rationale for these experiments was that inhibition of this complex with myxothiazol would lead to depletion of the coenzyme Q pool to its reduced, hydroquinone form (ubiquinol) (Kozlov et al., 1999) and so remove the quinone necessary for the ferricytochrome c-dependent oxidation of nitroxyl to nitric oxide. Consistent with this proposal, we found that treating aortic rings with myxothiazol did indeed inhibit relaxation to Angeli's salt. Caution must be exercised on interpreting this data, however, since the actions of myxothiazol were not entirely selective. Specifically, as is unsurprising for an agent that inhibits cellular respiration, myxothiazol led to a depression of muscle tone, with a consequent requirement for use of higher concentrations of the vasoconstrictor, phenylephrine. Of greater concern, however, was the ability of myxothiazol to inhibit relaxation to sodium nitroprusside and atrial natriuretic factor, vasodilators whose actions do not involve the production of nitroxyl anion. Moreover, relaxation to acetylcholine was abolished. This is almost certain to have occurred due to the known ability of metabolic inhibitors to block endothelium-dependent relaxation (Griffith et al., 1986; Richards et al., 1991) but, alternatively, it would be consistent with the production of nitroxyl by nitric oxide synthase (Schmidt et al., 1996). Relaxation to 8-bromo cGMP was, however, unaffected and that to papaverine was enhanced slightly. Thus, myxothiazol had a non-uniform effect on the actions of a range of vasodilators that act by different mechanisms. Consequently, firm evidence of our tentative suggestion that mitochondrial complex III might contribute to the oxidation of nitroxyl to nitric oxide by vascular tissue must await the outcome of a detailed study of nitric oxide/nitroxyl metabolism on isolated mitochondria and this is beyond the scope of the present study. Nevertheless, this study provides firm evidence that quinones can be added to the list of agents known to oxidize nitroxyl anion to nitric oxide and so potentially underlie the vasodilator actions of nitroxyl generators such as Angeli's salt.
In conclusion, our findings show that unstable quinones produced during the reaction of tyrosinase with several substrates have the ability to oxidize nitroxyl anion, generated from Angeli's salt, to nitric oxide. Experiments with coenzyme Qo, an analogue of the major endogenous quinone, coenzyme Q, suggest that it too can promote this oxidation reaction, but only in the presence of ferricytochrome c, i.e. under conditions present in mitochondrial complex III. These preliminary data suggest that a detailed study is warranted to determine if oxidation of nitroxyl to nitric oxide by mitochondrial complex III plays a role in the vasodilator actions of Angeli's salt.
Acknowledgments
This work was supported by the British Heart Foundation and the Wellcome Trust. K. Buyukafsar held a travel grant under the NATO Science Fellowship Programme of the Scientific and Technical Research Council of Turkey (TUBITAK).
Abbreviations
- ANF
atrial natriuretic factor
- 8-bromo cGMP
8-bromoguanosine-3′:5′-cyclic monophosphate
- EDTA
ethylenediaminetetraacetic acid
- HEPES
N-[hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- PE
phenylephrine
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
References
- ARNELLE D.R., STAMLER J.S. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- BORUTAITE V., BROWN G.C. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem. J. 1996;315:295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTI E., BROWN G.C., FEELISCH M., MONCADA S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIERKS E.A., BURSTYN J.N. Nitric oxide (NO.), the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- DOYLE M.P., MAHAPATRO S.N., BROENE R.D., GUY J.K. Oxidation and reduction of hemoproteins by trioxodinitrate (II). The role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- FUKUTO J.M., CHIANG K., HSZIEH R., WONG P., CHAUDHURI G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- FUKUTO J.M., HOBBS A.J., IGNARRO L.J. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem. Biophys. Res. Commun. 1993;196:707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- GHAFOURIFAR P., RICHTER C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- GRIFFITH T.M., EDWARDS D.H., NEWBY A.C., LEWIS M.J., HENDERSON A.H. Production of endothelium-derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc. Res. 1986;20:7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- JIMÉNEZ M., GARCÍA-CARMONA F. Hydroxylating activity of tyrosinase and its dependence on hydrogen peroxide. Arch. Biochem. Biophys. 2000;373:225–260. doi: 10.1006/abbi.1999.1519. [DOI] [PubMed] [Google Scholar]
- KHAN A.U., KOVACIC D., KOLBANOVSKIY A., DESAI M., FRENKEL K., GEACINTOV N.E. The decomposition of peroxynitrite to nitroxyl anion (NO−) and singlet oxygen in aqueous solution. Proc. Natl. Acad. Sci. USA. 2000;97:2984–2989. doi: 10.1073/pnas.050587297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZLOV A.V., STANIEK K., NOHL H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- LI C.G., KARAGIANNIS J., RAND M.J. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUNO-YAGI A., HATEFI Y. Ubiquinol:cytochrome c oxidoreductase. Effects of inhibitors on reverse electron transfer from the iron-sulfur protein to cytochrome b. J. Biol. Chem. 1999;274:9283–9288. doi: 10.1074/jbc.274.14.9283. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., SIES H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELLI S., HILLEN M., BUYUKAFSAR K., MARTIN W. Oxidation of nitroxyl anion to nitric oxide by copper ions. Br. J. Pharmacol. 2000;131:356–362. doi: 10.1038/sj.bjp.0703550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSI A., BELTRAN B., CLEMENTI E., HALLEN K., FEELISCH M., MONCADA S. Continuous exposure to high concentrations of nitric oxide leads to persistent inhibition of oxygen consumption by J774 cells as well as extraction of oxygen by the extracellular medium. Biochem. J. 2000;346:407–412. [PMC free article] [PubMed] [Google Scholar]
- PINO R.Z., FEELISCH M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- PUFAHL R.A., WISHNOK J.S., MARLETTA M.A. Hydrogen peroxide supported oxidation of NG-hydroxy-L-arginine by purified nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- RICHARDS J.M., GIBSON I.F., MARTIN W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br. J. Pharmacol. 1991;102:203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., HOFMANN H., SCHINDLER U., SHUTENKO Z.S., CUNNINGHAM D.D., FEELISCH M. No NO from NO synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARPE M.A., COOPER C.E. Reactions of nitric oxide with mitochondrial cytochrome c: A novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATARKO M., BUMPUS J.A. Further studies on the inactivation by sodium azide of lignin peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1997;339:200–209. doi: 10.1006/abbi.1996.9839. [DOI] [PubMed] [Google Scholar]
- XIA Y., ZWEIER J.L. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12705–12710. doi: 10.1073/pnas.94.23.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]


