Abstract
Mobilization of histamine from the ECL cells was monitored by gastric submucosal microdialysis in conscious rats. The ECL cells are known to operate under gastrin control and the purpose of the present study was to examine their in situ response to short-term (12 h) as well as long-term (28 days) hypergastrinaemia, induced by treatment with the proton pump inhibitor omeprazole.
Hypergastrinaemia promptly raised the histamine concentration in the microdialysate. The effect was prevented by CCK2 receptor blockade (YF476). On day 7 of omeprazole treatment the microdialysate histamine concentration reached a peak, five times higher than before treatment. Subsequently (14 and 28 days), less histamine was mobilized.
Gastrin infusion (4 h) raised the microdialysate histamine concentration in a dose-dependent manner in fasted rats and freely fed rats and in rats treated with omeprazole for a week. However, while fasted and fed rats responded to low doses of gastrin, the omeprazole-treated rats required large doses of gastrin to respond.
When the amount of histamine mobilized was related to the serum gastrin concentration the following EC50 values could be calculated: fasted rats 2.3×10−10 M, freely fed rats 2.5×10−10 M, omeprazole-treated rats 8.7×10−10 M. The maximal histamine responses in the three groups were 18.4 pmol 4 h−1±0.8, 21.9 pmol 4 h−1±1.2 and 68.0 pmol 4 h−1±3.5, respectively.
The results suggest that ECL cells, exposed to a high gastrin concentration for a week, respond with a shift in the receptor-ligand binding affinity from high to low. Apparently, CCK2 receptors of the ECL cells are subject to dynamic changes with respect to ligand-binding affinity.
Keywords: ECL cells, gastrin, CCK2 receptors, CCK2 receptor antagonist, histamine, histamine mobilization, omeprazole
Introduction
The ECL cells constitute the predominant endocrine/paracrine cell type in the oxyntic mucosa of the rat stomach (Håkanson et al., 1994; Chen et al., 1999). They produce and secrete histamine in response to gastrin. In fact, it is believed that gastrin stimulates acid secretion by mobilizing ECL-cell histamine that in turn stimulates histamine H2 receptors on adjacent parietal cells (Waldum et al., 1991; Andersson et al., 1996).
We have recently implemented a technique for gastric submucosal microdialysis which enables us to monitor the secretion of ECL-cell histamine in intact, conscious rats (Kitano et al., 2000a). The aim of the present study was to use this technique to examine the mobilization of ECL-cell histamine in conscious rats with either hypo- or hyper-gastrinemia. Hypogastrinemia was induced by food deprivation. Hypergastrinemia was induced by continuous intravenous infusion of gastrin or by treatment with the proton pump inhibitor omeprazole, which causes hyergastrinemia by eliminating the acid feedback inhibition of gastrin release (Ryberg et al., 1989). The receptor responsible for the effect of gastrin on the ECL cells is of the cholecystokinin 2-type (CCK2) (Sandvik & Waldum, 1991; Prinz et al., 1994; Ding et al., 1997a; Chen et al., 2000). The effect of CCK2-receptor blockade was investigated by the use of YF476, a potent and selective CCK2 receptor antagonist, which is known to prevent gastrin from mobilizing ECL-cell histamine (Lindström et al., 1999).
Methods
Animals
Male-Sprague-Dawley rats, weighing 250 – 300 g, were used in this study. The rats were housed in plastic cages under conditions of controlled temperature, humidity and 12 : 12 h light – dark cycle. They received standard laboratory chow and tap water ad libitum. Before the experiments, the rats had been thoroughly familiarized with Bollman-type restraining cages for at least 2 weeks. All rats were equipped with a microdialysis probe (MAB 3.8.10, membrane length 10 min, outer diameter 0.57 mm, cut-off 35 kDa, AgnTho's AB, Lidingö, Sweden), inserted in the submucosa of the acid-producing part of the stomach as previously described by Kitano et al. (2000a). At the same time the rats were fitted with an indwelling catheter in the right jugular vein. After implantation of the microdialysis probe, the rats were left to recover for 3 days in individual wire-mesh-bottom cages with free access to food and water. When rats were to be fasted (48 h) they were denied food but had free access to water. During sampling from the microdialysis probes (see below), they were kept in Bollman-type restraining cages. Blood samples for the determination of serum gastrin were drawn repeatedly from the tail. After completion of the study, the animals were killed by an overdose of chloral hydrate intraperitoneally. The experimental protocol was approved by the local Animal Welfare Committee of Lund/Malmoe.
Drugs
Human Leu15-gastrin-17 was purchased from Research Plus, Bayonne, NJ, U.S.A. It was dissolved in 0.9% saline, containing 0.1% bovine serum albumin (ICN, Aurora, OH, U.S.A.). The proton pump inhibitor omeprazole was obtained from AstraZeneca, Molndal, Sweden and dissolved in 0.25% Methocel (Dow Corning, Midland, MI, U.S.A.). Omeprazole or vehicle was administered once daily by oral gavage (400 mmol kg−1 day−1 (Larsson et al., 1986) between 0800 and 1000 h. The CCK2 receptor antagonist YF476, kindly provided by Dr A Harris (Ferring, Vanlose, Denmark), was dissolved in polyethylene glycol 300 (Acros Organics, Geel, Belgium). YF476 (or vehicle) was given by a single subcutaneous injection of 300 mmol kg−1, a dose known to cause sustained CCK2 receptor blockade for at least a month (Kitano et al., 2000b).
Microdialysis
Microdialysis was performed 3 days after implantation of the probe. All rats were conscious throughout the experiment since anaesthesia is known to suppress ECL-cell histamine mobilization (Norlén et al., 2000). The inlet tube of the microdialysis probe was connected to a microinfusion pump and the outlet tube was allowed to drain in 300 μl polyethylene vials. Perfusion of the microdialysis probe with 0.9% saline (72 μl h−1) started at 0800 h. After 2 h equilibration, collection of microdialysate commenced.
Determination of gastrin and histamine
Gastrin was determined in serum by radioimmunoassay (Stadil & Rehfeld, 1973). Histamine was determined in the microdialysate samples by radioimmunoassay (Morel & Delaage, 1988). The radioimmunoassay kit was from Immunotech, Marseille, France.
Experimental design
Effect of acute omeprazole
Sixteen freely fed rats were used. After equilibration for 2 h, two microdialysis samples were collected with hourly intervals. After the first two microdialysate samples (basal) had been collected, omeprazole or vehicle was administered. Microdialysate samples were then collected every 20 min in the first hour and then hourly for 6 h. After collecting the 6 h samples the rats were returned to their cages until they were again placed in the Bollman cages 1 h before collecting the 12 h samples. Blood samples for determining the serum gastrin concentration were drawn from the tail 0, 2, 4, 6 and 12 h after giving omeprazole or vehicle.
Effect of long-term omeprazole and/or YF476
One hundred and forty-eight fed rats were allocated to four groups; omeprazole treatment, YF476 treatment, treatment with omeprazole+YF476 or vehicle (controls). Rats received omeprazole (400 mmol kg−1) or vehicle orally every day (between 0900 and 1100 h) except on the day of sampling. Treatment started on day 0 and was discontinued after 1, 2, 4, 5, 7, 14 or 28 days. YF476 (300 mmol kg−1) was given as a single subcutaneous injection on day 0. Histamine in the microdialysate samples and gastrin in the serum were measured (6 – 8 rats in each group). Serum and microdialysate samples were collected between 0900 and 1100 h. Each rat was used for sampling once only.
Gastrin dose-response curves in hypo-, normo- and hyper-gastrinemic rats
One hundred and sixteen rats were allocated to three groups: fasted rats, freely fed rats and rats treated with omeprazole for 1 week. Omeprazole was not given on the day of the experiment. The rats were placed in Bollman cages at 0800 – 0900 h with free access to food and water during the experiment (except the fasted rats which were denied food). After collecting basal microdialysate samples for 2 h, continuous intravenous infusion of increasing doses of human Leu15-gastrin-17 (0.15, 0.5, 1.5, 5, 15, 50, 150 or 500 nmol kg−1 h−1, 1 ml h−1) was initiated. Microdialysate samples were collected every 20 min during the first hour and then hourly for the next 3 h. The basal serum gastrin concentration was determined in samples collected from the tail vein during the equilibration period preceding the first sampling of microdialysate, while the serum gastrin concentration at the conclusion of the 4-h gastrin infusion was determined after the last microdialysate sample had been collected. The amounts of histamine mobilized are expressed as integrated increment of histamine in the microdialysate over a 4 h time period of gastrin infusion or as integrated total histamine output in the microdialysate during the same time period.
Statistical analysis
All values are expressed as mean±s.e.mean. Analysis by ANOVA was followed by Dunnett's t-test for unpaired data. P values of <0.05 were considered significant. Dose-response curves, concentration-response curves, EC50 values (i.e. the concentration producing 50% of the maximal effect), and the 95% confidence interval (CI) were constructed/calculated using a GraphPad PRISM program (version 3.00, GraphPad Software, San Diego, CA, U.S.A.).
Results
Effects of a single dose of omeprazole
Changes in the microdialysate histamine concentration and the serum gastrin concentration were monitored for 12 h after the administration of a single dose of omeprazole. The histamine concentration increased until a plateau was reached after 6 h (3 fold increase). The changes in serum gastrin preceded those in microdialysate histamine with a few hours (Figure 1).
Figure 1.
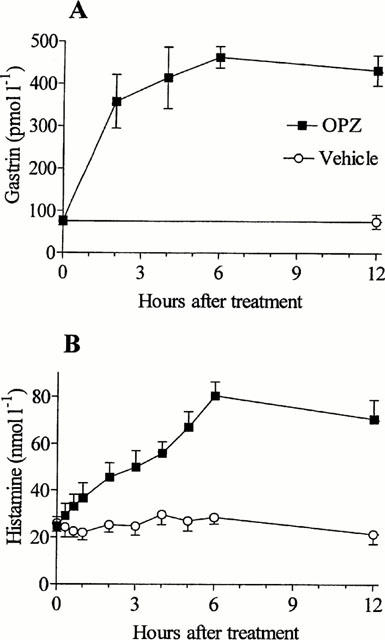
Serum gastrin (A) and microdialysate histamine (B) concentrations were monitored for 12 h after the administration of a single oral dose of omeprazole (OPZ, 400 μmol kg−1) or vehicle (at zero time). Means±s.e.mean (8).
Effects of long-term treatment with omeprazole and/or YF476
Daily omeprazole treatment gradually raised the serum gastrin concentration until a plateau was reached after about 7 days (15 fold elevation). Also the histamine concentration in the gastric submucosal microdialysate increased until day 7 when it reached a peak (151 nmol l−1±10), about five times higher than before treatment (Figure 2). On day 14 and 28, there was a decline in the amount of histamine mobilized; at this stage the histamine concentration was about three times higher than before treatment (89 nmol l−1±9). Treatment with YF476 raised the serum gastrin concentration to the same level as after omeprazole and lowered the amount of histamine mobilized compared to vehicle-treated controls already a few days after the start of the experiment. On day 28, the YF476-treated rats had a microdialysate histamine concentration of 15 nmol l−1±2; this value should be compared with that in vehicle-treated controls (32.6 nmol l−1±6.1) (P<0.05). YF476 also prevented the omeprazole-induced increase in microdialysate histamine (Figure 2).
Figure 2.
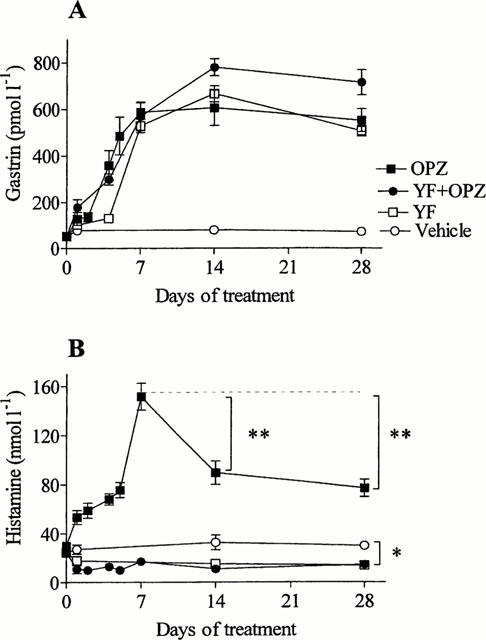
Serum gastrin (A) and microdialysate histamine (B) concentrations in response to treatment with omeprazole (OPZ), YF476 (YF), OPZ+YF or vehicle (control group), starting at zero time. OPZ was given daily by the oral route, YF was deposited subcutaneously once. OPZ, YF and OPZ+YF induced a slow, progressive increase in the serum gastrin concentration for about a week. Subsequently, the elevated serum gastrin concentration remained at a plateau for the duration of the experiment. The microdialysate histamine concentration reached a peak after 7 days of OPZ treatment and declined thereafter. The concentration at day 7 was compared to the concentrations at days 14 and 28, **P<0.01, (Dunnett's test). The microdialysate histamine concentration of the control rats was reduced by treatment with YF476 (*P<0.05, and the effect of OPZ was prevented by YF. Means±s.e.mean (8 – 20).
Gastrin dose-response curves in hypo-, normo- and hypergastrinemic rats
Fasted rats had lower serum gastrin concentration (11.0 pmol l−1±0.7, hypogastrinemia) than freely fed rats (76.6 pmol l−1±6.7, normogastrinemia). Rats treated with omeprazole for 1 week had greatly elevated serum gastrin concentration (589 pmol l−1±40, hypergastrinemia). Gastrin infusion promptly raised the microdialysate histamine concentration in a dose-dependent manner in fasted, freely fed and omeprazole-treated rats (Figure 3). Fasted and fed rats responded to quite low doses of gastrin, while omeprazole-treated rats required large doses of gastrin to respond. The data were used to plot the amount of histamine mobilized versus the serum gastrin concentration, and the EC50 values were calculated (Figure 4). The EC50 value in the omeprazole-treated rats was significantly higher (P<0.05) than in the fasted rats, and the maximal histamine response was much higher in the omeprazole-treated rats than in the two other groups (P<0.05).
Figure 3.
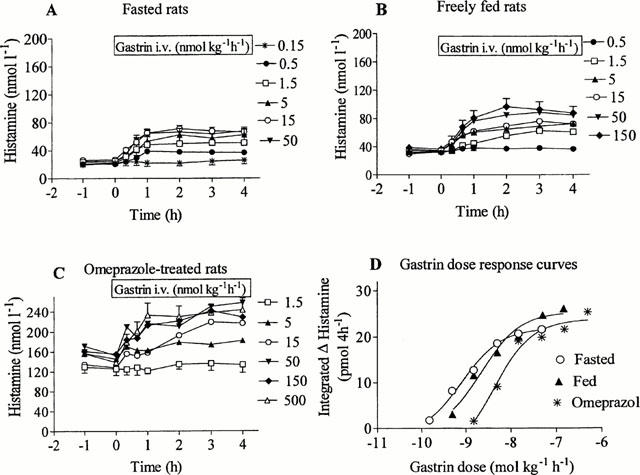
Gastrin-evoked mobilization of histamine in fasted rats (A), freely fed rats (B) and rats treated with omeprazole daily for 1 week (C). Omeprazole was given by the oral route. Gastrin-17 was given by continuous intravenous infusion in doses as indicated. The pre-gastrin level of histamine in the microdialysate of freely fed rats (34.3 nmol l−1±1.5) was 1.5 times higher than in fasted animals (22.7 nmol l−1±0.1); in omeprazole-treated rats the pre-gastrin histamine level (145 nmol l−1±6.2) was seven times higher than in the fasted rats. Means±s.e.mean (36 – 48). Gastrin dose-response curves (D) were constructed (using the GraphPad PRISM program) for the three groups of rats based on 4-h integrated increments in histamine.
Figure 4.
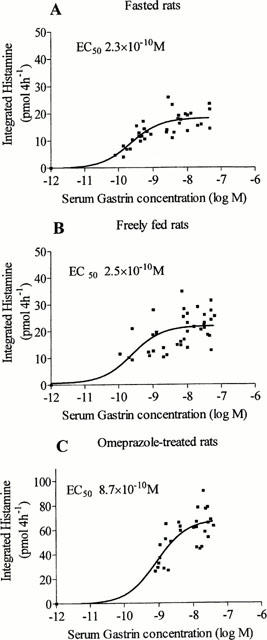
Gastrin concentration-response curves in hypo- (fasted, A), normo- (freely fed, B) and hypergastrinemic (1-week omeprazole treatment, C) rats. The curves were constructed from the serum gastrin and microdialysate histamine concentrations of the experiments in Figure 3 and from the assumption that no histamine would be released from the ECL cells at a gastrin concentration of 10−12 M. The EC50 values were calculated by identifying the gastrin concentration at the point of intersection of the concentration-response curve with the half-maximal level of histamine release. The EC50 values were 2.3×10−10 M in the fasted rats (95% confidence interval, CI: 1.3×10−10 - 3.0×10−10), 2.5×10−10 M in the freely fed rats (CI: 9.8×10−11 - 6.7×10−10) and 8.7×10−10 M in the omeprazole-treated rats (CI: 4.6×10−10 - 1.6×10−9). The maximal histamine response was 18.4 pmol 4 h−1±0.8, 21.9 pmol 4 h−1±1.2 and 68.0 pmol 4 h−1±3.5 in hypo-, normo- and hypergastrinemic rats, respectively.
Discussion
Using the technique of gastric submucosal microdialysis in conscious rats we were able to show that omeprazole mobilized gastric histamine. A single dose of omeprazole promptly raised the serum gastrin concentration and the microdialysate histamine concentration for up to 12 h. Thus, the omeprazole-evoked decrease in gastric acid secretion stimulated gastrin secretion and consequently also ECL-cell histamine mobilization. Long-term omeprazole treatment induced a progressive rise in the serum gastrin concentration until a plateau was reached 7 days after start of treatment. In this experiment serum samples were collected shortly before giving the subsequent dose, and the slow progressive rather than immediate rise in serum gastrin probably reflects the fact that it takes time to build up long-lasting acid inhibition by omeprazole given once a day. On the whole, the microdialysate histamine concentration changed according to the serum gastrin concentration with an initial slow rise and a peak after 7 days. That omeprazole-evoked mobilization of ECL-cell histamine depends on gastrin was supported by the finding that the CCK2 receptor antagonist YF476 prevented the histamine response to omeprazole. Gastrin interacts with two distinct receptor types, referred to as CCK1 and CCK2, which have been cloned and characterized (Kopin et al., 1992; Wank et al., 1994). ECL cells, which represent an important gastrin target, seem to have only CCK2 receptors (Chiba et al., 1991; Asahara et al., 1994; Ding & Håkanson, 1996, Ding et al., 1997b,1997c). YF476 is a potent and quite selective CCK2 receptor antagonist, which inhibits gastrin-induced gastric acid secretion (Kitano et al., 2000b) and also prevents gastrin-induced histamine mobilization in isolated ECL cells (Lindström et al., 1999) as well as in ECL cells in situ (Chen et al., 2000; Kitano et al., 2000a;2000b). The suppression of ECL-cell histamine mobilization in YF476-treated rats compared to vehicle-treated controls means that the drug blocks not only the effect of omeprazole-induced hypergastrinemia but also that of physiologically secreted gastrin in freely fed rats.
The microdialysate histamine concentration is likely to reflect the activity (and histamine content) of the ECL cells. Although the mobilization of histamine was stimulated as a result of omeprazole treatment, with a peak after 7 days, the histamine response was lower after 14 and 28 days of omeprazole treatment than after 7 days. We have previously examined the time course of omeprazole-induced effects on the ECL cells in intact rats and shown the ECL-cell HDC activity to be maximally stimulated after 7 days of omeprazole treatment followed by a progressive down-regulation (Kimura et al., 1997). Based on these results we suggested that sustained hypergastrinemia leads to a reduced ability of the ECL cells to respond to gastrin, perhaps through a reduced affinity of the CCK2 receptor for gastrin. In the present study, we made an attempt to explore the mechanism behind the reduced mobilization of histamine in response to omeprazole. Gastrin was infused intravenously in increasing doses to rats with varying serum gastrin concentrations: hypo- (fasted), normo- (fed), and hypergastrinemic (omeprazole-treated) rats. Gastrin dose-response and concentration-response curves were constructed, showing that the EC50 value of gastrin-induced histamine mobilization depended on the serum gastrin concentration prior to the administration of exogenous gastrin. In hypergastrinemic rats the EC50 value was 3.5 times higher than in hypogastrinemic (fasted) rats (P<0.05). Interestingly, the maximal histamine response was more than three times greater in the hypergastrinemic rats than in the hypo- and normo-gastrinemic rats. The increased histamine response (after 1 week of omeprazole treatment) does not reflect an increase in the number of ECL cells (Larsson et al., 1986; Kitano et al., 2000b) but reflects either an increased amount of releasable ECL-cell histamine or an increased receptor number per individual ECL cell. From the kinetic data we propose also that the CCK2 receptors of the ECL cells change from a high to a low affinity state when exposed to high concentrations of gastrin (see also Chen et al., 1998). Our observations indicate that the CCK2 receptors of the ECL cells are subject to dynamic changes with respect to ligand-binding affinity and that the serum gastrin concentration is a controlling factor.
Acknowledgments
This study was supported by grants from the Swedish MRC (04X-1007) and the A. Påhlsson Foundation.
Abbreviations
- CCK
cholecystokinin
- CCK2 receptor
cholecystokinin 2-type receptor
- CI
confidence interval
- ECL cell
enterochromaffin-like cell
- OPZ
omeprazole
- YF
YF476, a CCK2 receptor blocker
References
- ANDERSSON K., CABERO J.L., MATTSSON H, HÅKANSON R. Gastric acid secretion after depletion of enterochromaffin-like cell histamine. A study with α-fluoromethylhistidine in rats. Scand. J. Gastroenterol. 1996;31:24–30. doi: 10.3109/00365529609031622. [DOI] [PubMed] [Google Scholar]
- ASAHARA M., KINOSHITA Y., WAKATA H., MATSUSHIMA Y., NARIBAYASHI Y., NAKAMURA A., MATSUI H., CHIHARA K., YAMAMOTO J., ICHIKAWA A, CHIBA T. Gastrin receptor genes are expressed in gastric parietal cells and enterochromaffin-like cells of Mastomys natalensis. Dig. Dis. Sci. 1994;39:2149–2156. doi: 10.1007/BF02090363. [DOI] [PubMed] [Google Scholar]
- CHEN D., ZHAO C.-M., LINDSTRÖM E, HÅKANSON R. Rat stomach ECL cells: Up-date of biology and physiology. Gen. Pharmacol. 1999;32:413–422. doi: 10.1016/s0306-3623(98)00221-3. [DOI] [PubMed] [Google Scholar]
- CHEN D., ZHAO C.-M., NORLÉN P., BJÖRKQVIST M., DING X.-Q., KITANO M, HÅKANSON R. Effect of cholecystokinin-2 receptor blockade on rat stomach ECL cells. A histochemical, electron-microscope and chemical study. Cell. Tissue Res. 2000;299:81–95. doi: 10.1007/s004419900136. [DOI] [PubMed] [Google Scholar]
- CHEN D., ZHAO C.-M., YAMADA H., NORLÉN P, HÅKANSON R. Novel aspects of gastrin-induced activation of histidine decarboxylase in rat stomach ECL cells. Regul. Pept. 1998;77:169–175. doi: 10.1016/s0167-0115(98)00111-6. [DOI] [PubMed] [Google Scholar]
- CHIBA T., KINOSHITA Y., MORISHITA T., NAKATA H., NAKAMURA A, HOSODA S. Receptors for gastrin on gastric carcinoid tumor membrane of Mastomys natalensis. Biochem. Biophys. Res. Communs. 1991;177:739–744. doi: 10.1016/0006-291x(91)91850-c. [DOI] [PubMed] [Google Scholar]
- DING X.-Q, HÅKANSON R. Evaluation of the specificity and potency of a series of cholecystokinin-B/gastrin receptor antagonists in vivo. Pharmacol. Toxicol. 1996;79:124–130. doi: 10.1111/j.1600-0773.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E, HÅKANSON R. Time course of deactivation of rat stomach ECL cells following cholecystokinin B/gastrin receptor blockade. Brit. J. Pharmacol. 1997a;122:1–6. doi: 10.1038/sj.bjp.0701316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E, HÅKANSON R. Cholecystokinin-B/gastrin receptor blockade suppresses the activity of rat stomach ECL cells. Pharmacol. Toxicol. 1997b;81:19–25. doi: 10.1111/j.1600-0773.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E, HÅKANSON R. Evaluation of three novel cholecystokinin-B/gastrin receptor antagonists: A study of their effects on rat stomach enterochromaffin-like cell activity. Pharmacol. Toxicol. 1997c;81:232–237. doi: 10.1111/j.1600-0773.1997.tb00052.x. [DOI] [PubMed] [Google Scholar]
- HÅKANSON R., CHEN D, SUNDLER F.The ECL cells Physiology of the Gastrointestinal Tract 19942Raven Press; 1171–1184.(ed. LR Johnson) vol3rd et [Google Scholar]
- KIMURA K., CHEN D., LINDSTRÖM E., YAMADA H., ZHAO C.-M, HÅKANSON R. Functional impairment of the individual rat stomach ECL cells in response to sustained hypergastrinemia. Regul. Pept. 1997;72:69–77. doi: 10.1016/s0167-0115(97)01027-6. [DOI] [PubMed] [Google Scholar]
- KITANO M., NORLÉN P., DING X.-Q., NAKAMURA S, HÅKANSON R. Long-lasting cholecystokinin-2 receptor blockade after a single subcutaneous injection of YF476 or YM022. Brit. J. Pharmacol. 2000b;130:699–705. doi: 10.1038/sj.bjp.0703342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITANO M., NORLÉN P, HÅKANSON R. Gastric submucosal microdialysis: a method to study gastrin- and food-evoked mobilization of ECL-cell histamine in conscious rats. Regul. Pept. 2000a;86:113–124. doi: 10.1016/s0167-0115(99)00096-8. [DOI] [PubMed] [Google Scholar]
- KOPIN A.S., LEE Y.-M., MCBRIDE E.W., MILLER L.J., LU M., LIN H.Y., KOLAKOWSKI F, BEINBORN M. Expression cloning of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSSON H., CARLSSON E., MATTSSON H., LUNDELL L., SUNDLER F., SUNDELL G., WALLMARK B., WATANABE T, HÅKANSON R. Plasma gastrin and gastric enterochromaffin-like cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986;20:391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- LINDSTRÖM E., BJÖRKQVIST M., DING X.-Q, HÅKANSON R. Pharmacological analysis of CCK2 receptor antagonists using isolated ECL cells. Brit. J. Pharmacol. 1999;127:530–536. doi: 10.1038/sj.bjp.0702538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL A.M, DELAAGE M.A. Immunoanalysis of histamine through a novel chemical derivatization. J. Allergy Clin. Immunol. 1988;82:646–654. doi: 10.1016/0091-6749(88)90978-5. [DOI] [PubMed] [Google Scholar]
- NORLÉN P., KITANO M., LINDSTRÖM E, HÅKANSON R. Anaesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells. Brit. J. Pharmacol. 2000;130:725–730. doi: 10.1038/sj.bjp.0703347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINZ C., SCOTT D.-R., HURWITZ D., HELANDER H.F, SACHS G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am. J. Physiol. 1994;267:G663–G672. doi: 10.1152/ajpgi.1994.267.4.G663. [DOI] [PubMed] [Google Scholar]
- RYBERG B., MATTSSON H., LARSSON H, CARLSSON E. Correlation between inhibition of gastric acid secretion, plasma gastrin and oxyntic mucosal histidine decarboxylase activity in the rat. Scand. J. Gastroenterol. 1989;24:287–292. doi: 10.3109/00365528909093048. [DOI] [PubMed] [Google Scholar]
- SANDVIK A.K, WALDUM H.L. CCK-B (gastrin) receptor regulates gastric histamine release and acid secretion. Am. J. Physiol. 1991;260:G925–G928. doi: 10.1152/ajpgi.1991.260.6.G925. [DOI] [PubMed] [Google Scholar]
- STADIL F, REHFELD J.F. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand. J. Gastroenterol. 1973;8:101–112. [PubMed] [Google Scholar]
- WALDUM H.L., SANDVIK A.K., BRENNA E, PETERSEN H. The gastrin-histamine sequence in the regulation of gastric acid secretion. Gut. 1991;32:698–700. doi: 10.1136/gut.32.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANK S.A., PISEGNA J.R, DE WEERTH A. Cholecystokinin receptor family. Molecular cloning, structure and functional expression in rat, guinea pig and human. Ann. N.Y. Acad. Sci. 1994;713:49–66. doi: 10.1111/j.1749-6632.1994.tb44052.x. [DOI] [PubMed] [Google Scholar]


