Abstract
The present study examined the inhibitory effects of N-Hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016) on the renal metabolism of arachidonic acid by cytochrome P450 (CYP) enzymes. HET0016 exhibited a high degree of selectivity in inhibiting the formation of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) in rat renal microsomes. The IC50 value averaged 35±4 nM, whereas the IC50 value for inhibition of the formation of epoxyeicosatrienoic acids by HET0016 averaged 2800±300 nM. In human renal microsomes, HET0016 potently inhibited the formation of 20-HETE with an IC50 value of 8.9±2.7 nM. Higher concentrations of HET0016 also inhibited the CYP2C9, CYP2D6 and CYP3A4-catalysed substrates oxidation with IC50 values of 3300, 83,900 and 71,000 nM. The IC50 value for HET0016 on cyclo-oxygenase activity was 2300 nM. These results indicate that HET0016 is a potent and selective inhibitor of CYP enzymes responsible for the formation of 20-HETE in man and rat.
Keywords: 20-HETE, cytochrome P4504A, HET0016, kidney, EETs
Introduction
The metabolism of arachidonic acid (AA) by cytochrome P450 (CYP) enzymes in the various cells can be divided into two major categories, i.e. ω-hydroxylases that produce 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) and epoxygenases that catalyse the formation of epoxyeicosatrienoic acids (EETs) and dihydroxyeicosatetraenoic acids (di-HETEs). In the rat, there are four different CYP4A isozymes that can catalyse the ω-hydroxylation of AA to 20-HETE. CYP4A1, CYP4A2, CYP4A3 and CYP4A8 are all expressed in rat kidneys (Ito et al., 1998). A number of studies have demonstrated that 20-HETE has potent biological activities and contributes to the regulation of renal tubular and vascular function and the long-term control of arterial pressure (Omata et al., 1992; Ma et al., 1993; Zou et al., 1994; Lin et al., 1995). 20-HETE is also potent vasoconstrictor in the cerebral circulation and regulates vascular tone and autoregulation of cerebral blood flow (Harder et al., 1994; Alonso-Galicia et al., 1999; Gebremedhin et al., 2000). EETs have generally are considered to be vasodilators and inhibit sodium and water transport in the kidney (Carroll et al., 1992; Harris et al., 1990).
17-Octadecynoic acid (17-ODYA) was originally designed to be a ‘suicide substrate inhibitor' of the ω-hydroxylation of AA (Muerhoff et al., 1989) and has been widely used to investigate the role of CYP metabolites of AA in the regulation of renal and vascular function. However, 17-ODYA is not a specific inhibitor of the formation of 20-HETE and it is equally effective at inhibiting the formation of EETs (Zou et al., 1994). 1-Aminobenzotriazol (1-ABT) is a CYP inhibitor and its inactivation of CYP enzyme requires catalytic formation of benzyne (Ortiz de Montellano & Mathews, 1981; Su et al., 1998).
HET0016 (N-Hydroxy-N′-(4-butyl-2-methylphenyl) formamidine (Figure 1) was discovered using a high throughput screen of the compound library of the Taisho Pharmaceutical Co., Ltd. to be a potent inhibitor to the synthesis of 20-HETE by renal microsomes of the rat. In the present study, we compared the effects of HET0016 on CYP-derived AA metabolism in rat and human renal microsomes. We have also examined the selectivity of HET0016 by examining its effects on CYP2D6, CYP2C9 and CYP3A4 enzyme activity that are important in the metabolism of various compounds by human liver. The results indicate that HET0016 is a potent and selective inhibitor of CYP responsible for the formation of 20-HETE in man and rats.
Figure 1.

Chemical structure of HET0016 (N-Hydroxy-N′-(4-butyl-2-methylphenyl)formamidine).
Methods
Preparation of rat and human renal microsomes
Male spontaneously hypertensive rats (SHR, 5 weeks old, Charles-River Japan, Atsugi, Japan) were used in the present experiments. The rats were anaesthetized with sodium pentobarbitone (50 mg kg−1, i.p.). The kidneys were removed, and renal cortex was dissected and homogenized in a buffer containing 20 mM HEPES (pH 7.4), 1 mM EDTA, 100 μM p-(amidinophenyl)methanesulphonyl fluoride and 250 mM sucrose. The homogenate was centrifuged at 600×g for 10 min. The supernatant was then further centrifuged at 16,000×g for 30 min. The supernatant was collected and centrifuged at 200,000×g for 30 min. The resulting pellet was suspended in 50 mM MOPS buffer. All procedures were carried out at 4°C. Microsomes derived from human kidneys were purchased from the Human Cell Culture Center (Laurel, MD, U.S.A.). The microsomal protein concentration was determined using the Bradford method. All studies presented here have been reviewed by the Taisho Pharmaceutical Co. Ltd. Animal Care Committee and have met the Japanese Experimental Animal Research Association standards, as defined in the Guidelines for Animal Experiments (1987).
Arachidonic acid metabolism
Microsomes prepared from the kidneys of rats or humans were preincubated with or without HET0016 (10−9 – 10−4 M for rats; 10−11 – 10−6 M for human), 17-ODYA (10−7 – 10−4 M for rats; 10−9 – 10−4 M for human) or 1-aminobenzotriazole (1-ABT, 10−7 – 10−4 M for rats; 10−9 – 10−4 M for human) for 5 min at 37°C in 50 mM MOPS/5 mM MgCl2/1 mM EDTA (pH 7.4) buffer. [3H]-Arachidonic acid (5 μCi ml−1) and NADPH (1 mM) were added to the reaction and incubated for 10 min at 37°C. The reaction was terminated by the addition of formic acid (pH 3.5). One hundred per cent acetonitrile was added to the reaction buffer to adjust final concentration to 50% for HPLC separation. Metabolites of AA were separated on a Bio-sil C18HL90-5S column (150×4.6) at a flow rate of 0.7 ml min−1 using a gradient elution ranging from acetonitrile : water : acetic acid (48 : 52 : 0.1) to acetonitrile : water : acetic acid (75 : 25 : 0.1) over a 26 min period. The labelled metabolites were monitored using a radioactive flow detector ramona 93 (Raytest GmbH, Straubenhardt, Germany). The identity of each metabolite was confirmed by comigration with an authentic standard.
Effects of HET0016, 17-ODYA and 1-ABT on the CYP2D6, 2C9 and 3A4 activity
HET0016, 17-ODYA, and 1-ABT were tested for their ability to inhibit the catalytic activity of these enzymes important in the metabolism of a number of drugs by human liver. IC50 was estimated for each test substance and each enzyme, according to the method of Crespi et al. (1997). This method is described in detail on the Gentest Corporation website (www.gentest.com). Baculovirus/insect cell-expressed human CYP enzymes were obtained from GENTEST Corporation (Wirburn, MA, U.S.A.). The enzyme/substrate contained buffer, cDNA-expressed P450, substrate (CYP2C9: 7-methoxy-4-trifluoromethylcoumarin, CYP2D6: 3-[2-(N,N-diethyl-N-methyl-amino)ethyl]-7-methoxy-4-methyl-coumarin and CYP3A4: 7-benzyl-oxyquinoline), and the amount was adjusted to give the final concentration (CYP2C9: 1.0 pmol (enzyme) and 75 μM (substrate); CYP2D6: 1.5 pmol (enzyme) and 1.5 μM (substrate); CYP3A4: 3.0 pmol (enzyme) and 40 μM (substrate)) in a reaction volume of 200 μl. Reactions were terminated at 45 min by addition of a 4 : 1 acetonitrile: 0.5 M tris base solution. Fluorescence per well was measured using a fluorescent plate scannner (ARVO™ 1420 multilable counter, Wallac, Turku, Finland). Metabolite concentrations were measured using the excitation and emission wavelengths (CYP2C9: 405 nm and 535 nm; CYP2D6: 390 nm and 460 nm; CYP3A4: 405 nm and 535 nm), respectively. Detection of the products of either assay was linear over the range used for these assays.
Effects of HET0016 on COX activity
The effect of HET0016 on COX activity was examined using the COX inhibitor screening assay kit purchased from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). In brief, the purified PGH1 synthase enzyme from ram seminal vesicles was incubated with 100 μM of AA in 1.0 ml of incubation buffer (0.1 M Tris-HCl, pH 8, 5 mM EDTA, 2 mM phenol and 1 μM hematin) with or without various concentrations of HET0016 (10−10 – 10−4 M) and indomethacin (10−10 – 10−4 M). Reaction mixtures were incubated in a 37°C for 2 min before the addition of AA and for 2 min thereafter. All samples were run in duplicate. The amount of PGE2 generated in each sample was determined by enzyme immunoassay.
Data analysis
Data are expressed as mean±s.e.mean of the per cent of the control activity. Curve-fitting and parameter estimation were carried out by using Origin 5.0J (OriginLab Corp., MA, U.S.A.).
Drugs
20-HETE, 17-ODYA, 1-ABT and indomethacin were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). HET0016 was synthesized in Medicinal Research Laboratories, Taisho Pharmaceutical Co. Ltd. (Saitama, Japan). COX inhibitor assay kit was obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). [3H]-AA was obtained from Amersham Pharmacia Biotech (Tokyo, Japan).
Results
Microsomes prepared from the kidneys of SHR, avidly produced both [3H]-20-HETE and [3H]-11,12-EET when incubated with AA in the presence of NADPH. HET0016 selectively inhibited the formation of 20-HETE in a concentration-dependent manner with an IC50 value of 35.2±4.4 nM (n=11, Figure 2A). Higher concentrations of HET0016 also inhibited the formation of EETs but the IC50 value was approximately 100 times greater, 2800±300 nM (n=11). 17-ODYA inhibited both epoxygenase and ω-hydroxylase in a concentration-dependent manner. The IC50 values for inhibition of epoxygenase and ω-hydroxylase activity by 17-ODYA were 1.2±0.3 μM (n=11) and 6.9±1.0 μM, respectively (n=11, Figure 2B). 1-ABT slightly inhibited epoxygenase only at higher concentration (Figure 2C).
Figure 2.

Effects of HET0016 (A), 17-ODYA (B) and 1-ABT (C) on the formation of 20-HETE and EETs by microsomes prepared from the kidneys of SHR. Results are expressed as per cent of control and are the mean±s.e.mean, respectively n=10–11.
Microsomes prepared from human kidney only produced 20-HETE when incubated with [3H]-AA. A comparison of the effects of HET0016, 17-ODYA and 1-ABT on 20-HETE production are presented in Figure 3. HET0016 was a potent inhibitor of ω-hydroxylation of arachidonic acid. The IC50 value averaged 8.9±2.7 nM (n=6, Figure 3). 17-ODYA and 1-ABT also inhibited the ω-hydroxylation of arachidonic acid in a concentration-dependent manner with IC50 values of 1.8±0.8 μM (n=6) and 38.5±14.9 μM (n=5), respectively (Figure 3).
Figure 3.

Comparison of the effects of HET0016, 17-ODYA and 1-ABT on the production of 20-HETE in microsomes prepared from human kidneys. Results are expressed as per cent of control and are the mean±s.e.mean, respectively n=5–6.
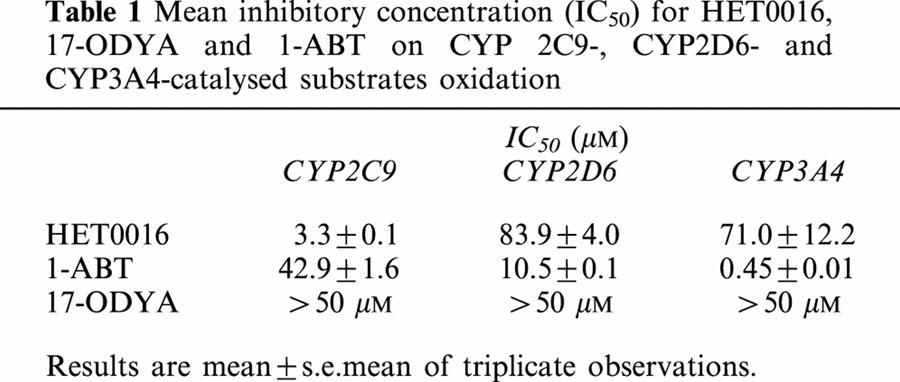
The inhibitory effects of HET0016, 17-ODYA and 1-ABT on human CYP2C9-, CYP2D6- and CYP3A4 activity is presented in Table 1. Higher concentrations of HET0016 and 1-ABT inhibited human CYP2C9-, CYP2D6- and CYP3A4 activity in a concentration-dependent manner. The IC50 values of HET0016 on CYP2C9-, CYP2D6- and CYP3A4- activities averaged 3.3±0.2, 83.9±7.0 and 71.0±21.2 μM, respectively. The IC50 values for 1-ABT on the CYP2C9-, CYP2D6- and CYP3A4-activities averaged 42.9±2.8, 10.5±0.2 and 0.45±0.01 μM, respectively. 17-ODYA had no significant effect on CYP2C9- CYP2D6- and CYP3A4- activity even at a concentration as high as 50 μM.
Table 1.
Mean inhibitory concentration (IC50) for HET0016, 17-ODYA and 1-ABT on CYP 2C9-, CYP2D6- and CYP3A4-catalysed substrates oxidation
We also examined the effects of HET0016 on COX activity by measuring the PGH1 catalysed conversion of AA to PGE2. Both indomethacin and HET0016 inhibited the COX activity in a concentration-dependent manner. The IC50 for HET0016 and indomethacin on the COX activity averaged 2300±285 and 40±25 nM, respectively n=3.
Discussion
The present study characterizes a new inhibitor of the formation of 20-HETE that was identified using the high throughput biochemical screening of a large industrial library of compounds. HET0016 is the most potent inhibitor of the formation of 20-HETE yet identified. The IC50 value inhibition of 20-HETE formation in rat renal microsomes was 35.2 nM. In microsomes prepared from human kidney with IC50 for inhibition of ω-hydroxylation of AA was 8.9 nM. In contrast, the IC50 of other known inhibitors of this pathway, 17-ODYA and ABT is greater than 5 μM. HET0016 is also a highly selective inhibitor of the CYP4A isoforms that produce 20-HETE. Indeed, the IC50 for HETE0016 for inhibition of the formation of EETs was 2800 nM. HET0016 had very little effect on the activity of CYP2C9, CYP2D6, CYP3A4 or COX even at very high concentrations (μM). These results suggest that HET0016 is a potent and selective inhibitor of the CYP enzymes that catalyse the formation of 20-HETE from AA in microsomes prepared from the kidneys of rats and humans.
Omega hydroxylation of AA to produce 20-HETE in rat kidney is mainly catalysed by isoforms of the CYP4A family (Omata et al., 1992; Laniado Schwartzman et al., 1996; Ito et al., 1998). In human kidney Lasker et al. (2000) has reported that the formation of 20-HETE is catalysed by CYP4A11 and CYP4F2. Epoxidation of the AA acid in the kidney is catalysed by enzymes of the CYP1A, 2B, 2C and 2J families (Laethem & Koop, 1992; Laethem et al., 1994) . In the present study, HET0016 potently and selectively inhibited the production of 20-HETE but not EETs. These results suggest that HET0016 has a selective inhibitory effect on CYP4A and 4F isoforms rather than CYP1A, 2B, 2C and 2J. In contrast, 17-ODYA was a nonselective inhibitor of the formation of 20-HETE and EETs. These results are consistent with the previous results of Zou et al. (1994) and Wang et al. (1998).
CYP are the principal enzymes for the oxidative metabolism of drugs and other xenobiotics. Although CYP2C9, CYP2C19 and CYP3A4 are not involved in the formation of 20-HETE in human renal microsome however, CYP2C9, CYP2C19 and CYP3A4 are the isoforms that are most important for the metabolism of most drugs in humans. To investigate the selectivity of the HET0016 on CYP isoforms that regulate drug metabolism, we compared the effects of HET0016, 17-ODYA and 1-ABT on CYP2C9-, CYP2D6- and CYP3A4-activity. HET0016 inhibited CYP2C9-, CYP2D6- and CYP3A4-activity but only at concentrations 100 times higher than those needed to inhibit the ω-hydroxylation of AA. In comparison, 1-ABT also inhibited CYP2C9-, CYP2D6- and CYP3A4-activities at concentrations similar to those needed to inhibit the ω-hydroxylation of AA. These results suggest that 1-ABT is a nonselective inhibitor of CYP enzymes. 17-ODYA exhibited no selectivity regarding inhibition of EETs and 20-HETE but it had no effect on CYP2C9, CYP2D6 and CYP3A4, even at concentrations as high as 50 μM.
Wang et al. (1998) recently reported that N-methylsulphonyl-12,12-dibromododec-11-enamide (DDMS) and 12,12-dibromododec-11-enoic acid (DBDD) selectively inhibits the ω-hydroxylation with an IC50 values of 2 μM, whereas the IC50 values for epoxidation were 60 and 51 μM. The inhibitory effects of DBDD and DDMS on ω-hydroxylation of AA was 30 times more selective than that of epoxidation of AA in rat renal microsomes. In contrast with these compounds, inhibitory effect of HET0016 on ω-hydroxylation of AA was 60 times more potent than these compounds and the selectivity was also better than those of compounds.
20-HETE is an important eicosanoid in regulating renal and cerebral circulation (Harder et al., 1995). As a pathological correlate, there are considerable evidence suggesting that an overproduction of 20-HETE plays an important role in the pathogenesis of hypertension, cyclosporin-induced renal failure and hepatorenal syndrome (Omata et al., 1992; Nakamura et al., 1994; Sacerdoti et al., 1997). The results of the present study, indicating that HET0016 is a potent and selective inhibitor of the formation of 20-HETE, suggest it may have some potential as a therapeutic agent in some of these diseases.
Acknowledgments
The author would like to thank Richard J. Roman, PhD, Department of Physiology, Medical College of Wisconsin for his helpful comments and critical reading of the manuscript.
Abbreviations
- AA
arachidonic acid
- 1-ABT
1 aminobenzotriazole
- COX
cyclo-oxygenase
- CYP
cytochrome p450
- di-HETEs
dihydroxyeicosatetraenoic acids
- EETs
epoxyeicosatrienoic acids
- 20-HETE
20-hydroxy-5,8,11,14-eicosatetraenoic acid
- 17-ODYA
17-octadecynoic acid
References
- ALONSO-GALICIA M., HUDETZ A.G., SHEN H., HARDER D.R., ROMAN R.J. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke. 1999;30:2727–2734. doi: 10.1161/01.str.30.12.2727. [DOI] [PubMed] [Google Scholar]
- CARROLL M.A., PILAR GRACIA M., FALCK J.R., MCGIFF J.C. Cycloxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonic metabolites. J. Pharmacol. Exp. Ther. 1992;260:104–109. [PubMed] [Google Scholar]
- CRESPI C.L., MILLER V.P., PENMAN B.W. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal. Biochem. 1997;248:188–190. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., LOWRY T.F., TAHERI M.R., BIRKS E.K., HUDERTZ A.G., NARAYANAN J., FALCK J.R., OKAMOTO H., ROMAN R.J., NITHIPATIKOM K., CAMPBELL W.B., HARDER D.R. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ. Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- HARRIS R.C., HOMMA T., JACOBSEN H.R., CAPDEVILA J. Epoxyeicosatrienoic acids activate Na+/H+ exchange and are mitogenic in cultured rat glomerular mesangial cells. J. Cell. Physiol. 1990;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., CAMPBELL W.B., ROMAN R.J. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J. Vasc. Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- HARDER D.R, , GEBREMEDHIN D., NARAYANAN J., JEFCOAT C., FALCK J.R., CAMPBELL W.B., ROMAN R.J. Formation and action of a P-450 4A metabolite of arachidonic acid in cat middle cerebral microvessels. Am. J. Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- ITO O., ALINSO-GALICIA M., HOPP K.A., ROMAN R.J. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am. J. Physiol. 1998;274:F395–F404. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- LAETHEM R.M., HALPERT J.R., KOOP D.R. Epoxydation of arachidonic acid as an active site probe of cytochrome p-450 2B isoforms. Biochim. Biophys. Acta. 1994;1206:42–48. doi: 10.1016/0167-4838(94)90070-1. [DOI] [PubMed] [Google Scholar]
- LAETHEM R.M., KOOP D.R. Identification of rabbit cytochrome P450 2C1 and 2C2 as arachidonic acid epoxygenase. Mol. Pharmacol. 1992;42:958–963. [PubMed] [Google Scholar]
- LANIADO SCHWARTZMAN M., DA SILVA J-L., LIN F., NISHIMURA M, ABRAHAM N.G. Cytochrome P450 4A expression and arachidonic acid ω-hydroxylation in the kidney of the spontaneously hypertensive rat. Nephron. 1996;73:652–663. doi: 10.1159/000189154. [DOI] [PubMed] [Google Scholar]
- LASKER J.M., CHEN W.B., WOLF I., BLOSWICK B.P., WILSON P.D., POWELL P.K. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of CYP4F2 and CYP4A11. J. Biol. Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- LIN F., RIOS A., FALCK J.R., BELOSLUDTSEV Y., SCHWARTZMAN M.L. 20-hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am. J. Physiol. 1995;269:F806–F816. doi: 10.1152/ajprenal.1995.269.6.F806. [DOI] [PubMed] [Google Scholar]
- MA Y.H., GEBREMEDHIN D., SCHWARTZMAN M.L., FALCK J.R., CLARK J.E., MASTERS B.S., HARDER D.R., ROMAN R.J. 20-hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ. Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- MUERHOFF A.S., WILLIAMS D.E., REICH N.O., CAJACOB C.A., ORIZ DE MONTELLANO P.R., MASTERS B.S. Prostaglandin and fatty acid ω- and (ω-1)-oxidation in rabbit lung. Acetylenic fatty acid mechanism-based inactivators as specific Inhibitors. J. Biol. Chem. 1989;264:749–756. [PubMed] [Google Scholar]
- NAKAMURA M., IMAOKA S., MIURA K., TANAKA E., MISAWA S., FUNAE Y. Induction of cytochrome P450 isozymes in rat renal microsomes by cyclosporin A. Biochem. Pharmacol. 1994;48:1743–1746. doi: 10.1016/0006-2952(94)90460-x. [DOI] [PubMed] [Google Scholar]
- OMATA K., ABRAHAM N.G., ESCALANTE B., SCHRTZMAN M.L. Age-related changes in renal cytochrome P-450 arachidonic acid metabolism in spontaneously hypertensive rats. Am. J. Physiol. 1992;262:F591–F599. doi: 10.1152/ajprenal.1992.262.1.F8. [DOI] [PubMed] [Google Scholar]
- ORTIZ DE MONTELLANO P.R., MATHEWS J.M. Autocatalytic alkylation of the cytochrome P-450 prosthetic haem group by 1-aminobenzotriazol. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem. J. 1981;195:761–764. doi: 10.1042/bj1950761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACERDOTI D., BALAZY M., ANGELI P., GATTA A., MCGIFF J.C. Eicosanoid excretion in hepatic cirrhosis. Predominance of 20-HETE. J. Clin. Invest. 1997;100:1264–1270. doi: 10.1172/JCI119640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU P., KAUSHAL K.M., KROETZ D.L. Inhibition of renal arachidonic acid ω- hydroxylase activity with ABT reduces blood pressure in the SHR. Am. J. Physiol. 1998;275:R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- WANG M.-H., BRAND-SCHIEBER E., ZAND B.A., NGUYEN X., FALCK J.R., BALU N., SCHWARTZMAN M.L. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: Characterization of selective inhibitors. J. Pharmacol. Exp. Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- ZOU A.P., MA Y.H., SUI Z.H., ORITIZ DE MONTELLANO P.R., CLARK J.E., MASTERS B.s., ROMAN R.J. Effects of 17-octadecynoic acid, a suicide-substrate inhibitor of cytochrome P450 fatty acid omega-hydroxylase on renal function in rats. J. Phamacol. Exp. Ther. 1994;268:474–481. [PubMed] [Google Scholar]


