Abstract
The induction of B1 receptors (B1Rs) and desensitization or down-regulation of B2 receptors (B2Rs) as a consequence of the production of endogenous kinins has been termed the autoregulation hypothesis. The latter was investigated using two models based on the rabbit: kinin stimulation of cultured vascular smooth muscle cells (SMCs) and in vivo contact system activation (dextran sulphate intravenous injection, 2 mg kg−1, 5 h).
Rabbit aortic SMCs express a baseline population of B1Rs that was up-regulated upon interleukin-1β treatment ([3H]-Lys-des-Arg9-BK binding or mRNA concentration evaluated by RT–PCR; 4 or 3 h, respectively). Treatment with B1R or B2R agonists failed to alter B1R expression under the same conditions.
Despite consuming endogenous kininogen (assessed using the kinetics of immunoreactive kinin formation in the plasma exposed to glass beads ex vivo) and producing hypotension mediated by B2Rs in anaesthetized rabbits, dextran sulphate treatment failed to induce B1Rs in conscious animals (RT–PCR in several organs, aortic contractility). By contrast, lipopolysaccharide (LPS, 50 μg kg−1, 5 h) was an effective B1R inducer (kidney, duodenum, aorta) but did not reduce kininogen reserve.
We tested the alternate hypothesis that endogenous kinin participate in LPS induction of B1Rs. Kinin receptor antagonists (icatibant combined to B-9858, 50 μg kg−1 of each) failed to prevent or reduce the effect of LPS on B1R expression. Dextran sulphate or LPS treatments did not persistently down-regulate vascular B2Rs (jugular vein contractility assessed ex vivo).
The kinin receptor autoregulation hypothesis is not applicable to primary cell cultures derived from a tissue known to express B1Rs in a regulated manner (aorta). The activation of the endogenous kallikrein-kinin system is ineffective to induce B1Rs in vivo in an experimental time frame sufficient for B1R induction by LPS.
Keywords: Lipopolysaccharide, kinin-induced vascular contraction, receptor for kinins, contact system activation, vascular smooth muscle cells, interleukin-1
Introduction
The kinins (peptides related to bradykinin, BK) are known to activate two types of G protein coupled receptors, termed B1 and B2 (Marceau et al., 1998). Lys-des-Arg9-BK (des-Arg10-kallidin) is the optimal agonist sequence of the human and rabbit B1 receptors (B1Rs), whereas BK and Lys-BK in low concentrations stimulate B2 receptors (B2Rs). While the latter are preformed in a wide variety of cells, a large body of evidence shows that the B1Rs are generally not expressed in normal physiological conditions, but rapidly induced following some types of injury; the cytokine network, mitogen-activated protein (MAP) kinases and specific transcription factors have been implicated in this phenomenon (Deblois et al., 1988; Larrivée et al., 1998; Marceau et al., 1998; Ni et al., 1998; Yang et al., 1998).
In several systems pertaining to persistent inflammation, there is a temporal shift of kinin effect mediation from preformed B2Rs to induced B1Rs over several hours or days (e.g., inflammatory hyperalgesia and intestinal inflammation models in rodents) (Kachur et al., 1996; Perkins et al., 1993). This also applies to the oedema induced by exogenous kinin injection into the rat paw (Campos et al., 1995). In the latter system, daily injection of the B2R agonist [Tyr8]BK induces oedema of decreasing amplitude; however, the B1R agonist des-Arg9-BK, initially inert, becomes a powerful inflammatory substance by the end of the protocol. These and other observations suggest a pattern of kinin receptor ‘autoregulation' by the agonists. Agonist-induced temporary desensitization of the B2R, involving receptor phosphorylation and endocytosis, is well documented (Blaukat et al., 1996; Faussner et al., 1998; Pizard et al., 1999), but B2R down-regulation is not defined at the molecular level (protein, mRNA). As for B1R induction, the agonist Lys-des-Arg9-BK has been shown to induce B1Rs (mRNA, protein) in the human cell line IMR-90 (Schanstra et al., 1998). This cell line expresses a certain baseline population of B1R, which may not be representative of normal tissues in vivo; NF-κB binding to some DNA sequences reproduced from the B1R gene promoter was activated by the B1R agonist in these cells. Further, the MAP kinase pathways that were found to determine post-isolation B1R induction in rabbit vascular tissue (Larrivée et al., 1998) may be also activated upon kinin receptor stimulation in HEK 293 cells expressing either recombinant B1 or B2Rs (Naraba et al., 1998). In these cells, either B1R or B2R agonists stimulate the nuclear translocation of the transcription factor AP-1, an effect which is blocked by PD98059, the MEK-1 inhibitor. We and others have produced complementary evidence for the role of an AP-1 site in the B1R gene promoter (Yang et al., 1998; Angers et al., 2000). Ultimately, the full model of autoregulation has been illustrated by Phagoo et al. (1999) based on IMR-90 cells: incubation with BK (100 nM) suppressed 89% of the surface B2Rs in a few minutes, but upregulated B1Rs (2–3 fold) in a few hours. Lys-des-Arg9-BK did not influence the population of B2Rs, but also upregulated B1Rs. The agonists increased the expression of IL-1β in the cells, and the natural IL-1 receptor antagonist, IRA, decreased the induction of B1Rs by BK (Phagoo et al., 1999). Therefore, this ‘autoregulation' model integrates several known or suspected regulatory events (autologous desensitization of B2R, involvement of cytokine, MAP kinases and transcription factors in B1R gene transcriptional control).
We have investigated whether intense activation of the endogenous kallikrein-kinin system using dextran sulphate (Kaplan et al., 1998) could result in the definite pattern of kinin receptor regulation described above (desensitization or down-regulation of B2Rs, up-regulation of B1Rs) in the live rabbit. Complementary testing of the autoregulation hypothesis has been performed using primary cultures of rabbit vascular SMCs. In addition, kinin participation in LPS induction of B1Rs has been tested as a secondary objective, as LPS treatment may activate the kallikrein-kinin system in rabbits (Erdös & Miwa, 1968).
Methods
Drugs
Dextran sulphate (500 kDa), amastatin, phosphoramidon, captopril and enalapril maleate were purchased from Sigma (St. Louis, MO, U.S.A.). LPS, extracted from Escherichia coli serotype O111:B4, was produced by Difco (Detroit, MI, U.S.A.). Several BK-related peptides were used: BK itself (B2R agonist; Bachem Bioscience Inc., King of Prussia, PA, U.S.A.), Lys-des-Arg9-BK (a B1R agonist; Peninsula Laboratories, Belmont, CA, U.S.A.), Sar-[D-Phe8]des-Arg9-BK (a metabolically stable B1R agonist; Drapeau et al., 1993; gift from Prof D. Regoli, Sherbrooke, Canada), the B1R antagonist B-9858 (Lys-Lys-[Hyp3, Igl5, D-Igl7, Oic8]des-Arg9-BK; Larrivée et al., 2000) and the B2R antagonist icatibant (Hoe 140; D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK; both icatibant and B-9858 were gifts from Laboratoires Fournier S.C.A., Daix, France).
B1R expression in cultured rabbit aortic SMCs
Rabbit aortic SMCs were cultured as previously described (Levesque et al., 1993); the identity of these cells was confirmed using immunohistochemistry for the marker α-actin (monoclonal antibody from Sigma). Cells were used at passages 3–6, at a stage where the B1R basal expression is relatively low and its hormonal induction (epidermal growth factor treatment) is high (Schneck et al., 1994). Separate protocols dealt with the effect of kinin receptor ligands or IL-1 on B1R expression; both mRNA and radioligand were assessed in these experiments which were based on confluent cells cultured in 6- or 12-well plates, respectively. In order to reduce the basal B1R expression level, the FBS containing medium was replaced by serum-free medium 199 for 24 h; then various drugs (kinin analogue, captopril or IL-1β) were added to the serum-free medium and the total RNA was extracted after 3 h according to Chomczynski & Sacchi (1987). These cells were also the basis of a binding assay to rabbit B1Rs (12-well plates; approximately 60 μg of total protein per well). The assay was conducted as described (Levesque et al., 1995a), except for the identity of the ligand which was [3H]Lys-des-Arg9-BK ([3H]des-Arg10-kallidin, NEN Biosciences, 64 Ci mmol−1). The cells were treated as outlined above for 3 h with a kinin analogue, captopril or IL-1β; then, the medium containing stimulants was replaced by serum-free medium 199 for one additional hour of incubation at 37°C before performing the binding assay to avoid binding interference from some of the kinin analogues used in pretreatments. There is evidence that a 1 h washout at 37°C is sufficient for a full dissociation of the antagonist Ac-Lys-[Leu8]des-Arg9-BK from the rabbit recombinant B1Rs (Larrivée et al., 2000), and it is assumed that this will apply to the moderate affinity ligands used in the present experiments. The radioactivity bound to adherent intact cells after incubation with a radioligand concentration (4 nM) sufficient to reveal the Bmax (Schneck et al., 1994) was determined; a cold competing peptide (1 μM Lys-des-Arg9-BK) was added to some cell wells to determine the non specific binding (amounting to 15–40% of the total binding).
Treatment of animals used as sources of tissues
Groups of male New Zealand White rabbits, weighing 1.5–2.2 kg were used as a source of tissues for all experiments. In order to potentiate endogenous kinins produced by the treatments, the ACE inhibitor enalapril maleate (2 mg kg−1 per day in the drinking water) was given to all animals for 5 days prior to acute treatments. Each rabbit was weighed before the acute treatments, which consisted in the intravenous injection of the following drugs: dextran sulphate (2 mg kg−1) or LPS (50 μg kg−1). The first substance is a contact system activator which has previously been used to produce hypotension mediated via both B1 and B2Rs in the LPS-treated pigs, thus presumably by producing endogenous kinins (Schmid et al., 1998); the second one was used as a control inducer of B1Rs (Marceau et al., 1999). Control animals received the saline vehicle (500 μl kg−1). Additional groups of animals were treated intravenously with a combination of kinin receptor antagonists: icatibant (50 μg kg−1, a B2R antagonist) and B-9858 (50 μg kg−1, a B1R antagonist). These drugs provide long lasting receptor blockade at these dose levels in the rabbit (Gobeil et al., 1999). The intravenous injection was followed 5 min later by an intravenous injection of LPS or saline, as described above. Five hours after the last injection, all rabbits were consecutively killed by CO2/O2 asphyxiation and several organs (heart, abdominal aorta, kidney, duodenum and psoas muscle) were quickly removed, frozen in liquid nitrogen and kept at −80°C until RNA isolation (methods described previously) (Marceau et al., 1999). The thoracic aorta and the jugular veins were immediately used in the contractility studies (two separate tissues of each type were used per animal), for their functional response to the B1 or B2R agonists, Sar-[D-Phe8]des-Arg9-BK or BK, respectively (the responses were assessed within 1 h of tissue equilibration and expressed as a per cent of an internal contraction standard, the maximal responses to phenylephrine or histamine, respectively; Marceau et al., 1999).
Semiquantitative duplex RT–PCR
The RT–PCR experiments were conducted using the Ready-To-Go™ RT–PCR Beads (Amersham Pharmacia Biotech) as indicated by the manufacturer. The general conditions (primers used, PCR conditions and Southern analysis of the RT–PCR) were previously reported (Marceau et al., 1999). Briefly, 2 μg of total RNA sample, 250 ng of sense and antisense primers for the amplification of rabbit B1R or B2R fragments, 25 ng of sense and antisense primers for the amplification of a rabbit GAPDH fragment (used as an internal standard) and 250 ng of an oligo (dT)15 were added to each tube of Ready-To-Go™ RT–PCR beads. The tubes were incubated for 30 min at 42°C for the RT reaction. The samples were then submitted to a PCR followed by a Southern analysis as described previously (Marceau et al., 1999).
Kininogen reserve in anaesthetized rabbits submitted to the acute treatments
Separate male rabbits were used for these experiments. The animals also received oral enalapril maleate treatment (2 mg kg−1 per day) for 5 days prior to the experiment. This chronic treatment allows complete inhibition of plasma ACE activity and potentiates the conversion of BK into des-Arg9-BK which constitutes a minor metabolic pathway in absence of ACE inhibition (Décarie et al., 1996). The rabbits were anaesthetized using sodium pentobarbitone (approximately 50 mg kg−1 i.v., individually adjusted). A throat incision made under lidocaine 2% local anaesthesia was followed by trachea intubation for ventilatory assistance and cannulation of the left common carotid artery for blood sampling and drug injection (Raymond et al., 1995). After 10 min of equilibration, 5 ml of arterial blood was sampled in a polyethylene tube containing buffered sodium citrate (final concentration 13 mM) for the measurement of blood kininogen reserve. These sampling procedures have been previously validated and do not result in significant kininogen consumption in vitro (Adam et al., 1985a, 1985b, 1985c). The intravenous injection of test drugs used to induce B1Rs, dextran sulphate (2 mg kg−1) or LPS (50 μg kg−1) was then performed, and the blood sampling was repeated after 90 min in order to document acute treatment-induced kininogen consumption. Euthanasia was then applied under the form of a lethal dose of sodium pentobarbitone. Blood samples were centrifuged, and the plasma frozen for later testing.
Rabbit plasma samples were exposed to acid-washed glass beads at 37°C to activate the contact system and several aliquots were removed at different intervals of time between 1–60 min to determine the kinetic release of BK from high molecular weight kininogen and the formation of des-Arg9-BK. The concentration of immunoreactive BK and des-Arg9-BK was determined using two separate enzyme immunoassays after extraction from plasma (Décarie et al., 1994; Raymond et al., 1995).
Hypotensive effect of dextran sulphate in anaesthetized rabbits
Other groups of male rabbits, orally dosed with enalapril maleate, then anaesthetized and surgically prepared as described above, were used to assess the effect of dextran sulphate on mean arterial blood pressure measured using a pressure transducer (EM 751, Elcomatic Ltd., Glasgow, U.K.) connected to the left carotid artery catheter. Upon blood pressure stabilization, dextran sulphate (2 mg kg−1) was injected in an ear vein to closely reproduce the treatment applied to conscious animals, and the blood pressure was monitored for the next 30 min period (limited by the need to re-administer the anaesthetic drug). The animals were then sacrificed with an overdose of pentobarbitone. To analyse the mechanism of dextran sulphate-induced haemodynamic changes, a separate group of anaesthetized rabbits submitted to the blood pressure recording were initially treated with icatibant (50 mg kg−1, i.v.) followed 5 min later by dextran sulphate (2 mg kg−1, i.v.).
Statistical analysis
Statistical analysis was performed using the Kruskal–Wallis test followed by Mann–Whitney test or ANOVA followed by Dunnett's test using the InStat 2.0 computer program (GraphPad Software, San Diego, CA, U.S.A.).
Results
Kinin B1R expression in cultured aortic SMCs
Cultured rabbit aortic SMCs express a regulated population of B1Rs mediating such effects as phosphoinositide hydrolysis, prostaglandin release and DNA synthesis (Levesque et al., 1993; 1995a, 1995b). Confluent cells maintained for 24 h in serum-free medium 199 expressed a relatively small population of B1Rs (Figure 1A). As reported previously (Levesque et al., 1995a), IL-1β treatment (5 ng ml−1, applied for 3 h and rinsed for an additional hour of incubation) increased the expression of the B1R, as assessed with a specific radioligand. A treatment with the B1R agonist Sar-[D-Phe8]des-Arg9-BK (100 nM) did not influence [3H]Lys-des-Arg9-BK binding (Figure 1A). The employed agonist concentration was maximally effective to increase DNA synthesis in these cells (Levesque et al., 1995b). Rabbit aortic SMCs possess a certain population of B2Rs mediating phosphoinositide metabolism (Emax of about 20% relative to B1R agonist Emax; Schneck et al., 1994); however, in other assays, there is no functional response to B2R agonists in these cells (Levesque et al., 1993; 1995b). The effect of B2R stimulation was checked in the present experiments to exclude any effect on B1R regulation. BK (100 nM) also failed to alter the B1R radioligand binding (Figure 1A). In these experiments, BK was combined with captopril (1 μM) in order to improve peptide stability in the cultured cell system (Bachvarov et al., 2001); captopril alone had no effect on the B1R ligand binding. The same set of treatments was applied for 3 h to rabbit aortic SMCs for the evaluation of B1R mRNA concentration (Figure 1B). Again, only IL-1β upregulated B1R mRNA, as kinin receptor agonists failed to increase B1R mRNA concentration above the control value.
Figure 1.
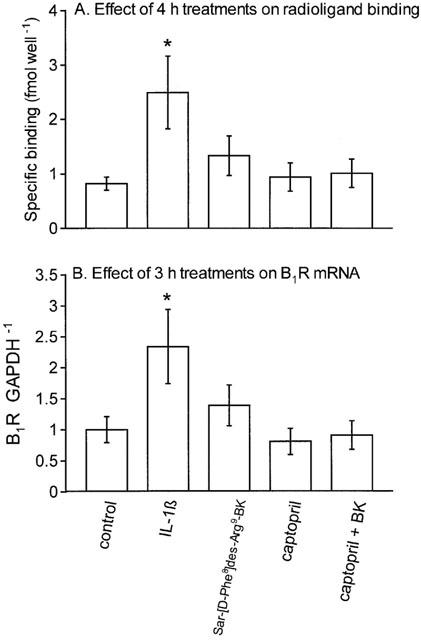
Investigation of the autoregulation hypothesis in cultured SMCs derived from the rabbit aorta. (A) Effect of 3 h drug treatments followed by 1 h drug washout incubation period on the specific binding of [3H]Lys-des-Arg9-BK (4 nM) to rabbit aortic SMCs maintained in serum-free medium for 24 h. Cells were treated with saline vehicle (2 μl per well), human recombinant IL-1β (5 ng ml−1), the B1R agonist Sar-[D-Phe8]des-Arg9-BK (100 nM), captopril (1 μM) alone or combined with the B2R agonist BK (100 nM). Results are the means±s.e.mean of four determinations based on cell lines derived from different animals. (B) Effect of 3 h drug treatments on B1R mRNA concentration in rabbit aortic SMCs maintained in serum-free medium for 24 h. Values (B1R GAPDH−1 ratio, derived from RT–PCR) are arbitrary scanning units normalized to saline group=1 and are the mean±s.e.mean of six determinations. The treatments were the same as in A. In both A and B, one-way analysis of variance showed that the experimental groups were different between them (P<0.05). Dunnett's test was applied to compare the effect of each treatment with the control values (*P<0.05).
Multiplex RT–PCR analysis of kinin receptor mRNA in organs from treated rabbits
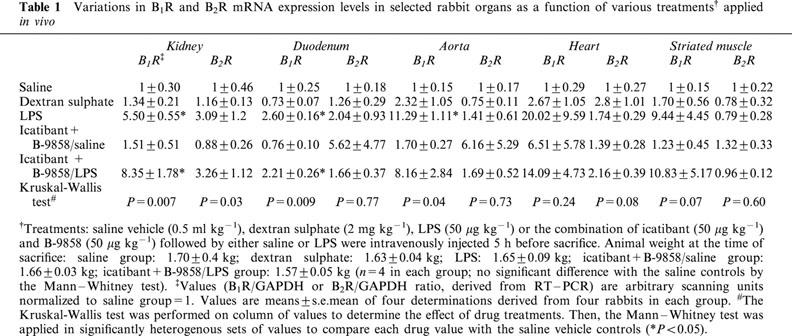
The B1 and B2R mRNA expression levels in several rabbit organs were determined by a semi-quantitative RT–PCR approach and the results are presented in Table 1. A baseline mRNA expression was detected in each organ for both receptors (normalized to 1 in Table 1). The dextran sulphate treatment failed to significantly induce the expression of B1R mRNA over the baseline. The 5 h LPS treatment significantly induced B1R expression over the baseline in the kidney, duodenum and aorta (values did not reach statistical significance for the two other organs, but the trends were similar). Animals pretreated with the combination of kinin receptor antagonists 5 min before LPS administration exhibited B1R mRNA expression levels similar to those of rabbits treated with LPS alone, whereas the antagonists given alone were ineffective in this respect. The short-term treatments applied (5 h) did not change significantly B2R mRNA concentrations in the five tested organs, although considerable value dispersion was observed in some groups (Table 1).
Table 1.
Variations in B1R and B2R mRNA expression levels in selected rabbit organs as a function of various treatments† applied in vivo
Vascular contractility mediated by B1 and B2Rs as a function of treatments applied in vivo to rabbits
Sigmoidal concentration-effect curves for each tested agonist (Figures 2 and 3) were characterized by the half-maximal effective concentration (EC50) and the maximal absolute contraction amplitude (Emax, per cent of internal standard) (Table 2). The aortic ring responses to the B1R agonist Sar-[D-Phe8]des-Arg9-BK were recorded within the first hour of tissue isolation. LPS treatment induced a definite state of responsiveness to this peptide when compared to saline-treated controls, even in animals pretreated with the combination of kinin antagonists (Emax values were not significantly different, P=0.08). Dextran sulphate or the mixture of kinin receptor antagonists were ineffective to induce responsiveness to the B1R agonist 5 h following the treatments (Figure 2, Table 2).
Figure 2.
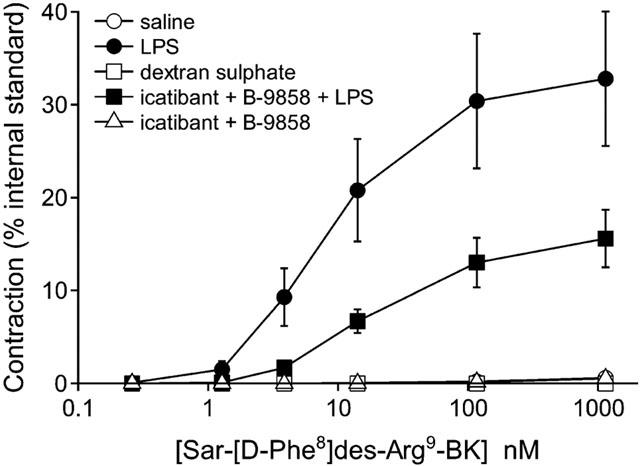
Initial responsiveness of aortic rings derived from control (saline vehicle i.v.), dextran sulphate-treated, LPS-treated or kinin antagonist-treated rabbits to an agonist of the B1 receptor. Kinin antagonist treatment was combined with either saline or LPS injection. Treatments were in the form of intravenous injections 5 h prior to sacrifice. The cumulative concentration-effect curve of the B1R agonist Sar-[D-Phe8]des-Arg9-BK was established after a short in vitro incubation (45 min) to minimize the influence of isolation and tissue incubation on the responses. Values (means±s.e.mean of eight determinations from four animals in each group) are expressed as a per cent of an internal contractile standard, the maximal effect of phenylephrine, established in each tissue. See Table 2 for statistical analysis.
Figure 3.
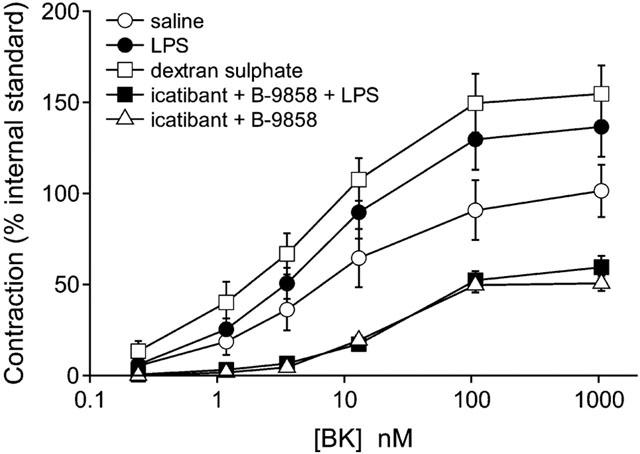
Initial responsiveness of external jugular vein strips derived from control (saline vehicle i.v.), dextran sulphate-treated, LPS-treated or kinin antagonist-treated rabbits to an agonist of the B2 receptor. Kinin antagonist treatment was combined with either saline or LPS injection. Treatments were in the form of intravenous injections 5 h prior to sacrifice. The cumulative concentration-effect curve of the B2R agonist BK was established after a short in vitro incubation (60 min). Values (means±s.e.mean of eight determinations from four animals in each group) are expressed as a per cent of an internal contractile standard established in each tissue, the maximal effect of histamine. See Table 2 for statistical analysis.
Table 2.
Effects of in vitro treatments on the contractile response of rabbit isolated blood vessels to kinins
The rabbit jugular vein stimulated with the B2R agonist BK revealed a similar state of sensitivity and maximal response in groups treated with saline, dextran sulphate or LPS (Figure 3, Table 2). The groups pretreated with the kinin antagonist combination and further treated with saline or LPS exhibited a trend towards reduced maximal effects of BK (significant only in the group treated with the antagonists alone; Figure 3, Table 2).
Kininogen reserve in rabbits submitted to the acute treatments
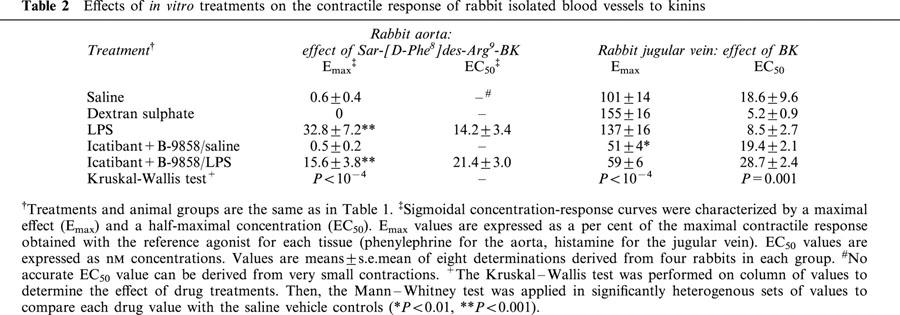
The kinetics of the appearance of immunoreactive BK, followed by its partial conversion into des-Arg9-BK, has been studied as an index of kininogen reserve in plasma exposed ex vivo to glass beads. Plasma samples were obtained from ACE-inhibitor treated rabbits in order to improve peptide stability and the conversion rate of BK into des-Arg9-BK (Décarie et al., 1996). As compared to pre-treatment values, acute dextran sulphate treatment reduced the capacity of the plasma to produce BK, but not to convert a part of BK into des-Arg9-BK (Figure 4). By contrast, LPS did not produce a significant consumption of the contact system components, as evidenced by an intact capacity to form immunoreactive kinins 90 min post-treatment.
Figure 4.
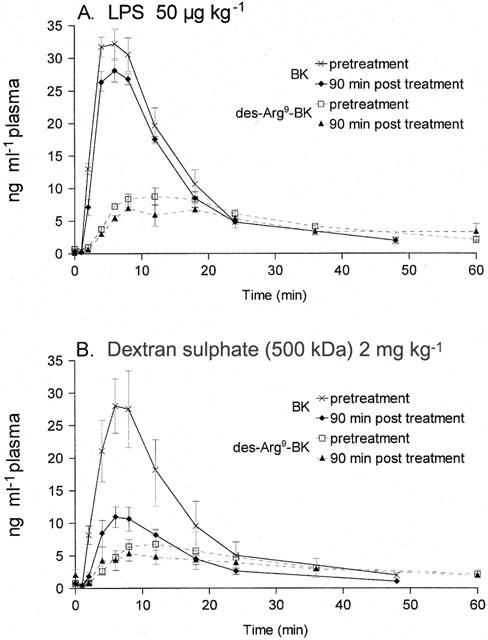
Consumption of the contact system components in rabbit plasma by in vivo treatments as assessed by in vitro formation of immunoreactive BK and des-Arg9-BK. Blood has been sampled in anaesthetized animals before and 90 min after injection of either LPS or dextran sulphate at the indicated doses. Then, the contact system was maximally activated using glass beads, and the plasma was periodically sampled for the measurement of immunoreactive kinins (see text). Values are the means±s.e.mean of three determinations in each group.
Hypotensive effect of dextran sulphate in anaesthetized rabbits
Anaesthetized rabbits treated with dextran sulphate exhibited a slow developing, but sustained hypotensive response over the 30 min observation period (Figure 5). Animals that were pretreated with the B2R antagonist icatibant initially responded to dextran sulphate by a decreased mean blood pressure, but this haemodynamic parameter returned towards baseline values within a few minutes (Figure 5). Thus, the blood pressure changes were significantly different between the two groups at time points 25 and 30 min.
Figure 5.
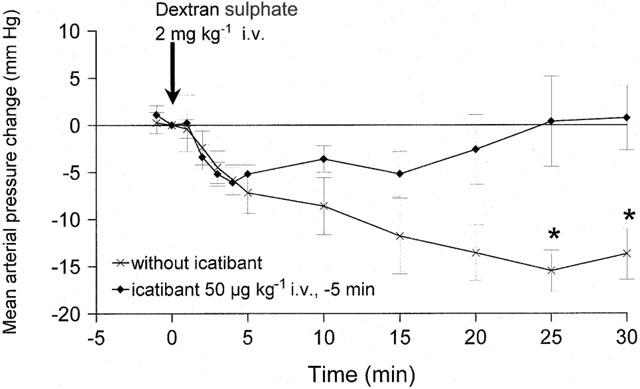
Change of mean blood pressure in anaesthetized rabbits treated with dextran sulphate. Values are the means±s.e.mean of seven (control) or five (icatibant) determinations in each group. A group of animals had been treated with icatibant (50 μg kg−1, i.v.) 5 min before dextran sulphate injection. *Values significantly different between groups (P<0.05, Mann–Whitney) at the considered time point.
Discussion
Rabbit aortic SMCs express a cytokine-regulated and functionally coupled population of B1Rs (Levesque et al., 1993; 1995a, 1995b; Schneck et al., 1994; Galizzi et al., 1994) and also a certain population of B2Rs coupled to phospholipase C activity (Schneck et al., 1994). These primary cultures are suitable to study the kinin receptor autoregulation hypothesis, as the stimulation of at least the B1Rs could regulate B1R expression. Our results are negative in this respect (Figure 1) in a time frame sufficient for a positive effect of IL-1β. The discrepancy between the cellular system, the IMR-90 cells (see Introduction), where the kinin receptor autoregulation has been documented and the present in vitro system, in which kinin receptor stimulation failed to up-regulate B1Rs, may be attributed to the biology of the cultured cell models. Either IMR-90 or SMCs exhibit a proliferative phenotype, as they grow in artificial medium supplemented with FBS rich in growth factors. However, IMR-90 cells themselves are a source of autocrine IL-1β, the IL-1 isoform usually produced by phagocytic leukocytes. Kinin-induced IL-1-dependent induction of B1Rs in IMR-90 cells (Phagoo et al., 1999) is not a specific phenomenon, as agonists for other G protein coupled receptors are active in this respect in IMR-90 cells (e.g., IL-8; Bastian et al., 1998). Therefore, a form of ‘immunological priming' may facilitate B1R expression in IMR-90 cells, a phenomenon that may not be widely applicable (most cells expressing B1Rs may not produce IL-1β in vivo). Indeed, the lack of effect of dextran sulphate administered in vivo on B1R expression in several organs does not generally support the coupling of cytokine synthesis with kinin receptor stimulation.
The rabbit has been previously used as a model organism to test whether endogenous levels of kinins can regulate the expression of either type of kinin receptors: chronic blockade of either B1 or B2Rs did not alter significantly receptor expression (Marceau et al., 1999). Further, angiotensin converting enzyme (ACE) inhibition also failed to induce B1Rs (Marceau et al., 1999), although this intervention is known to somewhat increase blood immunoreactive kinins in this species (Raymond et al., 1995). In the present experiments, we have attempted to simulate a higher, pathological level of endogenous kinins by activating in vivo the contact system using high-molecular dextran sulphate, a model previously described in the pig (Schmid et al., 1998). This treatment was effective in the rabbit, as evidenced by the consumption of contact system components necessary to ex vivo BK formation (Figure 4B) and the B2R-mediated slow-developing hypotension produced at least initially by this treatment; such a slow developing hypotensive effect was also evident in the pig and lasted less than 1 h (Schmid et al., 1998). Thus, dextran sulphate generated pharmacologically relevant concentration levels of endogenous kinin in vivo over an extended period. Dextran sulphate treatment did not induce B1R expression within an experimental time frame sufficient for B1R induction by LPS, thus failing to support the autoregulation hypothesis. The ex vivo experimental system that we used to assess kininogen reserve exhibits several distinctive features: it involves a maximal and rapid contact system activation, stronger that the ones that can be reasonably applied in vivo (Kaplan et al., 1998); the conversion of BK into des-Arg9-BK is relatively inefficient, as the relevant reaction is only one of several BK metabolic pathways in plasma (Décarie et al., 1996); des-Arg9-BK itself is slowly metabolized (Décarie et al., 1996), which explains the fact that it can accumulate to some extent (Figure 4). Could a higher intensity of in vivo B2R stimulation or a more persistent one be more effective to induce B1Rs? This possibility is supported by a system where exogenous [Tyr8]-BK is injected daily into rat tissues (Campos et al., 1995), but the latter system does not rely on endogenous formation of kinins. On the other hand, high concentrations of exogenous kinins, representing the maximal and persistent stimulation of kinin receptors in aortic SMCs, fail to upregulate B1Rs in primary cultures derived from a tissue known to express these receptors in a regulated manner (Figure 1).
The functional response to BK in the jugular vein from dextran sulphate-treated rabbits was not depressed, suggesting that autologous B2R desensitization observed in cellular systems (see Introduction) is rapidly and completely reversible. Supporting this view, experimental systems pertaining to the vascular function in the rat suggest that a specific time window (≈#38;15 min) of desensitization follows the administration of a B2R agonist in vivo, followed by complete resensitization (renal vasodilation, blood brain barrier opening) (Bartus et al., 1996; Praddaude et al., 1995). Further, our recent results, based on a rabbit B2R-green fluorescent protein conjugate stably expressed in an heterologous cell line, suggest that agonist-induced internalization is followed by complete recycling to the membrane in 1 h, with no significant down-regulation at the protein level (Bachvarov et al., 2001). This time frame is compatible with the possible ex vivo resensitization of the jugular vein in dextran sulphate-treated rabbits during the equilibration period allowed before BK stimulation, thus explaining the intact response of the preparation.
Our experimental data indicate that LPS was the only consistent treatment to induce B1R expression (functional response in aorta, mRNA induction in several organs) in the live rabbit. Low B1R mRNA levels measured in all tissues from control animals using the non-linear technique RT–PCR may not be associated with significant receptor populations, as suggested by the lack of functional response in control aortas (Table 2). LPS effects in vivo are particularly complex (Karima et al., 1999). However, at the low and sublethal dose used in the present experiments, the kallikrein-kinin system does not appear to mediate LPS effect to an important extent for the considered end points. Firstly, the kininogen reserve is not reduced to a significant extent in anaesthetized rabbits 90 min after LPS administration (Figure 4B); only considerably higher, lethal doses of LPS can deplete kininogen in rabbits (Erdös & Miwa, 1968). Secondly, combined blockade of B1 and B2Rs applied just before LPS administration exerted no modulatory (inhibitory) effect on B1R induction by LPS (rabbit aorta contractility, mRNA measurements in various organs). The two kinin receptor antagonists used in the present experiments, B-9858 and icatibant, are peptide drugs that work well in the rabbit, as either one exerts a prolonged action in vivo and in vitro (Gobeil et al., 1999; Houle et al., 2000; Larrivée et al., 2000). The depressed response to BK in jugular veins from animals treated with the kinin receptor antagonists (Figure 3) is likely to be determined by the practically irreversible effect of icatibant given in vivo several hours before the experiments (added to a 1 h washout period ex vivo applied for tissue equilibration). Similarly, in vivo treatment with both B-9858 and icatibant had a tendency to reduce the response to the B1R agonist Sar-[D-Phe8]des-Arg9-BK in aortic rings from LPS-treated rabbits, relative to those from animals treated with LPS alone (Figure 2), a fact probably explained by the insurmountable and slowly reversible effect of B-9858 on the rabbit B1R (Larrivée et al., 2000). Treatment with combined receptor antagonists did not influence B1R mRNA expression induced by LPS in various organs, supporting that the endogenous kinins do not participate in this effect of LPS. However, LPS may recruit other endogenous mediators that modulate B1R expression in vivo.
The present experiments support the exquisitely sensitive regulation of B1Rs by IL-1 in vitro and LPS in vivo; this has obvious implications for infectious disease physiopathology and immunopathology. Induction of vascular B1Rs following LPS treatment has been shown to occur in the mouse, rat, pig and a non-human primate (reviewed by Marceau et al., 1998; McLean et al., 2000; see also Schanstra et al., 2000; Deblois & Horlick, 2001). High sensitivity to endotoxin and the optimal stimulation of B1R by the sequence Lys-des-Arg9-BK are features of the rabbit model shared with primate species, as opposed to rodent models. B1R induction also occurs in complex pathological models, such as chemical inflammation of the urinary bladder (Bélichard et al., 1999), myocardial infarction (Tschöpe et al., 2000), or ischaemia-induced angiogenesis in the rat (Emanueli et al., 2001) or myocardial ischaemia-reperfusion in rabbits (Mazenot et al., 2001); the formation of both inflammatory cytokines and endogenous kinins is plausible in all these situations. The relatively simple experimental models presented here may help to clarify that endogenous kinins are not likely to be the major regulators of kinin receptor populations. The cytokine network is activated in a complex manner by LPS in the rabbit, and the relative importance of individual cytokines (IL-1, tumor necrosis factor-α, IL-6 etc.) (Dinarello, 1991), and of downstream effectors (MAP kinases, transcription factors) remains to be established for in vivo B1R induction. The occurrence of ligand-mediated B2R down-regulation in chronic inflammation remains uncertain. Persistent desensitization of the B2R may occur in vivo if endogenous kinins are continuously produced locally, perhaps accounting for some experimental systems where inflammation is long lasting (Kachur et al., 1996; Perkins et al., 1993). In addition, long term transcriptional suppression of the B2R mRNA may also be mediated by inflammatory cytokines and/or other mediators from infiltrating leukocytes; there is also preliminary evidence that limited proteolysis of the B2R by extracellular proteases results in its rapid degradation by cells (Bachvarov et al., 2001).
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR, grant MOP 14077). T. Sabourin and S. Houle are the recipients of Studentships from FCAR/FRSQ (Québec) and CIHR, respectively. D. R. Bachvarov is a Scholar of the FRSQ (Québec).
Abbreviations
- ACE
angiotensin converting enzyme
- B1R
B1 receptor
- B2R
B2 receptor
- BK
bradykinin
- FBS
foetal bovine serum
- IL
interleukin
- LPS
lipopolysaccharide
- MAP kinase
mitogen-activated protein kinase
- RT–PCR
reverse transcriptase-polymerase chain reaction
- Sar
sarcosine
- SMC
smooth muscle cell
References
- ADAM A., ALBERT A., BOULANGER J., GENOT D., DEMOULIN A., DAMAS J. Influence of oral contraceptives and pregnancy on constituents of the kallikrein-kininogen system in plasma. Clin. Chem. 1985a;31:1533–1536. [PubMed] [Google Scholar]
- ADAM A., ALBERT A., CALAY G., CLOSSET J., DAMAS J., FRANCHIMONT P. Human kininogens of low and high molecular mass: quantification by radioimmunoassay and determination of reference values. Clin. Chem. 1985b;31:423–436. [PubMed] [Google Scholar]
- ADAM A., AZZOUZI M., BOULANGER J., ERS P., ALBERT A., DAMAS J., FAYMONVILLE M.E. Optimized determination of plasma prokallikrein on a Hitachi 705 analyser. J. Clin. Chem. Clin. Biochem. 1985c;23:203–207. doi: 10.1515/cclm.1985.23.4.203. [DOI] [PubMed] [Google Scholar]
- ANGERS M., DROUIN R., BACHVAROVA M., PARADIS I., MARCEAU F., BACHVAROV D.R. In vivo protein-DNA interactions at the kinin B1 receptor promoter: no modification upon interleukin-1β or lipopolysaccharide induction. J. Cell. Biochem. 2000;78:278–296. doi: 10.1002/(sici)1097-4644(20000801)78:2<278::aid-jcb10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- BACHVAROV D.R., HOULE S., BACHVAROVA M., BOUTHILLIER J., ADAM A., MARCEAU F. Bradykinin B2 receptor endocytosis, recycling and down-regulation assessed using green fluorescent protein conjugates. J. Pharmacol. Exp. Ther. 2001;297:19–26. [PubMed] [Google Scholar]
- BARTUS R.T., ELLIOTT P.J., DEAN R.L., HAYWARD N.J., NAGLE T.L., HUFF M.R., SNODGRASS P.A., BLUNT D.G. Controlled modulation of BBB permeability using the bradykinin agonist, RMP-7. Exp. Neurol. 1996;142:14–28. doi: 10.1006/exnr.1996.0175. [DOI] [PubMed] [Google Scholar]
- BASTIAN S., PAQUET J.L., ROBERT C., CREMERS B., LOILLIER B., LARRIVÉE J.-F., BACHVAROV D.R., MARCEAU F., PRUNEAU D. Interleukin 8 (IL-8) induces the expression of kinin B1 receptor in human lung fibroblasts. Biochem. Biophys. Res. Commun. 1998;253:750–755. doi: 10.1006/bbrc.1998.9848. [DOI] [PubMed] [Google Scholar]
- BÉLICHARD P., LUCCARINI J.M., DEFRENE E., FAYE P., FRANCK R.M., DUCLOS H., PAQUET J.L., PRUNEAU D. Pharmacological and molecular evidence for kinin B1 receptor expression in urinary bladder of cyclophosphamide-treated rats. Br. J. Pharmacol. 1999;128:213–219. doi: 10.1038/sj.bjp.0702769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAUKAT A., ABD ALLA S., LOHSE M.J., MÜLLER-ESTERL W. Ligand-induced phosphorylation/dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. J. Biol. Chem. 1996;271:32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., MATA L.V., CALIXTO J.B. Expression of B1 kinin receptors mediating paw edema and formalin-induced nociception. Modulation by glucocorticoids. Can. J. Physiol. Pharmacol. 1995;73:812–819. doi: 10.1139/y95-110. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., BOUTHILLIER J., MARCEAU F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit aorta to des-Arg9-bradykinin. Br. J. Pharmacol. 1988;93:969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBLOIS D., HORLICK R.A. Endotoxin sensitization to kinin B1 receptor agonist in a non-human primate model: haemodynamic and pro-inflammatory effects. Br. J. Pharmacol. 2001;132:327–335. doi: 10.1038/sj.bjp.0703748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÉCARIE A., DRAPEAU G., CLOSSET J., COUTURE R., ADAM A. Development of digoxygenin-labelled peptides: Application to chemoluminescence immunoassay of bradykinin in inflamed tissues. Peptides. 1994;15:511–518. doi: 10.1016/0196-9781(94)90214-3. [DOI] [PubMed] [Google Scholar]
- DÉCARIE A., RAYMOND P., GERVAIS N., COUTURE R., ADAM A. Serum interspecies differences in metabolic pathways of bradykinin and [des-Arg9]BK: influence of enalaprilat. Am. J. Physiol. 1996;271:H1340–H1347. doi: 10.1152/ajpheart.1996.271.4.H1340. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. The proinflammatory cytokines interleukin-l and tumor necrosis factor and treatment of the septic shock syndrome. J. Infect. Dis. 1991;163:1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G., AUDET R., LEVESQUE L., GODIN D., MARCEAU F. Development and in vivo evaluation of metabolically resistant antagonists of B1 receptors for kinins. J. Pharmacol. Exp. Ther. 1993;266:192–199. [PubMed] [Google Scholar]
- EMANUELI C., MINASI A., ZACHEO A., CHAO J., CHAO L., SALIS M.B., STRAINO S., TOZZI M.G., SMITH R., GASPA L., BIANCHINI G., STILLO F., CAPOGROSSI M.C., MADEDDU P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- ERDÖS E.G., MIWA I. Effect of endotoxin shock on the plasma kallikrein-kinin system of the rabbit. Fed. Proc. 1968;27:92–95. [PubMed] [Google Scholar]
- FAUSSNER A., PROUD D., TOWNS M., BATHON J.M. Influence of the cytosolic carboxyl termini of human B1 and B2 kinin receptors on receptor sequestration, ligand internalization, and signal transduction. J. Biol. Chem. 1998;273:2617–2623. doi: 10.1074/jbc.273.5.2617. [DOI] [PubMed] [Google Scholar]
- GALIZZI J.P., BODINIER M.C., CHAPELAIN B., LY S.M., COUSSY L., GIRAUD S., NELIAT G., JEAN T. Up-regulation of [3H]-des-Arg10-kallidin binding to the bradykinin B1 receptor by interleukin-1β in isolated smooth muscle cells: correlation with B1 agonist-induced PGI2 production. Br. J. Pharmacol. 1994;113:389–394. doi: 10.1111/j.1476-5381.1994.tb17001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBEIL F., CHARLAND S, , FILTEAU C., PERRON S.I., NEUGEBAUER W., REGOLI D. Kinin B1 receptor antagonists containing α-methyl-L-phenylalanine: in vitro and in vivo antagonist activities. Hypertension. 1999;33:823–829. doi: 10.1161/01.hyp.33.3.823. [DOI] [PubMed] [Google Scholar]
- HOULE S., LARRIVÉE J.-F., BACHVAROVA M., BOUTHILLIER J., BACHVAROV D.R., MARCEAU F. Antagonist-induced intracellular sequestration of the rabbit bradykinin B2 receptor. Hypertension. 2000;35:1319–1325. doi: 10.1161/01.hyp.35.6.1319. [DOI] [PubMed] [Google Scholar]
- KACHUR J.F., ALLBEE W., GAGINELLA T.S.Effect of bradykinin and des-Arg9-bradykinin on ion transport across normal and inflamed rat colonic mucosa Gastroenterology 1996901481[abstract] [Google Scholar]
- KAPLAN A.P., JOSEPH K., SHIBAYAMA Y., NAKAZAWA Y., GHEBREHIWET B., REDDIGARI S., SILVERBERG M. Bradykinin formation. Clin. Rev. Allergy Immunol. 1998;98:403–429. doi: 10.1007/BF02737659. [DOI] [PubMed] [Google Scholar]
- KARIMA R., MATSUMOTO S., HIGASHI H., MATSUSHIMA K. The molecular pathogenesis of endotoxic shock and organ failure. Mol. Med. Today. 1999;5:123–132. doi: 10.1016/s1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- LARRIVÉE J.-F., BACHVAROV D.R., HOULE F., LANDRY J., HUOT J., MARCEAU F. Role of the mitogen-activated protein kinases in the expression of the kinin B1 receptors induced by tissue injury. J. Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- LARRIVÉE J.F., GERA L., HOULE S., BOUTHILLIER J., BACHVAROV D.R., STEWART J.M., MARCEAU F. Non-competitive pharmacological antagonism at the rabbit B1 receptor. Br. J. Pharmacol. 2000;131:885–892. doi: 10.1038/sj.bjp.0703656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVESQUE L., DRAPEAU G., GROSE J.H., RIOUX F., MARCEAU F. Vascular mode of action of kinin B1 receptors and development of a cellular model for the investigation of these receptors. Br. J. Pharmacol. 1993;109:1254–1262. doi: 10.1111/j.1476-5381.1993.tb13757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVESQUE L., HARVEY N., RIOUX F., DRAPEAU G., MARCEAU F. Development of a binding assay for the B1 receptors for kinins. Immunopharmacology. 1995a;29:141–147. doi: 10.1016/0162-3109(94)00053-i. [DOI] [PubMed] [Google Scholar]
- LEVESQUE L., LARRIVÉE J.-F., BACHVAROV D.R., RIOUX F., DRAPEAU G., MARCEAU F. Regulation of kinin-induced contraction and DNA synthesis by inflammatory cytokines in the smooth muscle of the rabbit aorta. Br. J. Pharmacol. 1995b;116:1673–1679. doi: 10.1111/j.1476-5381.1995.tb16390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARCEAU F., LARRIVÉE J.-F., BOUTHILLIER J., BACHVAROVA M., HOULE S., BACHVAROV D.R. Effect of endogenous kinins, prostanoids, and NO on kinin B1 and B2 receptor expression in the rabbit. Am. J. Physiol. 1999;277:R1568–R1578. doi: 10.1152/ajpregu.1999.277.6.R1568. [DOI] [PubMed] [Google Scholar]
- MAZENOT C., LOUFRANI L., HENRION D., RIBUOT C., MÜLLER-ESTERL W., GODIN-RIBUOT D. Endothelial kinin B1-receptors are induced by myocardial ischaemia-reperfusion in the rabbit. J. Physiol. (London) 2001;530:69–78. doi: 10.1111/j.1469-7793.2001.0069m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P.G., PERRETTI M., AHLUWALIA A. Kinin B1 receptors and the cardiovascular system: regulation of expression and function. Cardiovasc. Res. 2000;48:194–210. [Google Scholar]
- NARABA H., UENO A., KOSUGI Y., YOSHIMURA M., MURAKAMI M., KUDO I., OH-ISHI S. Agonist stimulation of B1 and B2 kinin receptors causes activation of the MAP kinase signaling pathway, resulting in the translocation of AP-1 in HEK 293 cells. FEBS Lett. 1998;435:96–100. doi: 10.1016/s0014-5793(98)01045-x. [DOI] [PubMed] [Google Scholar]
- NI A., CHAO L., CHAO J. Transcription factor nuclear factor κB regulates the inducible expression of the human B1 receptor gene in inflammation. J. Biol. Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., CAMPBELL E., DRAY A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and Hoe 140, in two models of persistent hyperalgesia in the rat. Pain. 1993;53:191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- PHAGOO S.B., POOLE S., LEEB-LUNDBERG L.M.F. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1β shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol. Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- PIZARD A., BLAUKAT A., MÜLLER-ESTERL W., ALHENC-GELAS F., RAJERISON R.M. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J. Biol. Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- PRADDAUDE F., TACK I., EMOND C., BASCANDS J.L., GIROLAMI J.-P., TRAN-VAN T., REGOLI D., ADER J.L. In vivo and in vitro homologous desensitization of rat glomerular bradykinin B2 receptors. Eur. J. Pharmacol. 1995;294:173–182. doi: 10.1016/0014-2999(95)00532-3. [DOI] [PubMed] [Google Scholar]
- RAYMOND P., DRAPEAU G., RAUT R., AUDET R., MARCEAU F., ONG H., ADAM A. Quantification of des-Arg9-bradykinin using a chemiluminescence enzyme immunoassay: application to its kinetic profile during plasma activation. J. Immunol. Methods. 1995;180:247–257. doi: 10.1016/0022-1759(94)00320-v. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLE E., MARIN CASTANO M.E., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.-P., BASCANDS J.L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-κB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHANSTRA J.P., MARIN-CASTANO M.E., PRADDAUDE F., TACK I., ADER J.L., GIROLAMI J.P., BASCANDS J.L., JEUNIER B. Bradykinin B1 receptor-mediated changes in renal hemodynamics during endotoxin-induced inflammation. J. Am. Soc. Nephrol. 2000;11:1208–1215. doi: 10.1681/ASN.V1171208. [DOI] [PubMed] [Google Scholar]
- SCHMID A., EICH-RATHFELDER S., WHALLEY E.T., CHERONIS J.C., SCHEUBER H.-P., FRITZ H., SIEBECK M. Endogenous B1 receptor mediated hypotension produced by contact system activation in the presence of endotoxemia. Immunopharmacology. 1998;40:131–137. doi: 10.1016/s0162-3109(98)00038-1. [DOI] [PubMed] [Google Scholar]
- SCHNECK K.A., HESS J.F., STONESIFER G.Y., RANSOM R.W. Bradykinin B1 receptors in rabbit aorta smooth muscle cells in culture. Eur. J. Pharmacol. 1994;266:277–282. doi: 10.1016/0922-4106(94)90137-6. [DOI] [PubMed] [Google Scholar]
- TSCHÖPE C., HERINGER-WALTHER S., WALTHER T. Regulation of the kinin receptors after induction of myocardial infarction: a mini-review. Braz. J. Med. Biol. Res. 2000;33:701–708. doi: 10.1590/s0100-879x2000000600011. [DOI] [PubMed] [Google Scholar]
- YANG X., TAYLOR L., POLGAR P. Mechanisms in the transcriptional regulation of bradykinin B1 receptor gene expression. J. Biol. Chem. 1998;273:10763–10770. doi: 10.1074/jbc.273.17.10763. [DOI] [PubMed] [Google Scholar]


