Abstract
The effects of K+ channel blockers, such as 4-aminopyridine (4-AP) and tetraethylammonium (TEA), and a reverse-mode Na+ – Ca2+ exchange blocker, 2-[2-[4-(4-nitrobenzyloxyl) phenyl] ethyl] isothiourea methanesulphonate (KB-R7943), on the responses of slowly adapting pulmonary stretch receptor activity to hyperinflation (inflation volume=3 tidal volumes) were investigated in anaesthetized, artificially ventilated, unilaterally vagotomized rats after pretreatment with a Na+ channel blocker flecainide. The administration of flecainide (9 mg kg−1) at a dose greater than that which abolished 50 μg kg−1 veratridine-induced SAR stimulation also inhibited hyperinflation-induced stimulation of SARs.
In flecainide-treated animals, administration of 4-AP (0.7 and 2 mg kg−1) stimulated SAR activity during normal inflation and also caused a partial blockade of hyperinflation-induced SAR inhibition.
The discharges of SARs during normal inflation in flecainide-treated animals were not significantly altered by administration of either TEA (2 and 7 mg kg−1) or KB-R7943 (1 and 3 mg kg−1), but both K+ channel and Na+-Ca2+ exchange blockers partially attenuated hyperinflation-induced SAR inhibition.
These results suggest that hyperinflation-induced SAR inhibition in the presence of flecainide (9 mg kg−1) involves the activation of several K+ conductance pathways.
Keywords: Slowly adapting pulmonary stretch receptor, Na+ channel blocker, hyperinflation, K+ channel blocker, Na+-Ca2+ exchange blocker
Introduction
During the generation of action potentials, Na+ conductance plays an important role in the depolarization of the nerve cell membranes. The Na+ channel opener veratridine in neural tissues is voltage-dependent (Corbett & Kreuger, 1989; Castillo et al., 1992) and tetrodotoxin (TTX)-sensitive (Narahashi, 1974; Gatteral, 1992). Pretreatment with flecainide, one of the Na+ channel blockers (Banitt et al., 1977; Campbell & Williams, 1983), prevents veratridine-induced slowly adapting pulmonary stretch receptor (SAR) stimulation in the rabbit (Matsumoto & Shimizu, 1995; Matsumoto et al., 1998) and rat (Matsumoto et al., 2000).
The ion channels responsible for mechanically activated currents in spider mechanoreceptors are mainly selective for Na+ (Juusola et al., 1994; Höger et al., 1997). The mechanical deformation through stretch or distention of the airway smooth muscle is thought to stimulate SARs which have stretch-activated (SA) channels on the receptor endings but the mechanism mediating the mechanosensitivity of SARs remains to be determined. In the rat, flecainide treatment (6 mg kg−1) sufficient to abolish 50 μg kg−1 veratridine-induced SAR stimulation has no significant effect on excitation of the receptor activity seen during hyperinflation (Matsumoto et al., 2000). The explanation for such an effect is that flecainide-resistant Na+ channels in SARs contribute to excitation of the receptor activity during hyperinflation.
Three prevalent types of K+ channels, characterized by kinetic and pharmacological properties, have been identified as ‘fast transient outward' (Connor & Stevens, 1971), ‘delayed recifier' (Hodgkin & Huxley, 1952) and ‘Ca2+-activated K+' (Meech & Standen, 1975) currents. In both the repolarization and after-hyperpolarization phases of action potentials, K+ conductances have the ability to regulate the number of spikes with a cycle of Na+ inflow and K+ outflow. Both a fast-inactivating A-type current (IA) and a sustained K+ current of delayed-rectifier type (IK) in addition to a Ca2+-activated K+ current (IKCa) are responsible for the hyperpolarizing effect which inhibits the number of action potentials. When considering together the hyperpolarization caused by the activation of IA, IK, or IKCa and the inhibitory effect of flecainide (9 mg kg−1) on hyperinflation-induced SAR stimulation (Matsumoto et al., 2000), it can be assumed that hyperinflation exerts an inhibitory effect on the activity of SARs through modification of K+ currents, particularly under conditions in which Na2+-dependent veratridine-induced SAR stimulation was blocked with flecainide.
To test this assumption, we performed three different types of experiments in anaesthetized, artificially ventilated rats after unilateral vagotomy. First, the responses of SARs to hyperinflation were examined before and after administration of 4-aminopyridine (4-AP), a K+ channel blocker inhibiting the IA, in the rats after flecainide treatment. Secondly, the responses of SARs to hyperinflation were compared before and after administration of tetraethylammonium (TEA), a K+ channel blocker inhibiting the IK, in flecainide-treated rats. Thirdly, in rats after flecainide treatment the changes in SAR activity in response to hyperinflation were examined before and after administration of 2-[2-[4-(4-nitrobenzyloxyl) phenyl] ethyl] isothiourea methanesulphonate (KB-R7943), which inhibits the inward Na+ – Ca2+ exchange current more potently than the outward Na+ – Ca2+ exchange current in guinea-pig ventricular cells (Watano et al., 1996). We selected either mostly inflationary SARs, firing throughout the whole respiratory cycle, or inflationary SARs, as described previously (Tsubone, 1986; Bergren & Peterson, 1993).
Methods
Animal preparation
The experiments were performed on 18 Wistar rats (280 – 350 g). All experimental protocols used in this study were approved by the Animal Use and Care Committee of Nippon Dental University. Each animal was first anaesthetized with sodium pentobarbital (45 – 50 mg kg−1) intraperitoneally. The trachea was reached through a middle incision in the neck and cannulated below the larynx. In order to obtain sufficient space for liquid paraffin, the trachea and esophagus were dissected free and retracted rostrally. Tracheal pressure (PT) was measured by connecting a polyethylene catheter inserted into the tracheal tube to a pressure transducer. The right carotid artery was cannulated for continuous monitoring of blood pressure (BP). The left vagus nerve was exposed and sectioned, whereas the right vagus nerve was left intact. Rectal temperature was maintained at around 37°C by means of a heating pad or lamp. The animals were paralyzed with intravenous administration of gallamine (5 – 10 mg kg−1) for 30 – 60 min, and additional doses (3 – 5 mg kg−1) of gallamine were administered to eliminate spontaneous respiratory movements, as required. The stroke volume of the respirator was set at 10 ml kg−1 and its frequency ranged from 50 to 60 cycles min−1. Tracheal CO2 was monitored with a CO2 gas analyser (Respina IH 26, Sanei) and kept at approximately 32 – 35 mmHg. Anaesthesia was maintained with additional doses of 15 – 20 mg kg−1 h−1 through a cannula inserted into the right jugular vein. In order to assess the level of anaesthesia, we used corneal and tail-pinch reflexes. Abrupt changes in BP induced by these two manoeuvres were monitored to ensure that the animals remained fully anaesthetized during neuromuscular blockade.
Measurement of slowly adapting pulmonary stretch receptors
The peripheral end of the cut left vagus nerve was desheathed. To record the single unit activity of SARs, thin strands containing afferent nerve fibres were separated, placed on a unipolar silver electrode and submerged in a pool of liquid paraffin (37 – 38°C). The SARs were identified, on the basis of their firing behaviour during inflation, as follows: (1) The most inflationary SARs increased their activity during inflation and decreased their discharge during deflation, and in the case of inflationary SARs, those receptors increased their activity during inflation only. (2) The increase in SAR activity was proportional to that in the inflation volume of the respirator. (3) The discharge of SARs continued as long as the tracheal tube was occluded in a hyperinflated condition. The SAR activity was amplified and selected by means of a window discriminator for counting the number of impulses.
Experimental design
The experiments were designed to test the roles of generalized K+ channels and a reverse-mode Na+ – Ca2+ exchanger in the SAR responses to hyperinflation, particularly under conditions in which flecainide treatment was more than sufficient to abolish veratridine-induced SAR stimulation. (1) In six SAR fibres in case of six rats, the effects of hyperinflation for approximately 10 respiratory cycles on SAR activity and PT were determined. Ten minutes after flecainide administration at a dose (9 mg kg−1) sufficient to block 50 μg kg−1 veratridine-induced SAR stimulation, the same sets of experiments were repeated, and we confirmed hyperinflation-induced SAR inhibition, which fell below the discharge rate of SARs during normal inflation in flecainide-treated animals. Finally, 10 min after 4-AP administration (0.7 and 2 mg kg−1) in flecainide-treated animals, the same tests were repeated under the same conditions. (2) In six SAR fibres in case of six rats, the changes in SAR activity and PT in response to hyperinflation were examined before and after administration of flecainide (9 mg kg−1) and, subsequently, after TEA (2 and 7 mg kg−1) in the presence of flecainide. (3) In six SAR fibres in case of six rats, the responses of SARs and PT to hyperinflation were determined. The absence of an effect of veratridine (50 μg kg−1) on SAR activity and the occurrence of hyperinflation-induced SAR inhibition were confirmed after administration of flecainide (9 mg kg−1). Subsequently, after KB-R7943 administration (1 and 3 mg kg−1) the effects of hyperinflation on SAR activity and PT were examined under the same conditions. In these experiments, the effectiveness of flecainide was indicated by the absence of increased SAR activity after veratridine administration.
Drugs
4-AP and TEA were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). 4-AP (10 mg) and TEA (10 mg) were dissolved in and diluted with 0.9% NaCl. Veratridine (Sigma) was dissolved in a small amount of weak HCl and diluted with 0.9% NaCl. Flecainide (10 mg, Eizai Pharmaceutical Co. Ltd., Tokyo, Japan) was dissolved in 5.0% glucose solution. KB-R7943 (10 mg, Kanebo, Osaka, Japan) was dissolved in dimethylsulphoxide (DMSO) and the final concentration of DMSO was 0.1% or less.
Statistical analysis
Under control conditions, the firing rates of SARs in each whole respiratory cycles were measured over several respiratory cycles and expressed as imp s−1. The SAR responses to hyperinflation (inflation volume=3 VT) for approximately 10 respiratory cycles and to veratridine administration (50 μg kg−1) were obtained by counting the firing rates of receptors during hyperinflation and between the onset of increased receptor activity and recovery to the control level, respectively, and the average activities of SARs during one whole respiratory cycle were expressed as imp s−1. Similarly, control values for PT were averaged over several respiratory cycles and expressed as cmH2O. The responses of PT to hyperinflation and veratridine administration were obtained by measuring the respiratory parameter, as described above, and the average values for PT were expressed as cmH2O. The statistical significance of the effects of flecainide (9 mg kg−1) on the responses of SAR activity and PT to veratridine administration (50 μg kg−1), normal inflation and hyperinflation was first calculated by a one-way ANOVA for repeated measurements. In flecainide (9 mg kg−1)-treated animals before and after administration of 4-AP (0.7 and 2 mg kg−1), TEA (2 and 7 mg kg−1) and KB-R7943 (1 and 3 mg kg−1), the changes in SAR activity during hyperinflation were compared those during normal inflation, by using a paired t-test. All values were expressed as the means±s.e.means. A value of less than 0.05 was considered statistically significant.
Results
Effects of flecainide on responses of SARs to veratridine administration and hyperinflation
Typical examples of the effects of veratridine on SAR activity and PT before and after pretreatment with flecainide are shown in Figure 1a,b. Intravenous administration of veratridine (50 μg kg−1) caused vigorous stimulation of the SAR activity during both inflation and deflation, and this effect lasted for about 100 s. Under these conditions, the value for PT did not change significantly (Figure 1a). Flecainide treatment at a dose which blocked veratridine-induced SAR stimulation decreased the discharge of SARs during normal inflation (Figure 1b). In the same preparation, before flecainide treatment, the SAR activity increased during hyperinflation (Figure 1c). As shown in Figure 1d, in a flecainide-treated animal the magnitude of decreased SAR activity provoked by hyperinflation became more prominent than that seen during normal inflation. Figure 2a,b summarize the effects of flecainide on the SAR and PT responses to veratridine administration (50 μg kg−1) during normal inflation (1 VT). During normal inflation, veratridine administration (50 μg kg−1) caused approximately 2.4 fold increase in the SAR activity and this increase was abolished by pretreatment with flecainide (9 mg kg−1), which significantly reduced the baseline discharges of SARs. The changes in PT in responses to normal ventilation were not significantly altered by administration of either flecainide or veratridine. The responses of 18 different SAR fibres to hyperinflation before and after pretreatment with flecainide at 9 mg kg−1 are summarized in Figure 2c. Before flecainide treatment, hyperinflation caused an approximately 3.6 fold increase in SAR activity but pretreatment with flecainide (9 mg kg−1), which resulted in a significant inhibition of SAR activity during normal inflation, caused a greater inhibition on hyperinflation-induced SAR stimulation. The Na+ channel blocker flecainide at a dose of 9 mg kg−1 inhibited the SAR responses to hyperinflation more efficaciously than that to normal inflation but had no significant effect on the responses of PT to normal inflation and hyperinflation (Figure 2c,d). The decreases in BP occurred during hyperinflation as well as after flecainide administration in normal inflation, but the hypotension evoked by hyperinflation in the presence of flecainide was small. The mean BP (MBP) values were 98.7±2.6 and 81.2±2.1 mmHg during normal inflation and hyperinflation, respectively, and in the presence of flecainide, 83.4±2.9 and 80.9±2.3 mmHg during normal inflation and hyperinflation, respectively.
Figure 1.
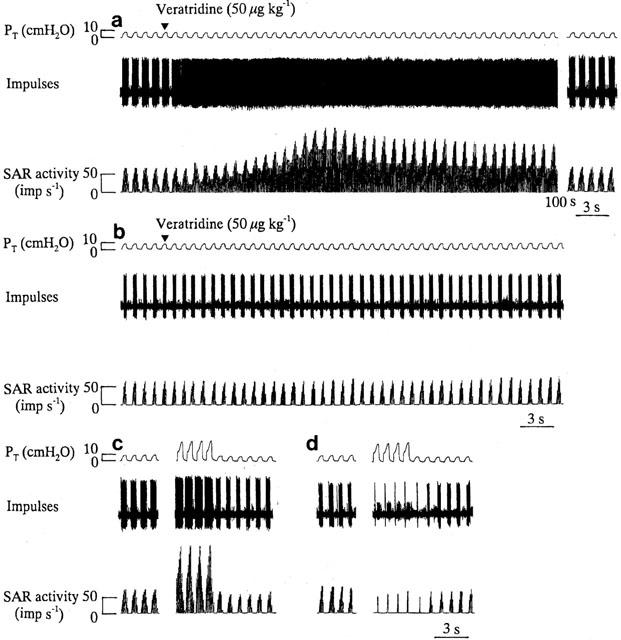
Responses in PT and SAR activity to veratridine before (a) and after (b) administration of flecainide (9 mg kg−1). 100 s indicates the elapsed time between recordings. Responses in PT and SAR activity to control inflation (inflation volume=1 VT) and hyperinflation (inflation volume=3 VT) before (c) and after (d) administration of flecainide (9 mg kg−1) in the same rat.
Figure 2.
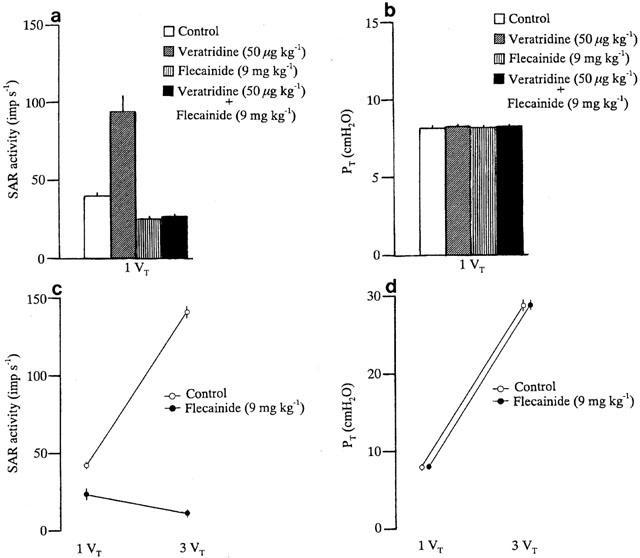
Responses in SAR activity (a) and PT (b) to veratridine at control inflation (inflation volume=1 VT). Changes in SAR activity (c) and PT (d) in response to control inflation and hyperinflation (inflation volume=3 VT) before and after pretreatment with flecainide (9 mg kg−1). Values are the means for 18 animals and the vertical bars show the s.e.means.
Effects of 4-AP on responses of SARs to hyperinflation in flecainide-treated animals
Figure 3a shows the responses of SAR activity and PT to normal inflation and hyperinflation in a flecainide (9 mg kg−1)-treated animal. Flecainide treatment caused hyperinflation-induced SAR inhibition. Under normal inflation, subsequent administration of 4-AP (2 mg kg−1) increased SAR activity during deflation but did not significantly alter PT (Figure 3b). In the presence of flecainide, the inhibitory response of SAR activity to hyperinflation was partially attenuated by 4-AP treatment (Figure 3c). Figure 4a summarizes the effect of 4-AP (0.7 and 2 mg kg−1) on the responses of SARs to hyperinflation in the presence of flecainide (9 mg kg−1). The K+ channel blocker 4-AP (0.7 and 2 mg kg) significantly reversed the inhibitory effect of hyperinflation on SAR activity in flecainide-treated animals (per cent change: absence, −41.6±5.2, n=6; in the presence of 4-AP, 0.7 mg kg−1, −7.8±1.2, n=6, P<0.05; 2 mg kg−1, +51.1±5.5, n=6, P<0.05). The administration of 4-AP in flecainide-treated animals caused an increase in BP, but this effect was short-lasting. During hyperinflation the MBP values were 80.4±4.9, 81.2±4.4 and 82.4±3.8 mmHg after flecainide treatment without and with 4-AP administration at 0.7 and 2 mg kg−1, respectively. No significant effects of 4-AP (0.7 and 2 mg kg−1) on the PT responses to hyperinflation were found in flecainide-treated animals (Figure 4b).
Figure 3.
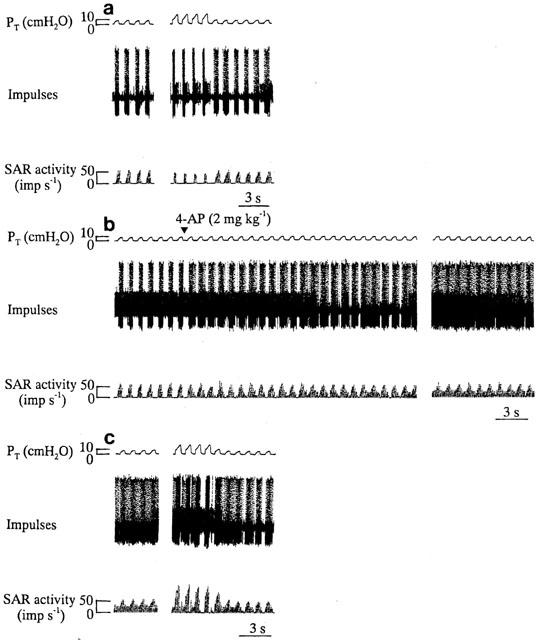
Responses in PT and SAR activity to control inflation (inflation volume=1 VT) and hyperinflation (inflation volume=3 VT) before (a), during (b) and after administration of 4-AP (2 mg kg−1) in a flecainide (9 mg kg−1)-treated animal.
Figure 4.
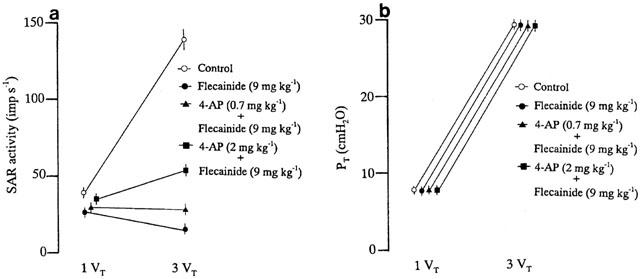
Changes in SAR activity (a) and PT (b) in response to control inflation (inflation volume=1 VT) and hyperinflation (inflation volume=3 VT) before and after pretreatment with 4-AP. Values are the means for six flecainide (9 mg kg−1)-treated animals and the vertical bars shown are the s.e.means.
Effects of TEA on responses of SARs to hyperinflation in flecainide-treated animals
After administration of flecainide (9 mg kg−1), hyperinflation inhibited SAR activity. Subsequent administration of TEA (7 mg kg−1) that did not change the discharge rate of SARs during normal inflation caused a partial blockade of hyperinflation-induced SAR inhibition in a flecainide-treated animal (Figure 5a,b). In six rats, pretreatment with flecainide (9 mg kg−1) greatly inhibited stimulation of SAR activity by hyperinflation. As shown in Figure 5c, subsequent administration of TEA (7 mg kg−1) in those animals partially attenuated hyperinflation-induced SAR inhibition (per cent change; absence, −56.3±6.2, n=6; in the presence of TEA, 2 mg kg−1, −49.8±5.4, n=6, P>0.05; 7 mg kg−1, +7.0±1.1, n=6, P<0.05). The MBP values were 82.6±3.5, 80.9±3.8 and 80.2±4.3 mmHg during hyperinflation after flecainide treatment without and with TEA administration 2 and 7 mg kg−1, respectively. TEA treatment had no significant effect on the PT and MBP responses to hyperinflation in flecainide-treated animals (Figure 5d).
Figure 5.
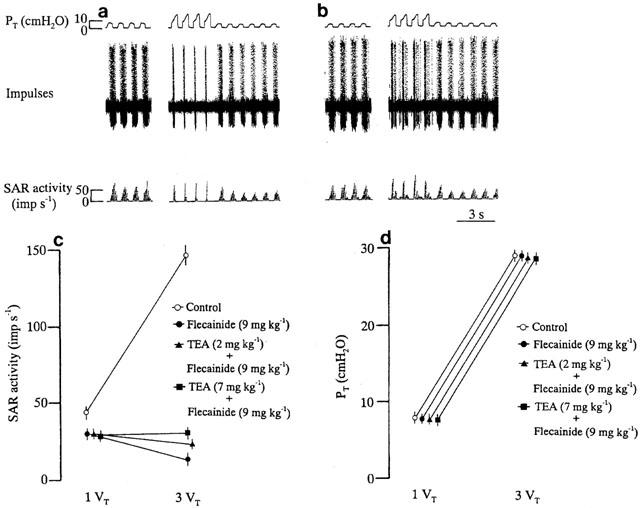
Responses in PT and SAR activity to control inflation (inflation volume=1 VT) and hyperinflation (inflation volume=3 VT) before (a) and after (b) administration of TEA (7 mg kg−1) in a flecainide (9 mg kg−1)-treated animal. Changes in SAR activity (c) and PT (d) in response to control inflation and hyperinflation before and after pretreatment with TEA. Values are the means for six flecainide (9 mg kg−1)-treated animals and the vertical bars show the s.e.means.
Effects of KB-R7943 on responses of SARs to hyperinflation in flecainide-treated animals
As shown in Figure 6a,b, hyperinflation caused inhibition of the SAR activity in a flecainide (9 mg kg−1)-treated animal, and this inhibitory effect was partially attenuated by subsequent administration of a reverse-mode Na+ – Ca2+ exchange blocker, KB-R7943 (3 mg kg−1). Figure 6c summarizes the effects of KB – R7943 (1 and 3 mg−1) on the responses of SAR activity to hyperinflation in six flecainide (9 mg kg−1)-treated animals. The inhibitory effect of hyperinflation on SAR activity in the presence of flecainide was partially attenuated by the Na+ – Ca2+ exchange blocker KB – R7943 (1 and 3 mg kg−1) (per cent change: absence, −57.4±6.3, n=6; in the presence of KB – R7943, 1 mg kg−1, −26.1±3.4, n=6, P<0.05; 3 mg kg, +43.2±5.5, n=6, P<0.05). In addition, no significant effects of KB-R7943 on the PT responses to normal inflation and hyperinflation were found in flecainide-treated animals (Figure 6d). During hyperinflation the MBP values were 81.1±3.9, 80.8±3.8 and 80.5±4.2 after flecainide treatment without and with KB-R7943 administration at 1 and 3 mg kg−1. In flecainide-treated animals, administration of DMSO, a solvent of KB-R7943, at a final concentration (0.1%) did not cause any significant change on SAR activity (Figure 6e).
Figure 6.
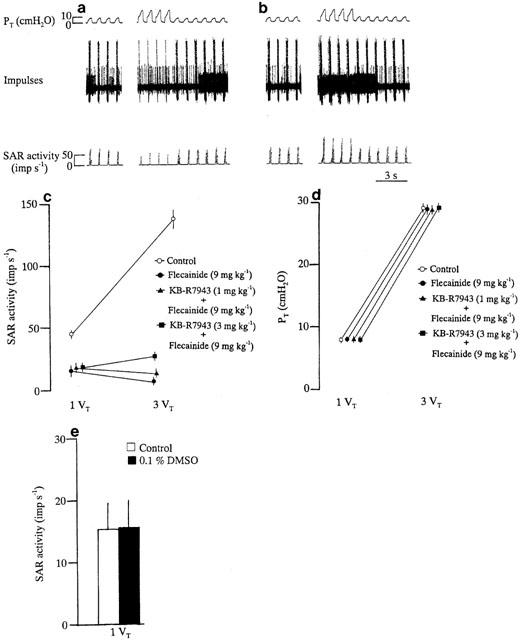
Responses in PT and SAR activity to control inflation (inflation volume=1 VT) and hyperinflation (inflation volume=3 VT) before (a) and after (b) administration of KB-R7943 (3 mg kg−1) in a flecainide (9 mg kg−1)-treated animal. Changes in SAR activity (c) and PT (d) in response to control inflation and hyperinflation before and after pretreatment with KB-R7943. Changes in SAR activity (e) induced by DMSO administration at control inflation. Values are the means for six flecainide (9 mg kg−1)-treated animals and the vertical bars show the s.e.means.
Discussion
In the present study we demonstrated that K+ channel blockers and a reverse mode Na+ – Ca2+ exchange blocker attenuated flecainide-induced inhibition of hyperinflation-induced SAR stimulation in rats. Our data suggest that K+ conductance pathways are involved in the hyperpolarization of SARs in the presence of flecainide.
Because TTX can block veratridine-induced increases in intracellular Na+ concentration [Na+]i in cultured rat hippocampal neurons (Rose & Ransom, 1997) and because vigorous stimulation in SARs by veratridine is completely blocked by flecainide treatment in the rabbit (Matsumoto & Shimizu, 1995; Matsumoto et al., 1998) and rat (Matsumoto et al., 2000), veratridine-induced SAR stimulation appears to be mediated by TTX-sensitive Na+ channels, but not by TTX-insensitive Na+ channels. In a previous study in rats, we found that flecainide treatment at a dose (6 mg kg−1), which abolished 50 μg kg−1 veratridine-induced SAR stimulation, had no significant effect on the excitation of SAR activity during hyperinflation (inflation volume=3 VT) (Matsumoto et al., 2000). This finding may be consistent with the observation that both TTX-sensitive and TTX-insensitive Na+ channel currents are present in the peripheral terminals of primary afferent nociceptive-like neurons (Brock et al., 1998). Presumably, TTX-resistant Na+ channels in SARs contribute at least to hyperinflation-induced stimulation of the receptor activity in flecainide (6 mg kg−1)-treated rats. However, the mechanism mediating the mechanosensitivity of SARs needs further elucidation. In in vivo preparations, the different flecainide concentration at the site of SAR endings is thought to be changed by several factors, for example, connective tissue barrier, dilution of tissue fluid and transport in the circulation. In this study, flecainide administration at a dose (9 mg kg−1) which blocked 50 μg kg−1 veratridine-induced SAR stimulation usually inhibited the basal discharge of SARs. Under these conditions, the magnitude of decreased SAR activity during hyperinflation became more prominent than that during normal inflation, indicating that hyperinflation itself in flecainide-treated animals may elicit the hyperpolarizing response which inhibits SAR activity.
Two broad classes of voltage-gated K+ channel currents have been distinguished on the basis of differences in time- and voltage-dependent properties: (1) a typical fast-inactivating A-type current (IA) and (2) a more sustained K+ current of the delayed-rectifier type (IK) (Halliwell, 1990; Storm, 1988). Two pharmacologically different types of K+ channels have also been identified in mammalian myelinated axons in the peripheral (Baker et al., 1987; Kocsis et al., 1987) and central (Gordon et al., 1989) nervous system: IA is blocked by 4-AP and IK is blocked by TEA. There is a functional difference between IA and IK in the myelinated axons of the rat sciatic nerve fibres because IA is responsible for action potential repolarization and I causes the afterhyperpolarization (AHP) after repetitive firing (Kocsis et al., 1987). Although the application of 4-AP is known to elicit the broad spike of action potentials (Kocsis et al., 1987; Poulter & Padjen, 1995), such an effect could not be confirmed in this study because we were measuring extracellular action potentials. The administration of 4-AP in the presence of flecainide increased the discharge of SARs, particularly in the deflation phase, in a dose-dependent manner, as reported in a previous study in the rabbit without flecainide treatment (Matsumoto et al., 1999), and caused a pressor effect (Chang et al., 1996; Matsumoto et al., 1999), which was short lasting. Because 4-AP can elicit both membrane depolarization and repetitive firing in squid axons (Yeh et al., 1976a,1976b), blockade of IA by 4-AP, even in the presence of Na+ channel blocker flecainide, could increase the expiratory discharge of SARs, in which there was no mechanical deformation due to lung inflation. We also found that 4-AP treatment resulted in a partial blockade of hyperinflation-induced SAR inhibition in flecainide-treated animals. This finding may be similar to a report demonstrating that administration of 4-AP antagonizes saxitoxin (STX)- and TTX-induced cardiorespiratory depression in anaesthetized guinea-pigs (Chang et al., 1996). Accordingly, a partial blockade of hyperinflation-induced SAR inhibition by 4-AP in the presence of flecainide is mediated by attenuation of the hyperpolarizing effect.
There are some difficulties in separating IA and IK, pharmacologically but the slow TEA-sensitive K+ conductance channel is prevalent in the nodal membrane of rat myelinated nerve fibres (Roper & Schwartz, 1989). Baker et al. (1987) also reported a similar localization of TEA-sensitive K+ channels. In a study using sucrose gap and intra-axonal recording of the isolated sciatic nerve fibres, TEA application had little effect on the wave form of compound action potentials in neither immature nor mature rats but blocked 4-AP-induced postspike positivity, and a pronounced AHP seen after repetitive stimulation was blocked by TEA (Eng et al., 1988). From these observations, it is possible to conclude that the TEA-sensitive IK does not activate the early phase of action potential repolarization but contributes to the later phase of AHP, back to the resting potential. Indeed, in this study the administration of TEA at any of the doses examined did not significantly alter the activity of SARs in flecainide-treated animals, but we found that TEA treatment caused a partial blockade of hyperinflation-induced SAR inhibition in those animals. The results indicate that blockade of TEA-sensitive K+ channels partially inhibits the hyperpolarizing response of SARs to hyperinflation in the presence of the Na+ channel blocker flecainide.
The existence of an endogenous Na+ – Ca2+ exchanger has been demonstrated in myelinated axons (Stys et al., 1991; 1992). The exchanger maintains intracellular Ca2+ concentration ([Ca2+]i) homeostasis by pumping Ca2+ out of cells under normal conditions. However, when the Na ion gradient is reversed, the exchanger increases the level of [Ca2+]i (Mullins et al., 1985; Dipolo & Beaugé, 1987). In an anoxic optic nerve model, Stys et al. (1990) suggested that Ca2+ enters the intracellular component via a route that is independent of voltage-gated Ca2+ channels. Furthermore, in vivo experiments on guinea-pigs, KB-R7943 can block the inward Na+ – Ca2+ exchange current providing protection of Ca2+ overload on ouabain-induced arrhythemias (Watano et al., 1999). If Ca2+ influx into the SAR endings occurred during hyperinflation in flecainide-treated animals, one could expect that pharmacological inhibition of the inward Na+ – Ca2+ exchange current should reduce Ca2+ entry responsible for the hyperpolarizing response stimulating Ca2+-activated K+ channel (Leblond & Krnjevic, 1989). In flecainide-treated rats, we found that administration of KB-R7943 (1 and 3 mg kg−1), which had no significant effect on SAR activity during normal inflation, produced a partial blockade of hyperinflation-induced SAR inhibition. This implies that during hyperinflation in flecainide-treated animals, the presumed rise in free [Ca2+]i in the SAR endings via the activation of a reverse-mode of the Na+ – Ca2+ exchanger may stimulate Ca2+-activated K+ channels and, as a result, cause the hyperpolarizing effect of SARs. On the other hand, there is a report that the solvent DMSO of KB – R7943 has the ability to inhibit Na+ – K+ ATPase activity (Chiou & Vesely, 1995). In this study we could not find any evidence that administration of DMSO alone at a final concentration (0.1%) alters the discharge of SARs.
In conclusion, we have shown that administration of flecainide at a dose sufficient to abolish veratridine-induced SAR stimulation resulted in strong inhibition of hyperinflation-induced SAR stimulation, and that the two K+ channel blockers (4-AP and TEA) and a reverse mode Na+ – Ca2+ exchange blocker (KB-R7943) partially attenuated hyperinflation-induced SAR inhibition. The results suggest that the effect of hyperinflation on SAR activity in the presence of flecainide at a higher dose would promote hyperpolarizing response of SARs via the activation of K+ conductance pathways.
Acknowledgments
The authors thank Eizai Pharmaceutical Co. Ltd. for the gift of flecainide as well as Kanebo for the gift of KB-R7943.
Abbreviations
- 4-AP
4-aminopyridine
- IA
a fast-inactivating A-type current
- IK
a sustained K+ current of delayed-rectifier type
- KB-R7943
2-[2-[4-(4-nitrobenzyloxyl) phenyl] ethyl] isothiourea methanesulphonate
- MBP
mean blood pressure
- PT
tracheal pressure
- SAR
slowly adapting pulmonary stretch receptor
- TEA
tetraethylammonium
- VT
tidal volume
References
- BANITT E.H., BRONN W.R., COYNE W.E., ACHMID J.R. Antiarrhythmic. 2. Synthesis and antiarrhythmic activity of N-(piperidylalkyl) trifluoroethyoxybenzamides. J. Med. Chem. 1977;20:821–826. doi: 10.1021/jm00216a016. [DOI] [PubMed] [Google Scholar]
- BAKER M., BOSTOC H., GRAFE P., MARTIUS P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J. Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGREN D.R., PETERSON D.F. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors. J. Physiol. 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., MCLACHLAN E.M., BELMONTE C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J. Physiol. 1998;512:211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL T.T., WILLIAMS E.M.V. Voltage- and time-dependent depression of maximum rate of depolarization of guinea-pig ventricular action potential by two new antiarrhythmic drugs, flecainide and lorcainide. Cardiovasc. Res. 1983;17:251–258. doi: 10.1093/cvr/17.5.251. [DOI] [PubMed] [Google Scholar]
- CASTILLO C., VILLEGAS R., RECIO-PINTO E. Alkaloid-modified sodium channels from lobster walking leg nerves in planar liquid bilayers. J. Gen. Physiol. 1992;99:897–930. doi: 10.1085/jgp.99.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG F.-C.T., BAUER R.M., BENTON B.J., KELLEN S.A., CAPACIO B.R. 4-aminopyridine antagonizes saxitoxin- and tetrodotoxin-induced cardiorespiratory depression. Toxicon. 1996;34:671–690. doi: 10.1016/0041-0101(95)00167-0. [DOI] [PubMed] [Google Scholar]
- CHIOU S., VESELY D.L. Dimethyl sulfoxide inhibits renal Na+/K+-ATPase at a site different from ouabain and atrial peptides. Life Sci. 1995;57:945–955. doi: 10.1016/0024-3205(95)02029-i. [DOI] [PubMed] [Google Scholar]
- CONNOR J.A., STEVENS C.F. Inward and delayed outward membrane currents in isolated neural somata under voltage-clamp. J. Physiol. 1971;213:1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBETT A.M., KREUGER B.K. Polypeptides neurotoxins modify gating and apparent single-channel conductance of veratridine-activated channels in planar liquid bilayers. J. Mem. Biol. 1989;110:199–207. doi: 10.1007/BF01869150. [DOI] [PubMed] [Google Scholar]
- DIPOLO R., BEAUGÉ L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Cai and ATP. Ca i-dependent Na i/Ca o and Na i/Na o exchange modes. J. Gen. Physiol. 1987;90:505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENG D.D., GORDON T.R., KOCSIS J.D., WAXMAN S.G. Development of 4-AP and TEA sensitivities in mammalian myelinated nerve fibers. J. Neurophysiol. 1988;60:2168–2179. doi: 10.1152/jn.1988.60.6.2168. [DOI] [PubMed] [Google Scholar]
- GATTERAL W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992;72:S15–S18. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- GORDON T.R., KOCSIS J.D., WAXMAN S.G. Pharmacological sensitivities of two afterhyperpolarizations in rat optic nerve. Brain Res. 1989;502:252–257. doi: 10.1016/0006-8993(89)90620-3. [DOI] [PubMed] [Google Scholar]
- HALLIWELL J.V.K+ Channels in the central nervous system Potassium Channels: structure, classification, function and therapeutic potential 1990Wiley: New York; 348–381.Cook, N.S. (ed) [Google Scholar]
- HODGKIN A.L., HUXLEY A.F. A quantitative description of membrane current and its application to conductance and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÖGER U., TORKKELI P.H., SEYFARTH E.-A., FRENCH A.A. Ionic selectivity of mechanically activated channels in spider neurons. J. Neurophysiol. 1997;78:2079–2085. doi: 10.1152/jn.1997.78.4.2079. [DOI] [PubMed] [Google Scholar]
- JUUSOLA M., SEYFARTH E.-A., FRENCH A.A. Sodium-dependent receptor current in a new mechanoreceptor preparation. J. Neurophysiol. 1994;72:3026–3028. doi: 10.1152/jn.1994.72.6.3026. [DOI] [PubMed] [Google Scholar]
- KOCSIS J.D., ENG D.L., GORDON T.R., WAXMAN S.G. Functional differences between 4-aminopyridine and tetraethylammonium-sensitive potassium channels in myelinated axons. Neurosci. Lett. 1987;75:193–198. doi: 10.1016/0304-3940(87)90296-5. [DOI] [PubMed] [Google Scholar]
- LEBLOND J., KRNJEVIC K. Hypoxic changes in hippocampal neurons. J. Neurophysiol. 1989;62:1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S., IKEDA M., NISHIKAWA T. Effects of sodium and potassium channel blockers on hyperinflation-induced slowly adapting pulmonary stretch receptor stimulation in the rat. Life Sci. 2000;67:2167–2175. doi: 10.1016/s0024-3205(00)00813-4. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S., SHIMIZU T. Flecainide blocks the stimulatory effect of veratridine on slowly adapting pulmonary stretch receptors in anaesthetized rabbits without changing lung mechanics. Acta Physiol. Scand. 1995;155:297–302. doi: 10.1111/j.1748-1716.1995.tb09977.x. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S., TAKAHASHI T., TANIMOTO T., SAIKI C., TAKEDA M. Effects of potassium channel blockers on CO2-induced slowly adapting pulmonary stretch receptor inhibition. J. Pharmacol. Exp. Ther. 1999;290:974–979. [PubMed] [Google Scholar]
- MATSUMOTO S., TAKAHASHI T., TANIMOTO T., SAIKI C., TAKEDA M., OJIMA K. Excitatory mechanism of veratridine on slowly adapting pulmonary stretch receptors in anesthetized rabbits. Life Sci. 1998;63:1431–1437. doi: 10.1016/s0024-3205(98)00410-x. [DOI] [PubMed] [Google Scholar]
- MEECH R.W., STANDEN N.B. Potassium activation in Helix aspersa neurons under voltage clamp: A component mediated by calcium influx. J. Physiol. 1975;249:211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L.J., REQUENA J., WHITTEMBURY J. Ca2+ entry in squid axons during voltage-clamp pulses in mainly Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 1985;82:1847–1851. doi: 10.1073/pnas.82.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T. Chemical as tools in the study of excitable membranes. Physiol. Rev. 1974;54:813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- POULTER M.O., PADJEN A.L. Different voltage-dependent potassium conductances regulate action potential repolarization and excitability in frog myelinated axon. Neuroscience. 1995;68:497–504. doi: 10.1016/0306-4522(95)00139-a. [DOI] [PubMed] [Google Scholar]
- ROPER J., SCHWARTZ J.R. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibers. J. Physiol. 1989;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE C.R., RANSOM B.R. Regulation of intracellular sodium in cultured rat hippocampal neurones. J. Physiol. 1997;499:573–587. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORM J.F. Temporal integration by a slow inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–382. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- STYS P.K., RANSOM B.R., WAXMAN S.G., DAVIS P.K. Role of extracellular calcium in anoxic injury of mammalian central white matter. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4212–4216. doi: 10.1073/pnas.87.11.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STYS P.K., WAXMAN S.G., RANSOM B.R. Na+-Ca2+ exchanger mediates Ca2+ influx during anoxia in mammalian central nervous system white matter. Ann. Neurol. 1991;30:375–380. doi: 10.1002/ana.410300309. [DOI] [PubMed] [Google Scholar]
- STYS P.K., WAXMAN S.G., RANSOM B.R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na+-Ca2+ exchanger. J. Neurosci. 1992;12:430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUBONE H. Characteristics of vagal afferent activity in rats: three types of pulmonary receptors responding to collapse, inflation, and deflation of the lung. Exp. Neurol. 1986;92:541–542. doi: 10.1016/0014-4886(86)90296-7. [DOI] [PubMed] [Google Scholar]
- WATANO T., HARADA Y., HARADA K., NISHIMURA N. Effect of Na+/Ca2+ exchange inhibitor, KB-R7943, on ouabain-induced arrhythemias in guinea-pigs. Br. J. Pharmacol. 1999;127:1846–1850. doi: 10.1038/sj.bjp.0702740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANO T., KIMURA J., MORITA T., NAKANISHI H. A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br. J. Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEH J.Z., OXFORD G.S., WU C.H., NARAHASHI T. Interactions of amino-pyridines with potassium channels of squid axon membranes. Biophys. J. 1976a;16:77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEH J.Z., OXFORD G.S., WU C.H., NARAHASHI T. Dynamics of amino-pyridine block of potassium channels in squid axon membranes. J. Gen. Physiol. 1976b;65:519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


