Abstract
Adenosine produced a biphasic lowering of the mean BP with a drastic bradycardic effect at the highest doses. The first phase hypotensive response was significantly reduced by the nitric oxide (NO) synthase inhibitor L-NAME.
The A2a/A2b agonist NECA produced hypotensive and bradycardic responses similar to those elicited by adenosine, which were not significantly modified by the A2b antagonist enprofylline.
The A2a agonist CGS 21680 did not significantly influence basal HR while induced a hypotensive response antagonized by the A2a selective antagonist ZM 241385, and reduced by both L-NAME and the guanylate cyclase inhibitor methylene blue.
The A1 agonist R-PIA showed a dose-dependent decrease in BP with a drastic decrease in HR at the highest doses. The A1 selective antagonist DPCPX significantly reduced the bradycardic activity and also the hypotensive responses obtained with the lowest doses while it increased those obtained with the highest ones.
The A1/A3 agonist APNEA, in the presence of the xanthinic non-selective antagonist 8-pSPT, maintained a significant hypotensive, but not bradycardic, activity, not abolished by the histamine antagonist diphenhydramine.
The selective A3 agonist IB-MECA revealed a weak hypotensive and bradycardic effect, but only at the highest doses.
In conclusion, in the systemic cardiovascular response to adenosine two major components may be relevant: an A2a- and NO-mediated hypotension, and a bradycardic effect with a consequent hypotension, via atypical A1 receptors. Finally, an 8-pSPT-resistant hypotensive response not attributable to A3 receptor-stimulation or to release of histamine by mastocytes or other immune cells was observed.
Keywords: Adenosine receptors, hypotension, bradycardia, NO, guinea-pig
Introduction
Four classes of membrane surface adenosine receptors are described, defined A1, A2a, A2b and A3, through which the nucleoside influences many functions in humans and other animals (Ralevic & Burnstock, 1998).
As far as the cardiovascular system is concerned, adenosine slows heart rate (HR) and atrioventricular conduction and antagonizes the cardio-stimulatory effects of catecholamines via cardiac A1 receptors in different mammals (Belardinelli et al., 1989; Olsson & Pearson, 1990). An increase in blood pressure (BP) and HR has been described in rats and in cats via central A1 receptors (St Lambert et al., 1994; Silva-Carvalho et al., 1993). In contrast, a dose-related decrease in BP and HR is observed after microinjection of adenosine into the caudal nucleus of tractus solitarii in the rat (Lo et al., 1998).
As regards a direct effect on blood vessels, A1 purinoceptors mediate vasodilation in the porcine coronary artery (Merkel et al., 1992) but they are involved, on the contrary, in vasoconstrictor responses, i.e. in the rat kidney (Jackson, 1991), in guinea-pig pulmonary artery and aorta (Biaggioni et al., 1989; Stoggall & Shaw, 1990) and in hamster skin (Stojanov & Proctor, 1990; Proctor & Stojanov, 1991).
Moreover, a significant role of A1 receptors in the regulation of BP also appears to be linked to a prejunctional regulation of transmitter release, such as from perivascular sympathetic nerves (Goncalves & Queiroz, 1996) or capsaicin-sensitive sensory neurons (Rubino et al., 1993).
A2 receptors (according to the old nomenclature used by Fredholm et al., 1994) are implicated in many vessels in the hypotensive activity of adenosine, due to the presence of specific membrane receptors in many vessels (Rongen et al., 1997). A nitric oxide-dependent vasorelaxant effect via A2-adenosine receptors on the vascular endothelium has been described in porcine coronary vasculature (Abebe et al., 1995), while an endothelium-independent A2-receptor-mediated vasodilation is reported in human and guinea-pig coronaries (Sabouni et al., 1990; Vials & Burnstock, 1993). As regards the particular A2 receptor subtype involved in the adenosine-mediated hypotension, there are different indications: A2a receptors are reported to mediate relaxation of rat aorta and bovine, rat and pig coronary artery (Hutchison et al., 1989; Conti et al., 1993) while A2b receptors mediate adenosine-induced relaxation in guinea-pig pulmonary artery (Szentmiklosi et al., 1995) and rat mesenteric arterial bed (Rubino et al., 1995).
A role for A3 receptors at the cardiovascular level has also been suggested by different authors: a hypotensive effect of adenosine analogues in the presence of both A1 and A2 receptor blockade has been reported in anaesthetized and pithed rats, which was antagonized by the A3 antagonist BW-A522 (Fozard & Hannon, 1994) and was linked to mediator release from mast cells (Hannon et al., 1995); in contrast, a vasoconstrictor response to adenosine via A3-mediated mast cell degranulation has been described in in vivo hamster (Shepherd et al., 1996). At the cardiac level, A3 receptor stimulants, administered prior to and during an ischaemic episode, have shown a protective action on heart cells in human and other animals (Strickler et al., 1996; Stambaugh et al., 1997; Tracey et al., 1997; Carr et al., 1997). Finally, a decrease in BP without any change in HR, mediated by A3 receptor stimulation was observed in the rat by Stella et al. (1998).
The overall evidence reported above seems to indicate that all the four subclasses of P1 purinoceptors may be involved in cardiovascular responses to adenosine with differences, depending on experimental animals and conditions employed.
As no data have been reported about the systemic effects of the nucleoside on BP and HR in in vivo guinea-pig, the aim of the present work was to study the influence of P1 receptor subtypes stimulation on the above parameters in anaesthetized guinea-pigs, evaluating also the participation of an NO-mediated component in the adenosinic responses.
Methods
Male Dunkin-Hartley guinea-pigs, weighing 300 – 400 g, were used in the present study; the experiments were carried out in accordance with the legislation of the Italian authorities (D.L. 27/01/1992 n° 116) concerning the care and use of laboratory animals, in conformity also with the CEE Directive 86/609.
Groups of 2 – 3 animals were housed in cages, with a grid on the bottom, and kept at a temperature of 20±2°C with a light – dark cycle of 12 h. A standard guinea-pig diet was given to the animals, and drinking water was supplied ad libitum.
All animals were anaesthetized with sodium pentobarbitone (50 – 70 mg kg−1 i.p.) and their trachea cannulated and connected to a rodent ventilator pump (mod. 7025 Basile, Varese, Italy). The animals were paralyzed with 2 mg kg−1 i.v. of pancuronium bromide, in order to block the spontaneous breathing and to obtain a standardized ventilation provided by the above ventilator pump operating at 50 strokes min−1, with a volume per stroke of 1 ml of room air per 100 g of animal body weight.
All drugs were injected i.v. as a bolus through a cannula connected to the right jugular vein at the cervical level. Blood pressure (BP) and heart rate (HR) monitoring was performed via the left carotid artery, which was cannulated with an heparinized catheter (20 IU ml−1 heparin in 0.9% NaCl solution) connected to a pressure transducer (mod. Keller 7016 Basile), in turn connected to a Bichannels microdynamometer (mod. Gemini, Basile). After surgery and immediately subsequent pancuronium injection, at least 15 min was allowed before treatment with other drugs or simply with the drug vehicle. An additional dose (25 mg kg−1 i.p.) of the anaesthetic was administered during this period in order to extend the deep anaesthesia.
After the stabilization period, adenosine antagonists, or other substances tested for their inhibiting ability vs adenosinic effects, or their vehicle, were injected i.v.; after a further 10 min, dose-response curves to the agonists were performed.
Only a single dose-response curve in each animal was carried out, the intervals between doses being sufficient to allow a plateau response to develop, and in any case not shorter than 5 min.
Drugs and solutions
The following drugs were used: adenosine hemisulphate, L-NAME (L-NG-Nitro-arginine methyl ester) hydrochloride, enprofylline (3-propylxanthine) and methylene blue, obtained from Sigma-Aldrich, Italy; R-PIA ((−)-N6-(R-phenylisopropyl)adenosine), NECA (5′-N-ethylcarboxamidoadenosine), 8-pSPT (8-p-sulphophenyltheophylline), APNEA (N6-(2-(4-aminophenyl)ethyl)-adenosine) and DPCPX (8-cyclopentyl-1,3 dipropylxanthine) from Sigma-RBI, Italy; CGS 21680 (2-[p-(2-carboxyethyl)-phenethylamino]-5′N-ethyl-carboxamidoadenosine), ZM241385 (4-[2-[7-amino-2-(2-furil)-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-oyl-amino]ethyl]phenole) and IB-MECA (N6-(3-iodobenzyl)adenosine-5′-N-methyluromide) from Tocris Cookson, U.K.; sodium pentobarbitone from Carlo Sessa, Italy; pancuronium bromide (Pavulon) from Organon Teknika, Italy. A concentrated calcium heparin solution (25000 IU ml−1) was obtained from Italfarmaco, Italy. L-NAME hydrochloride, methylene blue and sodium pentobarbitone were dissolved completely in saline (0.9% NaCl w v−1). All the adenosine receptor ligands stock solutions (10−2 M) were prepared in DMSO and then diluted to the required concentration with saline immediately before use, with the exception of 8-pSPT which was dissolved directly in saline at 37°C and enprofylline which was dissolved in 10% NaOH 1N in saline. All the drugs were administered at a volume of 1 ml kg−1.
Data evaluation and statistics
Blood pressure (BP) was recorded as mean (diastolic-systolic) arterial pressure and measured as mmHg decrease or increase from baseline. Heart rate (HR) was measured in beats min−1. In the dose-response curves, the responses to single doses of agonists were reported as percentages of the resting BP or HR, measured immediately before the first dose of the agonist.
Differences between groups were evaluated by the unpaired Student's t-test (for two groups) or by variance analysis (ANOVA) (for more than two groups). A P value ⩽0.05 was taken to be significant.
The ED50 values reported in Table 2 represent the mean doses at which a 50% decrease in the BP and HR baseline values was obtained; they were calculated by means of the computer-aided program Prism 3.0 (GraphPad Software, San Diego, CA, U.S.A.), after a non-linear regression analysis of the curves.
Table 2.
−Log ED50 on blood pressure and heart rate for adenosinic agonoists
Results
The basal mean blood pressure (BP) and heart rate (HR) were 52.2±1.4 mmHg and 367.6±8.3 beats min−1, respectively (n=60).
For all the drugs used, control experiments, carried out employing the specific solvent solution, did not reveal any significant influence on the baseline BP and HR.
Adenosine
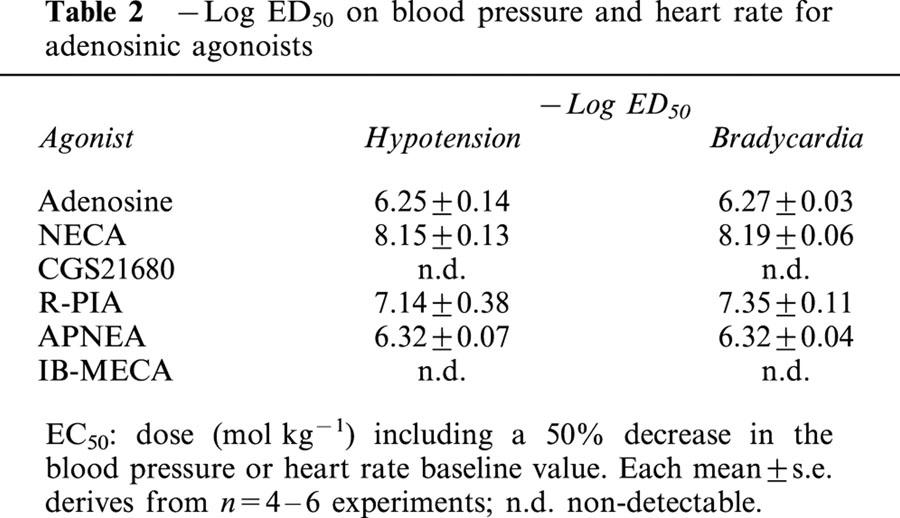
The curve obtained with exogenously administered adenosine (10−9 – 10−5 mol kg−1 i.v.) produced a biphasic lowering of the basal mean BP: the lowest doses (10−9 – 3×10−7 mol kg−1) induced a gradual decrease to about 70% of the resting value, while the highest doses had a powerful hypotensive effect, with a reduction to about 20% of the baseline (Figure 1A). Contemporaneously to the latter phase, a drastic bradycardic activity was observed (Figure 1B), with a total block of heart activity at the two highest doses (3×10−6, 10−5 mol kg−1).
Figure 1.
Cardiovascular responses to adenosine (10−9 – 10−5 mol kg−1 i.v.) in anaesthetized guinea-pig in the absence and in the presence of L-NAME (50 mg kg−1 i.v.). Each point in the curves is the mean·±s.e. of 4 – 6 experiments; *P⩽0.05. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 47.7±4.5 mmHg and 338.8±27.0 beats min−1 (control); 75.8±1.4 mmHg and 338.5±18.2 beats min−1 (L-NAME-treated).
After pretreatment of the animals with the nitric oxide synthase inhibitor L-NAME (50 mg kg−1 i.v.), a significant enhancement of the baseline BP (to 76.6±1.4 mmHg; n=4) and a reduction in the basal HR (to 320±5 beats min−1; n=4) were observed. Moreover, this pretreatment induced a modified influence of adenosine on the BP: a significant reduction by L-NAME of the BP decrease induced by the lowest doses of adenosine was observed (Figure 1A), while no different response to the nucleoside was present in the HR parameter. (Figure 1B).
Synthetic agonists
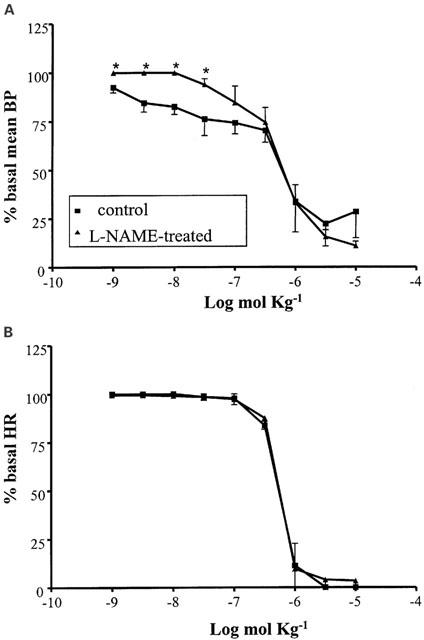
The A2a/A2b agonist NECA (10−10 – 10−7 mol kg−1 i.v.) had an influence on BP and HR similar to that observed with adenosine, although in a range of doses two orders of magnitude higher and with a less clear distinction between the two phases (Figure 2A, B). A significant decrease in the BP baseline (to 37.0±4.1 mmHg; n=4) and a poor and non-significant increase in the HR were shown after enprofylline treatment (10 mg kg−1 i.v.), while the A2b antagonist did not significantly modify the response to NECA either at the BP or the HR level.
Figure 2.
Cardiovascular responses to NECA (10−10 – 10−7 mol kg−1 i.v.) in anaesthetized guinea-pig in the absence and in the presence of enprofylline (10 mg kg−1 i.v.). Each point in the curves is the mean±s.e. of 4 – 6 experiments. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 56.3±1.2 mmHg and 360.0±12.8 beats min−1 (control); 38.8±1.4 mmHg and 390.5±16.2 beats min−1 (enprofylline-treated).
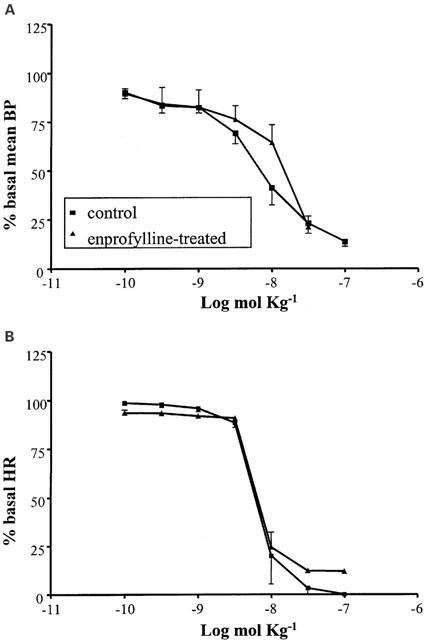
The A2a selective agonist CGS 21680 (10−11 – 10−7 mol kg−1 i.v.) produced an hypotension similar to that observed with the lowest doses of adenosine and NECA, with a maximal reduction to about 65% of the baseline (n=6) (Figure 3A). The A2a agonist did not produce, on the contrary, any significant effect on HR (Figure 3B).
Figure 3.
Cardiovascular responses to CGS21680 (10−11 – 10−7 mol kg−1 i.v.) in anaesthetized guinea-pig; in the absence of pretreatment and in the presence of L-NAME (50 mg kg−1 i.v.), methylene blue (10 mg kg−1 i.v) or ZM 241385 (1 mg kg−1 i.v). Each point in the curves is the mean±s.e. of 4 – 6 experiments; *P⩽0.05. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 48.3±8.5 mmHg and 371.7±15.0 beats min−1 (control); 65.6±4.4 mmHg and 368.5±14.2 beats min−1 (L-NAME-treated); 70.0±6.3 and 390±11.6 beats min−1 (methylene blue-treated); 65.6±4.2 and 375.5±11.6 beats min−1 (ZM 241385-treated).
The hypotensive response to CGS21680 was almost totally abolished by the A2a selective antagonist ZM241385 (1 mg kg−1; n=4), by the nitric oxide synthase inhibitor L-NAME (50 mg kg−1 i.v.; n=4) and by the guanylyl cyclase and NO-synthase inhibitor methylene blue (10 mg kg−1 i.v.; n=4). All the three drugs, L-NAME, methylene blue and ZM241385, enhanced the baseline BP (Table 1).
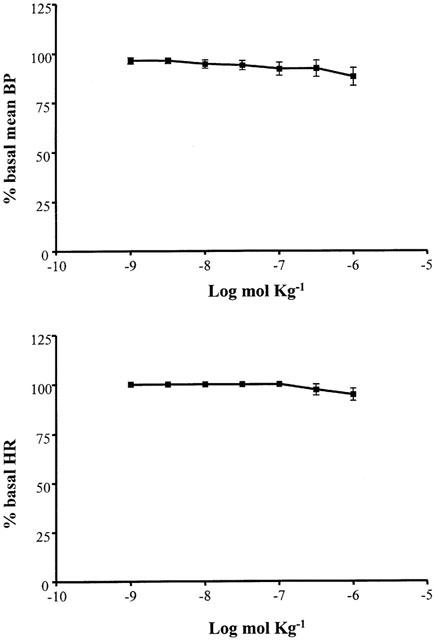
Table 1.
Effects on baseline blood pressure and heart rate of drugs used before dose-response curve to adenosine or adenosine agonists
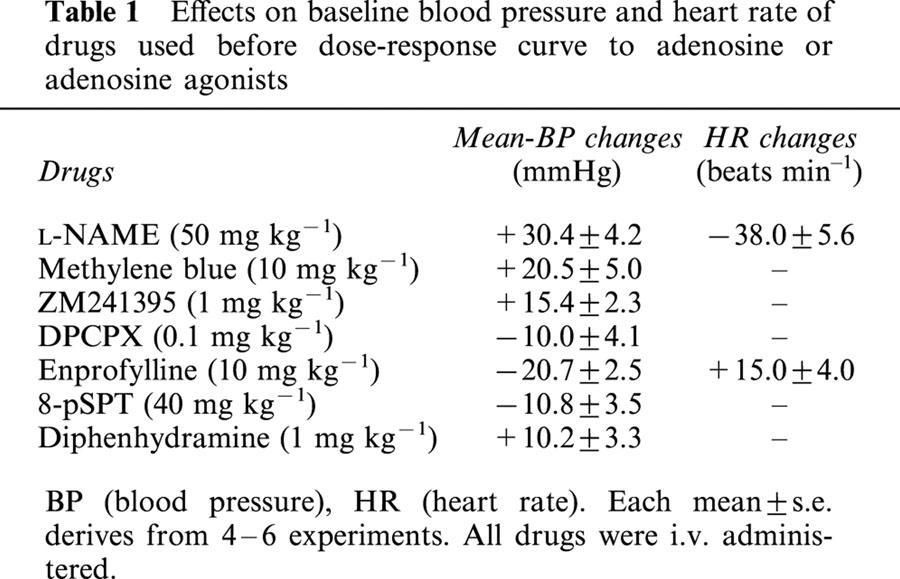
The A1 agonist R-PIA (10−10 – 3×10−7 mol kg−1 i.v.) induced an apparently monophasic response with a drastic decrease in BP contemporaneously to a drastic reduction in heart activity. Pretreatment with the A1 selective antagonist DPCPX (0.1 mol kg−1 i.v.; n=4) significantly modified the response to R-PIA, increasing the hypotensive effect of the lowest doses and decreasing that of the highest ones (Figure 4A). The R-PIA-mediated bradycardic effect was significantly reduced, too (Figure 4B).
Figure 4.
Cardiovascular responses to R-PIA (10−10 – 3×10−7 mol kg−1 i.v.) in anaesthetized guinea-pig in the absence and in the presence of DPCPX (0.1 mg kg−1 i.v.). Each point in the curves is the mean·±s.e. of 4 – 6 experiments; *: P⩽0.05. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 52.7±4.1 mmHg and 355.8±17.1 beats min−1 (control); 44.8±1.8 mmHg and 342.5±15.2 beats min−1 (DPCPX-treated).
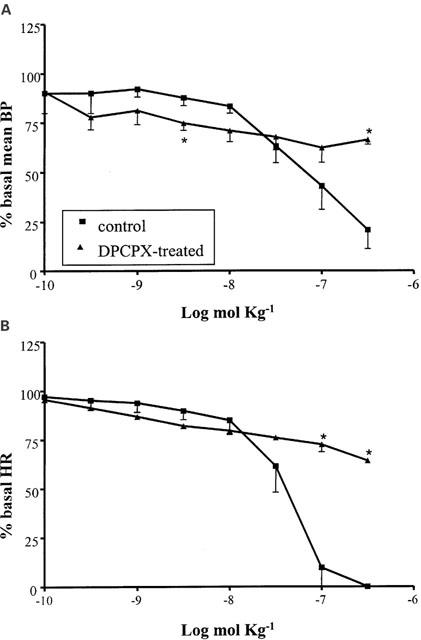
The A1/A3 agonist APNEA (10−9 – 10−6 mol kg−1 i.v.), induced a dose-dependent BP and HR decrease similar to that observed with R-PIA, but at higher doses. (Figure 5). The responses to this agonist were examined also in animals pretreated with a high dose (40 mol kg−1 i.v.) of the non selective adenosine antagonist 8-pSPT. This drug almost completely abolished the response to APNEA at the cardiac level, while at the vascular level, it had a lower antagonistic efficacy (Figure 5). The residual BP response to APNEA in the presence of 8-pSPT was, then, re-evaluated in the presence of the histamine antagonist diphenhydramine, in order to verify an analogy with the APNEA response reported in experiments in anaesthetized rats (Fozard & Hannon, 1994; Fozard et al., 1996).
Figure 5.
Cardiovascular responses to APNEA (10−9 – 10−6 mol kg−1 i.v.) in anaesthetized guinea-pig in the absence of pretreatment and in the presence of 8-pSPT (40 mg kg−1 i.v.) alone or in addition to diphenhydramine (1 mg kg−1 i.v). Each point in the curves is the mean·±s.e. of 4 – 6 experiments; *P⩽0.05. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 53.1±5.5 mmHg and 379.8±24.3 beats min−1 (control); 44.1±2.0 mmHg and 368.4±7.2 beats min−1 (8-pSPT-treated); 48.1±3.0 mmHg and 377.4±7.2 beats min−1 (8-pSPT+diphenhydramine-treated).
As shown in Figure 5A diphenhydramine had no effect on the 8-pSPT-resistant response to APNEA.
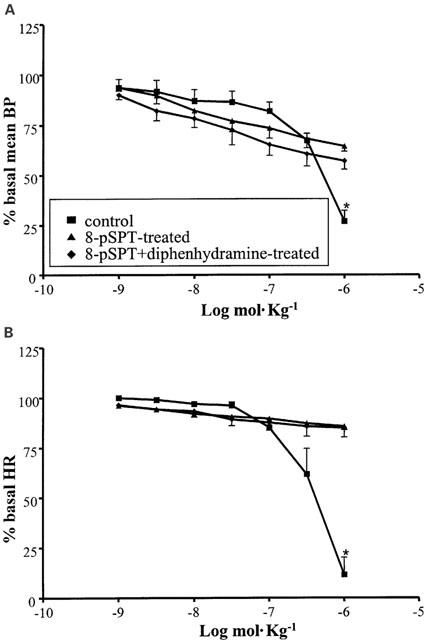
Finally, the selective A3 agonist IB-MECA (10−9 – 10−6 mol kg−1 i.v.) had very weak hypotensive and bradycardic effects and only at the highest doses employed (Figure 6).
Figure 6.
Cardiovascular responses to IB-MECA (10−9 – 10−6 mol kg−1 i.v.) in anaesthetized guinea-pig in the absence of pretreatment. Each point in the curves is the mean·±s.e. of 4 – 6 experiments; *P⩽0.05. Values are reported as per cent of resting mean blood pressure (BP) or heart rate (HR) just prior to starting the agonist dose-response curve: 47.7±4.5 mmHg and 338.8±27.0 beats min−1.
Discussion
The biphasic dose-response curve obtained for BP with adenosine suggested that more than one receptor subtype is implicated in the hypotensive response to the nucleoside; the involvement of at least two subtypes can be hypothesized: one mediating a slight hypotensive response which maximally reduced the basal BP value by about 30%, and another subtype mediating a powerful decrease of the mean artery pressure by about 80% of basal value. A clear distinction between the first and the second phase of the hypotensive response to adenosine was supported by the drastic bradycardic activity that was contemporaneous with the second phase. A hypotensive response closely linked to an action of adenosine at the cardiac level has been already reported in the rat (Webb et al. (1990)). A variety of evidence demonstrates the presence of adenosinic receptors responsible both for the negative chronotropic and the inotropic and dromotropic effects on cardiac function; they are mostly described as belonging to the A1 subtype in experiments in different mammals including humans (Belardinelli et al., 1989; Pelleg & Belardinelli, 1993; Olsson & Pearson, 1990; Linden, 1991); these receptors represent the basis for the therapeutic use of adenosine in the treatment of supraventricular tachycardia, and for the use of adenosine receptor antagonists in the treatment of bradyarrhythmias (Ralevic & Burnstock, 1998). Thus, the bradycardic effect of adenosine in our experiments is in agreement with literature where the occurrence of a cardiac block following high doses has already been reported (Belardinelli et al., 1989). In spite of the inhibitory activity on adenosine response by the selective A1 antagonist DPCPX, in our experiments, the order of potency of the synthetic adenosine agonists (NECA>R-PIA>APNEA=adenosine, Table 2) is not compatible with A1 receptor subtype stimulation, but rather with an A2 stimulation (Fredholm et al., 1994). However, the involvement of A2a or A2b receptors was not confirmed: the A2a agonist CGS 21680 did not show any bradycardic effect, and the A2b antagonist enprofylline did not significantly influence the bradycardic effect of NECA (Feoktistov & Biaggioni, 1997).
A protective effect following A3 receptor stimulation at the cardiac level has been described in different animal models (Strickler et al., 1996; Stambaugh et al., 1997; Tracey et al., 1997) but the lack of any significant effect of the A3 selective agonist IB-MECA (Gallo-Rodriguez et al., 1994) suggests that an A3-mediated influence of adenosine on cardiac function can be excluded.
It is difficult to justify our results invoking influences by pharmacokinetic variables, because the i.v. administration of the drugs excludes differences in the absorption phase; moreover, the heart is the drug target, and it is promptly reachable after i.v. administration by all the compounds; perhaps a blood-protein bond giving a different free drug component could be hypothesized, but as no data is now available in literature in this connection it should be speculative. On the other hand, another possible explanation, pharmacodynamic in nature, has been suggested by recent evidence obtained by Gardner & Broadley (1999): they described in the guinea-pig isolated atria atypical characteristics of adenosine receptors mediating negative inotropic and chronotropic responses of the tissue. Only a knowledge of the primary structure of these receptors or molecular biology studies will clarify their exact nature. Indeed, the cloning and characterization of a pharmacologically distinct A1 adenosine receptor from guinea-pig brain has already been reported (Meng et al., 1994): this ‘A1 receptor' displayed a high affinity for the antagonist DPCPX, but a very low affinity for some selective agonists, including R-PIA.
Martynyuk et al. (1996) and Shimoni et al. (1996) reported an effect by adenosine at the cardiac level mediated by the synthesis of NO; in our experiments, we can exclude the involvement of an NO-mediated action since the NO-synthase inhibitor L-NAME was ineffective on the adenosinic HR decrease. On the contrary, a role for nitric oxide has been clearly evident as regards the first phase response to the nucleoside at the vascular level, which was significantly reduced by L-NAME. This response reproduced by the A2a agonist CGS21680 (Collis & Hourani, 1993) was antagonized by the A2a antagonist ZM 241385 (Poucher et al., 1995) and blocked not only by L-NAME but also by methylene blue, a guanylate cyclase and NO-synthase inhibitor (Mayer et al., 1993). We can exclude that the effect of methylene blue, L-NAME and ZM 241385 is due to the hypertensive action of these drugs since similar hypertensive activity induced by the α1-adrenergic agonist methoxamine did not modify the dose-response curve to adenosine (data not shown).
The A2a-mediated hypotension was not accomplished by any activity at the cardiac level, confirming the results obtained in anaesthetized rats (Patel et al., 1994); in conscious rats, on the contrary, Monopoli et al. (1998) reported that the stimulation of A2a receptors produced, in addition to a systemic hypotension, a tachycardic action probably reflex in nature. Then, in our experimental model, the absence of a cardiac activity, in response to a direct hypotensive action, is probably to be reconduced to a cardiovascular reflex abolition by anaesthesia.
A very different profile in the response to the A2a selective agonist CGS 21680 was observed in our guinea-pig model with respect to the data reported for rats. In our experiments, CGS21680 gave a maximal fall in BP of about 30% of the baseline while in rat the same agonist elicited a reduction in basal BP similar to that observed with NECA and CPA (about 75% of baseline) (Patel et al., 1994). In spite of these differences, our data confirm an important role for A2a receptors in vascular tone regulation, and suggest that the systemic response mediated by these receptors is prevalently linked to the release of NO, probably from endothelial cells. The role of endothelial receptors in the response to adenosine was previously suggested by the evidence from Nees (1989), who observed that after i.v. administration, the nucleoside was largely entrapped inside endothelial cells. The participation of A2a receptors in the maintenance of physiological BP values has also demonstrated using transgenic mice: high blood pressure was, in fact, observed in A2a receptor gene knockout-mice (Ledent et al., 1997).
A possible role also for A3 receptors in the adenosinic response at the BP level was investigated in our work, since a residual hypotension to APNEA persisted in spite of treatment with a high dose of the xanthinic antagonist 8-pSPT (40 mg kg−1 i.v.). Nevertheless, the very weak cardiovascular activity of the A3 selective agonist IB-MECA, allow us to exclude an involvement of A3 receptor stimulation at peripheral and central levels; IB-MECA, in fact, is reported to elicit central effects after i.p. administration (Jacobson et al., 1993), thus revealing the ability to pass the blood brain barrier. In the presence of the histamine antagonist diphenhydramine, the 8-pSPT-resistant component of the response to APNEA was not modified. These data are not in agreement with those reported for experiments in anaesthetized rats, where the hypotensive xanthine-resistant response to APNEA was well correlated with histamine plasma increase and blocked by the A3 antagonist BW-A522, and by the mast cell stabilizer sodium chromoglycate (Fozard & Hannon, 1994; Patel et al., 1994; Hannon et al., 1995). It should be underlined that the role observed for A3 receptors on rat mast cells (Fozard et al., 1996) is not confirmed in other animals such as dogs and humans (Auchampach et al., 1997; Feoktistov & Biaggioni, 1997) and, as far as the guinea-pig is concerned, there is no report about a clear characterization of adenosinic receptor subtypes on mast cells.
The hydrophilicity of the xanthinic compound 8-pSPT powerfully limits its blood brain penetration: the dose used in our experiments (40 mg kg−1 i.v.) is reported not to give significant concentrations in rat brain (Evoniuk et al., 1987). The 8-pSPT-resistant response, in our experiments, could therefore be the result of an action by APNEA in the CNS (central nervous system) through receptors different from A3. Another possibility is the existence of a xanthine-resistant response not attributable to A3 receptor stimulation, as already suggested by evidence from in vitro experiments in isolated rat aorta (Prentice & Hourani, 1996).
Conclusion
In summary, present data though outlining the existence of remarkable differences in cardiovascular responses to adenosinic agonists between different animals, confirm a vasorelaxant property of A2a receptor agonists, via NO release, which may be considered for a new pharmacological approach to the treatment of the hypertensive conditions unlinked to a defect in NO production. Finally, the finding of a negative chronotropic effect via atypical A1 receptors and of an 8-pSPT-resistant response need further examination to explain the exact nature of the receptors involved.
Abbreviations
- APNEA
N6-(2-(4-aminophenyl)ethyl)-adenosine
- BP
blood pressure
- CGS 21680
2-[p-(2-carboxyethyl)-phenethylamino]-5′N-ethyl-carboxamidoadenosine
- DPCPX
8-cyclopentyl-1,3dipropylxanthine
- HR
heart rate
- IB-MECA
N6-(3-iodobenzyl)adenosine-5′-N-methyluromide
- L-NAME
L-NG-Nitro-arginine methyl ester
- NECA
5′-N-ethylcarboxamidoadenosine
- NO
nitric oxide
- 8-pSPT
8-p-sulphophenyltheophylline
- R-PIA
(−)-N6-(R-phenylisopropyl)adenosine
- ZM241385
4-[2-[7-amino-2-(2-furil)-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-oyl-amino]ethyl]phenol
References
- ABEBE W., HUSSAIN T., OLANREWAJU H., MUSTAFA S.J. Role of nitric oxide in adenosine receptor-mediated relaxation of porcine coronary artery. Am. J. Physiol. 1995;269:H1672–H1678. doi: 10.1152/ajpheart.1995.269.5.H1672. [DOI] [PubMed] [Google Scholar]
- AUCHAMPACH J.A., JIN X., WAN T.C., CAUGHEY G.H., LINDEN J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol. Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- BELARDINELLI L., LINDEN J., BERNE R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- BIAGGIONI I., KING L.S., ENAYAT N., ROBERTSON D., NEWMAN J.H. Adenosine produces pulmonary vasoconstriction in sheep. Evidence for thromboxane A2/prostaglandin endoperoxide-receptor activation. Circ. Res. 1989;65:1516–1525. doi: 10.1161/01.res.65.6.1516. [DOI] [PubMed] [Google Scholar]
- CARR C.S., HILL R.J., MASAMUNE H., KENNEDY S.P., KNIGHT D.R., TRACEY W.R., YELLON D.M. Evidence for a role for both the adenosine A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischaemia. Cardiovasc. Res. 1997;36:52–59. doi: 10.1016/s0008-6363(97)00160-0. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., HOURANI S.M. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- CONTI A., MONOPOLI A., GAMBA M., BOREA P.A., ONGINI E. Effects of selective A1 and A2 adenosine receptor agonists on cardiovascular tissues. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:108–112. doi: 10.1007/BF00168545. [DOI] [PubMed] [Google Scholar]
- EVONIUK G., VON BORSTEL R.W., WURTMAN R.J. Antagonism of the cardiovascular effects of adenosine by caffeine or 8-(p-sulfophenyl)-theophylline. J. Pharmacol. Exp. Ther. 1987;240:428–432. [PubMed] [Google Scholar]
- FEOKTISTOV I., BIAGGIONI I. Adenosine A2B receptors. Pharmacol. Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- FOZARD J.R., HANNON J.P. BW-A522 blocks adenosine A3 receptor-mediated hypotensive responses in the rat. Eur. J. Pharmacol. 1994;252:R5–R6. doi: 10.1016/0014-2999(94)90604-1. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R., PFANNKUCHE H.J., SCHUURMAN H.J. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GALLO-RODRIGUEZ C., JI X.D., MELMAN N., SIEGMAN B.D., SANDERS L.H., ORLINA J., FISCHER B., PU Q., OLAH M.E., VAN GALEN P.J. , et al. Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER N.M., BROADLEY K.J. Analysis of the atypical characteristics of adenosine receptors mediating negative inotropic and chronotropic responses of guinea-pig isolated atria and papillary muscles. Br. J. Pharmacol. 1999;127:1619–1626. doi: 10.1038/sj.bjp.0702719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONCALVES J., QUEIROZ G. Purinoceptor modulation of noradrenaline release in rat tail artery: tonic modulation mediated by inhibitory P2Y- and facilitatory A2A-purinoceptors. Br. J. Pharmacol. 1996;117:156–160. doi: 10.1111/j.1476-5381.1996.tb15168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNON J.P., PFANNKUCHE H.J., FOZARD J.R. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br. J. Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHISON A.J., WEBB R.L., OEI H.H., GHAI G.R., ZIMMERMAN M.B., WILLIAMS M. CGS 21680, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J. Pharmacol. Exp. Ther. 1989;251:47–55. [PubMed] [Google Scholar]
- JACKSON E.K. Adenosine: a physiological brake on renin release. Annu. Rev. Pharmacol. Toxicol. 1991;31:1–35. doi: 10.1146/annurev.pa.31.040191.000245. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., NIKODIJEVIC O., SHI D., GALLO-RODRIGUEZ C., OLAH M.E., STILES G.L., DALY J.W. A role for central A3-adenosine receptors. Mediation of behavioral depressant effects. FEBS Lett. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.M., SCHIFFMANN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- LINDEN J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- LO W.C., JAN C.R., WU S.N., TSENG C.J. Cardiovascular effects of nitric oxide and adenosine in the nucleus tractus solitarii of rats. Hypertension. 1998;32:1034–1038. doi: 10.1161/01.hyp.32.6.1034. [DOI] [PubMed] [Google Scholar]
- MARTYNYUK A.E., KANE K.A., COBBE S.M., RANKIN A.C. Nitric oxide mediates the anti-adrenergic effect of adenosine on calcium current in isolated rabbit atrioventricular nodal cells. Pflugers Arch. 1996;431:452–457. doi: 10.1007/BF02207285. [DOI] [PubMed] [Google Scholar]
- MAYER B., BRUNNER F., SCHMIDT K. Novel actions of methylene blue. Eur. Heart J. 1993;14 Suppl I:22–26. [PubMed] [Google Scholar]
- MENG F., XIE G.X., CHALMERS D., MORGAN C., WATSON S.J., JR, AKIL H. Cloning and characterization of a pharmacologically distinct A1 adenosine receptor from guinea pig brain. Mol. Brain Res. 1994;26:143–155. doi: 10.1016/0169-328x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- MERKEL L.A., LAPPE R.W., RIVERA L.M., COX B.F., PERRONE M.H. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J. Pharmacol. Exp. Ther. 1992;260:437–443. [PubMed] [Google Scholar]
- MONOPOLI A., CASATI C., LOZZA G., FORLANI A., ONGINI E. Cardiovascular pharmacology of the A2A adenosine receptor antagonist, SCH 58261, in the rat. J. Pharmacol. Exp. Ther. 1998;285:9–15. [PubMed] [Google Scholar]
- NEES S. Coronary flow increases induced by adenosine and adenine nucleotides are mediated by the coronary endothelium: a new principle of the regulation of coronary flow. Eur. Heart J. 1989;10 Suppl F:28–35. doi: 10.1093/eurheartj/10.suppl_f.28. [DOI] [PubMed] [Google Scholar]
- OLSSON R.A., PEARSON J.D. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- PATEL M., SHEEHAN M.J., STRONG P. Failure of CGS15943A to block the hypotensive action of agonists acting at the adenosine A3 receptor. Br. J. Pharmacol. 1994;113:741–748. doi: 10.1111/j.1476-5381.1994.tb17056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLEG A., BELARDINELLI L. Cardiac electrophysiology and pharmacology of adenosine: basic and clinical aspects. Cardiovasc. Res. 1993;27:54–61. doi: 10.1093/cvr/27.1.54. [DOI] [PubMed] [Google Scholar]
- POUCHER S.M., KEDDIE J.R., SINGH P., STOGGALL S.M., CAULKETT P.W., JONES G., COLL M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRENTICE D.J., HOURANI S.M. Activation of multiple sites by adenosine analogues in the rat isolated aorta. Br. J. Pharmacol. 1996;118:1509–1517. doi: 10.1111/j.1476-5381.1996.tb15567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCTOR K.G., STOJANOV I. Direct vasoconstriction evoked by A1-adenosine receptor stimulation in the cutaneous microcirculation. Circ. Res. 1991;68:683–688. doi: 10.1161/01.res.68.3.683. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RONGEN G.A., FLORAS J.S., LENDERS J.W., THIEN T., SMITS P. Cardiovascular pharmacology of purines. Clin. Sci. (Colch). 1997;92:13–24. doi: 10.1042/cs0920013. [DOI] [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. The P1-purinoceptors that mediate the prejunctional inhibitory effect of adenosine on capsaicin-sensitive nonadrenergic noncholinergic neurotransmission in the rat mesenteric arterial bed are of the A1 subtype. J. Pharmacol. Exp. Ther. 1993;267:1100–1104. [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABOUNI M.H., RAMAGOPAL M.V., MUSTAFA S.J. Relaxation by adenosine and its analogs of potassium-contracted human coronary arteries. Naunyn Schmiedebergs Arch. Pharmacol. 1990;341:388–390. doi: 10.1007/BF00180667. [DOI] [PubMed] [Google Scholar]
- SHEPHERD R.K., LINDEN J., DULING B.R. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ. Res. 1996;78:627–634. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- SHIMONI Y., HAN X., SEVERSON D., GILES W.R. Mediation by nitric oxide of the indirect effects of adenosine on calcium current in rabbit heart pacemaker cells. Br. J. Pharmacol. 1996;119:1463–1469. doi: 10.1111/j.1476-5381.1996.tb16059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA-CARVALHO L., DAWID-MILNER M.S., GOLDSMITH G.E., SPYER K.M. Hypothalamic-evoked effects in cat nucleus tractus solitarius facilitating chemoreceptor reflexes. Exp. Physiol. 1993;78:425–428. doi: 10.1113/expphysiol.1993.sp003696. [DOI] [PubMed] [Google Scholar]
- ST LAMBERT J.H., DAWID-MILNER M.S., SILVA-CARVALHO L., SPYER K.M. Action of adenosine receptor antagonists on the cardiovascular response to defence area stimulation in the rat. Br. J. Pharmacol. 1994;113:159–164. doi: 10.1111/j.1476-5381.1994.tb16188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMBAUGH K., JACOBSON K.A., JIANG J.L., LIANG B.T. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am. J. Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STELLA L., DE NOVELLIS V., MARABESE I., BERRINO L., MAIONE S., FILIPPELLI A., ROSSI F. The role of A3 adenosine receptors in central regulation of arterial blood pressure. Br. J. Pharmacol. 1998;125:437–440. doi: 10.1038/sj.bjp.0702126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOGGALL S.M., SHAW J.S. The coexistence of adenosine A1 and A2 receptors in guinea-pig aorta. Eur. J. Pharmacol. 1990;190:329–335. doi: 10.1016/0014-2999(90)94197-6. [DOI] [PubMed] [Google Scholar]
- STOJANOV I., PROCTOR K.G. Temperature-sensitive adenosine-mediated vasoconstriction in the skin microcirculation. J. Pharmacol. Exp. Ther. 1990;253:1083–1089. [PubMed] [Google Scholar]
- STRICKLER J., JACOBSON K.A., LIANG B.T. Direct preconditioning of cultured chick ventricular myocytes. Novel functions of cardiac adenosine A2a and A3 receptors. J. Clin. Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZENTMIKLOSI A.J., UJFALUSI A., CSEPPENTO A., NOSZTRAY K., KOVACS P., SZABO J.Z. Adenosine receptors mediate both contractile and relaxant effects of adenosine in main pulmonary artery of guinea pigs. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:417–425. doi: 10.1007/BF00169083. [DOI] [PubMed] [Google Scholar]
- TRACEY W.R., MAGEE W., MASAMUNE H., KENNEDY S.P., KNIGHT D.R., BUCHHOLZ R.A., HILL R.J. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc. Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- VIALS A., BURNSTOCK G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br. J. Pharmacol. 1993;109:424–429. doi: 10.1111/j.1476-5381.1993.tb13586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB R.L., MCNEAL R.B., JR, BARCLAY B.W., YASAY G.D. Hemodynamic effects of adenosine agonists in the conscious spontaneously hypertensive rat. J. Pharmacol. Exp. Ther. 1990;254:1090–1099. [PubMed] [Google Scholar]


