Abstract
This study was designed to assess the influence of activation and blockade of the endogenous opioid system in the brain on two key proteins involved in the regulation of programmed cell death: the pro-apoptotic Fas receptor and the anti-apoptotic Bcl-2 oncoprotein.
The acute treatment of rats with the μ-opioid receptor agonist morphine (3 – 30 mg kg−1, i.p., 2 h) did not modify the immunodensity of Fas or Bcl-2 proteins in the cerebral cortex. Similarly, the acute treatment with low and high doses of the antagonist naloxone (1 and 100 mg kg−1, i.p., 2 h) did not alter Fas or Bcl-2 protein expression in brain cortex. These results discounted a tonic regulation through opioid receptors on Fas and Bcl-2 proteins in rat brain.
Chronic morphine (10 – 100 mg kg−1, 5 days, and 10 mg kg−1, 13 days) induced marked increases (47 – 123%) in the immunodensity of Fas receptor in the cerebral cortex. In contrast, chronic morphine (5 and 13 days) decreased the immunodensity of Bcl-2 protein (15 – 30%) in brain cortex. Chronic naloxone (10 mg kg−1, 13 days) did not alter the immunodensities of Fas and Bcl-2 proteins in the cerebral cortex.
The concurrent chronic treatment (13 days) of naloxone (10 mg kg−1) and morphine (10 mg kg−1) completely prevented the morphine-induced increase in Fas receptor and decrease in Bcl-2 protein immunoreactivities in the cerebral cortex.
The results indicate that morphine, through the sustained activation of opioid receptors, can promote abnormal programmed cell death by enhancing the expression of pro-apoptotic Fas receptor protein and damping the expression of anti-apoptotic Bcl-2 oncoprotein.
Keywords: Morphine, naloxone, opioid receptors, opioid addiction, apoptosis, Fas receptor, Bcl-2 oncoprotein, rat brain
Introduction
Apoptosis, or programmed cell death, is an active process of normal cell death during development and also occurs as a consequence of the cytotoxic effect of various neurotoxins (e.g., MPTP/MPP+, MDMA, ethanol and cocaine) (Sastry & Rao, 2000). Among the drugs of abuse, cocaine has been shown to cause a direct cytotoxic effect on the foetal rat heart, and to induce apoptosis in foetal rat myocardial cells in a dose-dependent manner (Xiao et al., 2000). The induction of apoptosis in neurones has been demonstrated to share the same basic mechanisms with all other cell types (Sastry & Rao, 2000; Yuan & Yankner, 2000). Recent in vitro studies also indicate that exposure to μ- and/or κ-opioid receptor agonists of neuronal cultures from embryonic chick brain (Goswami et al., 1998) and specific cell lines (Dawson et al., 1997; Yin et al., 1997; Singhal et al., 1998; 1999) increases their vulnerability to death by apoptotic mechanisms.
The molecular mechanisms of apoptosis (i.e., the detailed cascade of events from the cell surface to final changes in the nucleus) have not been established yet, but various key proteins are involved in the regulation of programmed cell death (Kinloch et al., 1999; Sastry & Rao, 2000). Thus, some members of the Bcl-2 family of proteins, such as Bcl-2 and Bcl-xL, supresses apoptosis, while the expression of other, such as the homologues Bax and Bak, are pro-apoptotic (Adams & Cory, 1998). Specifically, the Bcl-2 oncoprotein, localized mainly to the mitochondrial membranes, has been shown to play a important role in protecting neurones from apoptotic cell death (Hockenbery et al., 1990), probably by preventing the release of cytochrome c (induced by Bax) and the subsequent activation of specific proteases termed caspases, the proteolytic enzymes which are crucial for the execution of nuclear fragmentation and apoptosis (see Adams & Cory, 1998; Sastry & Rao, 2000; Yuan & Yankner, 2000). In fact, Bax mRNA and Bax protein are increased in the substantia nigra of MPTP-treated mice (degeneration of dopamine neurones by apoptosis) (Hassouna et al., 1996), and the release of cytochrome c from the mitochondria and the subsequent activation of caspases-3/9 was shown to play a key role in cocaine-induced apoptosis in foetal rat myocardial cells (Xiao et al., 2000). Another key element involved in the regulation of apoptosis is the Fas glycoprotein (also known as CD95 or Apo1), a cell surface receptor that belongs to the tumour necrosis factor receptor family (death receptors) and that is expressed abundantly in various tissues (Nagata & Golstein, 1995; Nagata, 1999; Orlinick et al., 1999). In contrast to Bcl-2 mitochondrial protein, the Fas receptor triggers cell apoptosis when it binds to its ligand FasL, and Fas-mediated death bypasses the usual long sequence of signalling enzymes and immediately activates a pre-existing caspase cascade (Nagata, 1999; Krammer, 2000). In the context of the induction of aberrant apoptosis in opioid addiction, it was of great interest the recent in vitro study demonstrating the ability of morphine to increase, through a naloxone-sensitive mechanism, the expression (mRNA) of the pro-apoptotic receptor Fas in mouse splenocytes and in human blood lymphocytes (Yin et al., 1999). A relevant consequence of the morphine-induced potentiation of apoptosis in lymphocytes (Singhal et al., 1999; Yin et al., 1999) is the reduction of the immune response (and the increase in recurrent infections) observed in heroin addicts (Govitrapong et al., 1998).
Against this background, and because various chronic effects of morphine on the structure of neurones have been interpreted to indicate that opiate drugs might induce neuronal damage after long-term exposure (see Nestler, 1996; Ferrer-Alcón et al., 2000), the present study was designed to assess the in vivo influence of the activation and blockade of the endogenous opioid system (i.e. the acute and chronic effects of the agonist morphine and the antagonist naloxone) on the immunodensities of the pro-apoptotic Fas receptor and the anti-apoptotic Bcl-2 oncoprotein in the rat brain.
Methods
Animals and treatments
Male Sprague-Dawley rats (250 – 300 g) were used. The animals received a standard diet with water freely available and were housed under controlled environmental conditions (20±2°C, 70% humidity and 12 h light/dark cycle). For the acute treatments, the rats received a single intraperitoneal (i.p.) injection of morphine (3 and 30 mg kg−1) or naloxone (1 and 100 mg kg−1). For the chronic treatment with morphine, the rats were injected i.p. three times (at 08:00, 14:00 and 20:00 h) during 5 consecutive days with increasing doses of the opiate as follows: day 1: 10, 10 and 10 mg kg−1; day 2: 10, 20 and 20 mg kg−1; day 3: 20, 20 and 40 mg kg−1; day 4: 40, 40 and 80 mg kg−1; day 5: 80 and 100 mg kg−1. In another series of chronic experiments, the animals were injected i.p. every 12 h with morphine (10 mg kg−1) during 13 days. In rats, the 5-day treatment with morphine resulted in a high degree of tolerance and dependence (Ulibarri et al., 1987; Escribá et al., 1994) and the 13-day treatment in a marked degree of tolerance (Boronat et al., 1998). For the chronic treatment with naloxone (10 mg kg−1), the animals received the opiate antagonist i.p. every 12 h during 13 days. In a parallel experiment, a group of rats received i.p. every 12 h and during 13 days an injection of naloxone (10 mg kg−1) followed by a morphine injection (10 mg kg−1) 30 min later. In all series of experiments, control rats received 0.9% saline vehicle i.p. at the indicated treatment times. The animals were killed by decapitation 2 h after the last dose in the acute and in the 5-day chronic morphine treatments, and 24 h after the last injection in the 13-day chronic treatments (morphine and naloxone). The brains were rapidly removed and the parieto-occipital cortex dissected on ice and stored at −70°C until assay. These experiments in rats were performed according to the guidelines of the University of Balearic Islands.
Immunoblotting of Fas, Bcl-2 and 68 kDa neurofilament (NF-L) proteins
For the immunodetection of the target proteins 250 – 350 mg of cerebral cortex was homogenized (30 s) with an Ultraturrax homogenizer in 5 volumes of 10 mM Tris HCl buffer, pH 7.4, containing 150 mM NaCl, 0.03% Nonidet P-40 (NP-40), and the following protease inhibitors: 1 mM phenylmethylsulphonylfluoride (PMSF), 5 mM iodoacetamide, 10 μg ml−1 of trypsin-chymotrypsin inhibitor and 1 μg ml−1 of each leupeptin and aprotinin. The samples were then sonicated (10 s) and centrifuged at 4°C and 14,900×g for 15 min. The supernatant was recovered and the protein content determined by the method of Lowry et al. (1951) with bovine serum albumin as the standard. An aliquot (400 μl) of the supernatant was mixed with 50 μl of 160 mM Tris HCl, 8% SDS, pH 6.8, and 50 μl of electrophoresis loading buffer (500 mM Tris HCl, 8% SDS, 30% glicerol, 20% 2-mercaptoethanol, 0.02% bromophenol blue, pH 6.8) and boiled. The samples were then submitted to SDS – PAGE in a 12% Laemmli gel (1.5 mm thickness). Proteins were transferred to 0.45 micron (for Fas immunoblotting) or 0.2 micron (for Bcl-2 immunoblotting) nitrocellulose membranes and blocked at room temperature for 1 h with phosphate buffered saline solution (PBS in mM: NaCl 137, KCl 2.7, Na2HPO4 12, KH2PO4 1.38, pH 7.4) containing 5% nonfat dry milk, 0.5% bovine serum albumin and 0.2% Tween 20 (blocking solution). Then, the membranes were incubated overnight at 4°C in blocking solution containing the primary antibody: anti-Fas (M-20; batch D 219) dilution of 1 : 2000 (Santa Cruz Biotechnology, CA, U.S.A.) and anti-Bcl-2 (ΔC 21; batch C 309) dilution of 1 : 2000 (Santa Cruz Biotechnology). The secondary antibody, horseradish peroxidase-linked donkey anti-rabbit immunoglobulin G (IgG), was incubated at 1 : 5000 dilution in blocking solution at room temperature for 2 h. Immunoreactivity of Fas protein was detected with the Enhanced Chemiluminiscence (ECL) Western Blot Detection system (Amersham International, U.K.) followed by exposure to Hyperfilm ECL film (Amersham). For Bcl-2 detection the more sensitive ECL-Plus System was used (Amersham).
Fas and Bcl-2 antibodies are rabbit affinity-purified polyclonal antisera raised against a peptide of Fas carboxy terminus (mouse origin) or against a recombinant protein corresponding to amino acids 1 – 205 of Bcl-2 (human origin). In rat brain, these antisera labelled proteins with relative molecular masses of ≈48/49 kDa (Fas) and ≈25/26 kDa (Bcl-2) (see Figure 1), which were in good agreement with previous findings in rat tissues (Prehn et al., 1994; Taylor et al., 1999). In order to test the selectivity of anti-Fas antibody with specific proteins, the antigenic peptide was used in excess to block the binding of the antibody to the specific protein species tested. Thus, previous preincubation of the antibody with the antigenic peptide (preabsorbed antibody) resulted in the blockade of the immunoreaction for the specific protein (Fas, 48/49 kDa) and other unknown related peptides (≈35 – 40 kDa) (Figure 1A). In the case of Bcl-2, because of the lack of the antigenic peptide availability, omission of the primary antibody was used as a negative control; i.e. the immunoreactivity was absent under this condition (Figure 1B).
Figure 1.
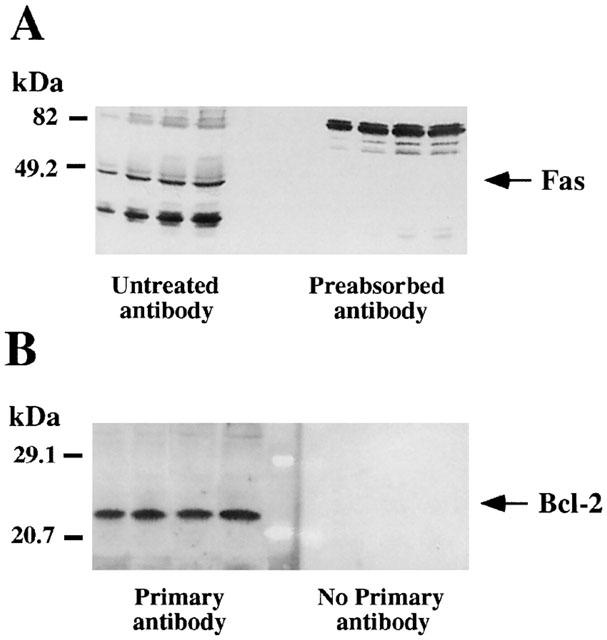
Representative autoradiographs of Western blots depicting labelling of immunodetectable pro-apoptotic Fas receptor (A) and anti-apoptotic Bcl-2 oncoprotein (B) in rat brain membranes. Samples from the cerebral cortex were subjected to SDS – PAGE, transferred to nitrocellulose membranes (immunoblotting), incubated with the specific primary and secondary antibodies, and visualized by the Enhanced Chemiluminiscence (ECL or ECL-Plus) method. The apparent molecular masses of Fas (48/49 kDa) and Bcl-2 (25/26 kDa) proteins were determined by calibrating the blots with prestained molecular weight markers as shown on the left hand side. For Fas the amounts of total protein loaded per gel well were: 31.4; 62.7; 94.1; 125.4 (μg) and the corresponding IOD: 0.37; 0.75; 1.08; 1.21 (arbitrary units) (standard curve; μg protein vs IOD, r=0.98). For Bcl-2 the amounts of total protein loaded per gel well were: 31.4; 62.7; 94.1; 125.4 (μg) and the corresponding IOD: 0.56; 1.29; 2.48; 2.90 (arbitrary units) (standard curve; μg protein vs IOD, r=0.99). (A) The specificity of the antibody anti-Fas was assessed by preincubating the antibody with the antigenic peptide (preabsorbed antibody), which resulted in the blockade of the immunoreaction for the specific protein (48/49 kDa) and other unknown related peptides (≈35 – 40 kDa). (B) For Bcl-2 immunodetection, omission of the primary antibody was used as a negative control and the immunoreactivity was absent under this condition.
Immunodetection of 68 kDa neurofilament (NF-L) protein, a specific neuronal cytoskeletal protein used as a positive control for the cellular effects of morphine (Boronat et al., 1998; Jaquet et al., 2001) was performed by stripping and reprobing the same nitrocellulose membranes that had been used for the immunodetection of Fas and Bcl-2 proteins. Briefly, the membranes were washed three times for 10 min with PBS and then incubated in stripping buffer (2-mercaptoethanol 100 mM, SDS 2%, Tris HCl 62.5 mM, pH 6.8) at 50°C for 30 min with occasional agitation. After two quick rinses with PBS and a further three 10 min washings, the membranes were blocked at room temperature for 1 h with blocking solution and the immunodetection of NF-L protein was performed similarly as described above using a specific monoclonal anti-68 kDa NF-L (Amersham) diluted at 1 : 500 as the primary antibody, followed by a horseradish peroxidase-linked sheep anti-mouse IgG secondary antibody (1 : 2000 dilution). The immunoreactivity was detected with the ECL system as described above.
Quantitation of specific immunoreactivity for Fas, Bcl-2 and 68 kDa neurofilament (NF-L) proteins
Specific protein immunoreactivity was quantitated by scanning densitometry in the image analyser Bio Image (Millipore, Ann Arbor, MI, U.S.A.). After scanning, standard curves were constructed using samples from saline-treated rats. In these curves, the total protein loaded in at least four wells (25 – 125 μg) was plotted against the integrated optical density (IOD). For Fas, Bcl-2 and NF-L linear relationships (correlation coefficients: r=0.98 – 0.99) between the amount of protein loaded in the gel and the IOD were found all over the range of protein content used (Figure 1). For the quantitation of the immunoreactivity of the target proteins, samples from saline-treated and drug-treated rats were loaded in the same gel as well as the standard curve and, for every sample, a theoretical amount of protein loaded in the gel (Pt) was obtained by intrapolation of its IOD in the standard curve. The percentage of target protein immunoreacivity of a given sample respect to the standard (saline-treated) samples was calculated as (Pt/Pr)×100; where Pr is the real amount of protein loaded in the gel well. This quantitation procedure was repeated at least five times for each rat brain sample in different gels and the mean value calculated. The mean intra- and inter-assay coefficients of variation were 7 – 10% and 18 – 20%, respectively, for the different target proteins.
Statistics
Results are expressed as mean±s.e.mean values. One-way ANOVA, followed by Scheffé's multiple comparison test, was used for the statistical evaluations. The level of significance was chosen as P=0.05.
Materials and drugs
Morphine HCl was from Unión Químico-Farmacéutica S.A.E. (Madrid, Spain) and naloxone HCl was from Endo Laboratories (Garden City, NY, U.S.A.). Polyclonal rabbit antisera against Fas and Bcl-2 proteins were purchased from Santa Cruz Biotechnology (U.S.A.). Anti-68 kDa NF-L mouse monoclonal antibody, horseradish peroxidase-linked donkey anti-rabbit or sheep anti-mouse IgG antibodies, Enhanced Chemiluminescence (ECL) reagents and Hyperfilm ECL film were suplied by Amersham International (U.K.). Other reagents were obtained from Sigma Chemical Co. (U.S.A.).
Results
Acute effects of morphine and naloxone on Fas, Bcl-2 and NF-L protein immunoreactivity in rat brain
The acute treatment with the μ-opioid receptor agonist morphine (3 and 30 mg kg−1, i.p. for 2 h), compared with saline solution administration, did not modify significantly the immunodensity of Fas or Bcl-2 proteins in the cerebral cortex (Figure 2A,B). Similarly, the acute treatment with low and high doses of naloxone (1 and 100 mg kg−1, i.p. for 2 h), a non-selective opioid receptor antagonist, did not alter significantly the immunoreactive levels of Fas and Bcl-2 in the cerebral cortex (Figure 2C,D). These results indicated the absence of a tonic regulation induced by endogenous opioid peptides (i.e., endomorphines), through opioid receptors, on Fas and Bcl-2 proteins in rat brain. Furthermore, the acute treatments with morphine or naloxone did not modify significantly the immunoreactivity of NF-L proteins in cerebral cortex (Figure 2) (see Jaquet et al., 2001).
Figure 2.

(A) Representative immunoblots using antisera against Fas, Bcl-2 and NF-L proteins in the cerebral cortex of saline-treated rats (two animals, S1 and S2) and morphine-treated rats (3 and 30 mg kg−1, 2 h) (two animals for each acute treatment, M1 and M2). The amount of total protein loaded per gel well ranged from 87 to 89 μg for the different treatments. Note that the immunodensities of Fas, Bcl-2 and NF-L did not change significantly in morphine-treated rats (IOD, percentage of saline-treated rats, range 95 – 106%). (B) Columns are means±s.e.mean of 6 – 8 experiments per group performed in duplicate with an animal per experiment, and expressed as percentage of saline-treated rats. One-way ANOVA did not detect significant differences for Fas (F=0.35, P=0.71), Bcl-2 (F=0.96, P=0.39) and NF-L (F=1.09, P=0.34) immunodensities after the acute treatments with morphine. (C) Representative immunoblots using antisera against Fas, Bcl-2 and NF-L proteins in the cerebral cortex of saline-treated rats (two animals, S1 and S2) and naloxone-treated rats (1 and 100 mg kg−1, 2 h) (two animals for each acute treatment, N1 and N2). The amount of total protein loaded per well ranged from 88 to 91 μg for the different treatments. Note that the immunodensities of Fas, Bcl-2 and NF-L did not change significantly in naloxone-treated rats (IOD, percentage of saline-treated rats, range 98 – 102%). (D) Columns are means±s.e.mean of 6 – 8 experiments per group performed in duplicate with an animal per experiment, and expressed as percentage of saline-treated rats. One-way ANOVA did not detect significant differences for Fas (F=1.04, P=0.36), Bcl-2 (F=1.58, P=0.21) and NF-L (F=3.06, P=0.05) immunodensities after the acute treatments with naloxone.
Chronic effects of morphine and naloxone on Fas, Bcl-2 and NF-L protein immunoreactivity in rat brain
Chronic treatment (5 days) with morphine (increasing doses from 10 to 100 mg kg−1), compared with saline solution administration, induced a marked increase in the immunodensity of Fas receptor protein in the cerebral cortex (47±15%, P<0.05) (Figure 3A,B). A prolonged chronic treatment (13 days) with morphine (10 mg kg−1, every 12 h) resulted in a much greater up-regulation in Fas immunoreactivity (123±15%, P<0.001) in the cortex (Figure 3A,B). In contrast, chronic morphine treatment (13 days) markedly decreased the immunodensity of Bcl-2 protein (30±2%, P<0.001) (Figure 3A,B). Treatment for 5 days with morphine only showed a tendency to decrease Bcl-2 immunoreactivitiy in the cerebral cortex (15±3%, P>0.05) (Figure 3A,B). On the other hand, chronic treatment with naloxone (10 mg kg−1, i.p. for 13 days), compared with saline solution administration, did not modify significantly the immunodensities of Fas, Bcl-2 and NF-L proteins in the cerebral cortex (Figure 3C,D), suggesting further the absence of a tonic regulation of endogenous opioids on these proteins which are involved in the regulation of apoptosis.
Figure 3.
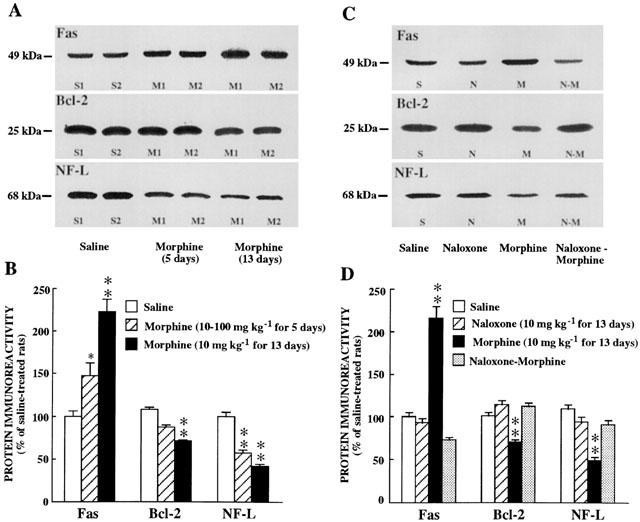
(A). Representative immunoblots using antisera against Fas, Bcl-2 and NF-L proteins in the cerebral cortex of saline-treated rats (two animals, S1 and S2) and chronic morphine-treated rats (10 to 100 mg kg−1 for 5 days or 10 mg kg−1 every 12 h for 13 days) (two animals for each chronic treatment, M1 and M2). The amount of total protein loaded per gel well ranged from 92 to 96 μg for the different treatments. Note that the immunodensity of Fas increased (IOD, percentage of saline-treated rats: 155 – 238%) and that of Bcl-2 (96 – 80%) or NF-L (58 – 45%) decreased in chronic morphine-treated rats. (B) Columns are means±s.e.mean of 6 – 8 experiments per group performed in duplicate with an animal per experiment, and expressed as percentage of saline-treated rats. One-way ANOVA detected significant differences between groups with respect to protein immunodensities after the chronic treatments with morphine: Fas (F=27.56, P<0.0001), Bcl-2 (F=51.52, P<0.0001) and NF-L (F=54.81, P<0.0001). * P<0.05; ** P<0.001 when compared with the saline group (ANOVA followed by Scheffé's test). (C) Representative immunoblots using antisera against Fas, Bcl-2 and NF-L proteins in the cerebral cortex of saline-treated (S), naloxone-treated (N, 10 mg kg−1 for 13 days), morphine-treated (M, 10 mg kg−1 for 13 days) and naloxone plus morphine-treated (N – M) rats. The amount of total protein loaded per gel well ranged from 90 to 91 μg for the different treatments. Note that naloxone completely prevented the effects of morphine (group N – M) on Fas, Bcl-2 and NF-L immunoreactivity. (D) Columns are means±s.e.mean of 6 – 8 experiments per group performed in duplicate with an animal per experiment, and expressed as percentage of saline-treated rats. One-way ANOVA detected significant differences between groups: Fas (F=53.77, P<0.0001), Bcl-2 (F=36.66, P<0.0001) and NF-L (F=25.82, P<0.0001). ** P<0.001 when compared with the saline group (ANOVA followed by Scheffé's test).
To prove further the efficacy of these chronic morphine treatments, the immunodensity of NF-L, a specific neuronal cytoskeletal protein and a neurochemical marker of opioid adiction (Boronat et al., 1998; Jaquet et al., 2001), was also quantitated. Both chronic morphine treatments (5 and 13 days), compared with saline solution administration, induced marked decreases in NF-L immunoreactivity in the cerebral cortex (42±3%, and 59±3%, respectively, P<0.001) (Figure 3A,B).
Prevention by naloxone of morphine-induced up-regulation of Fas and down-regulation of Bcl-2 and NF-L protein immunoreactivity in rat brain
The concurrent chronic treatment (13 days) of naloxone (10 mg kg−1, i.p.) and morphine (10 mg kg−1, i.p.), completely prevented the morphine-induced increase in Fas receptor protein immunoreactivity in the cerebral cortex, which even resulted in Fas levels below, but not significantly different, the saline control values (Figure 3C,D). Similarly, the concurrent administration of naloxone and morphine also antagonized completely the morphine-induced decreases in Bcl-2 and NF-L protein immunoreactivities in the cerebral cortex (Figure 3C,D).
Discussion
The main finding of this study is the demonstration that chronic treatment of rats with morphine (tolerant and dependent states) is associated with a remarkable opposite modulation in brain of two key proteins involved in the regulation of the programmed cell death: i.e., a strong up-regulation of the pro-apoptotic Fas receptor and a moderate down-regulation of the anti-apoptotic Bcl-2 oncoprotein. Moreover, these chronic effects of morphine in the rat brain, which also decreased the abundance of the specifc marker of opioid addiction NF-L proteins, were prevented by the concurrent administration of naloxone, an opioid receptor antagonist. At present and because of the time-schedules for the various morphine treatments it cannot completely be discarded an effect of repeated short periods of spontaneous opiate withdrawal on the observed changes on Fas and/or Bcl-2 proteins in brain. However, these in vivo findings most probably indicate that the stimulatory and inhibitory effects of chronic morphine on brain pro-apoptotic Fas receptor and anti-apoptotic Bcl-2 oncoprotein, respectively, and on NF-L proteins, are mediated through the sustained activation of opioid receptors and specifically of the μ-type.
Previous in vitro studies have demonstrated the ability of morphine and DAMGO, a specific μ-opioid receptor agonist, to induce apoptosis in T lymphocytes and/or Jurkat cells, through mechanisms associated with a decrease in the expression of anti-apoptotic protein Bcl-2 and an enhancement in that of pro-apoptotic protein Bax (Singhal et al., 1999). Moreover, morphine has also been shown to increase the expression of pro-apoptotic Fas receptor mRNA in lymphocytes, through the activation of opioid receptors, as well as that in the spleen, lung and heart of mice (Yin et al., 1999). On the other hand, Fas mRNA expression also was shown to be markedly enhanced in splenic lymphocytes of stressed mice (with increased levels of endogenous opioids), an effect that was antagonized by naltrexone or naloxone, which suggested that Fas-mediated lymphocyte apoptosis is dependent on endogenous opioids (Yin et al., 2000). This finding could also imply that in stressed mice there is a tonic regulation of Fas expression mediated by endogenous opioids acting on opioid receptors. In the current study, however, the acute treatment of rats with moderate and high doses of morphine or naloxone did not alter the abundance of immunoreactive Fas receptor or Bcl-2 oncoprotein in brain. Moreover, sustained blockade of opioid receptors with naloxone (13 days) did not result in significant changes in Fas or Bcl-2 protein expression. These negative results indicated the absence of a tonic regulation induced by endogenous opioid peptides (i.e., endomorphines), through opioid receptors, on Fas and Bcl-2 proteins in rat brain. However, it is also possible that the lack of effects after the acute treatments with morphine could be due to the partial agonist character and/or low efficacy of this prototypical opiate drug (Yu et al., 1997; Kovoor et al., 1998).
During the last few years, various effects of opiate drugs on the structure (cytoskeleton) of neurones have been interpreted to indicate that morphine and other opiates might induce neuronal damage after long-term exposure (see García-Sevilla et al., 1997). Recently, cell-matrix interactions has been suggested to play an important role in apoptosis, specifically in the regulation of anchorage-dependent apoptosis. In fact, cytoskeletal elements (e.g. intermediate filaments and microfilaments) are substrates for both Fas ligand and caspases, and disruption of the cytoskeleton can induce cytotoxicity and apoptotic cell death (Kothakota et al., 1997; Pike et al., 1998; Abbracchio et al., 1999 and other references therein). In this context, chronic treatment with morphine in rats has been shown to result in marked reductions in the immunodensity of NF proteins, the major intermediate filaments of the neuronal cytoskeleton, in brain regions relevant to opioid addiction (Beitner-Johnson et al., 1992; Boronat et al., 1998; Jaquet et al., 2001; present results). Similarly, the immunodensities of nonphosphorylated NF proteins also were shown to be decreased in postmortem brains of chronic heroin abusers (García-Sevilla et al., 1997; Ferrer-Alcón et al., 2000). Moreover, aberrant hyperphosphorylation of NF-H and NF-M was demonstrated in brains of opioid addicts (Ferrer-Alcón et al., 2000) and in brains of morphine-dependent rats (Jaquet et al., 2001). In opioid-dependent patients, an enlargement of pericortical space and both lateral ventricles was revealed by use of cranial computerized tomography, which indicates volume loss (frontal cortex) of brain (Pezawas et al., 1998). Chronic morphine in rats was also shown to reduce the size and calibre of dendrites and soma of mesolimbic dopamine neurones (Sklair-Tavron et al., 1996), and to decrease the number of dendritic spines on neurones in various brain regions (Robinson & Kolb, 1999), which could be related to the ability of opiate drugs to alter NF proteins (see Ferrer-Alcón et al., 2000). In the neocortex of mice, chronic morphine was shown to reduce the number of calbindin D-28 kDa-positive neurones, a neuroprotective calcium-binding protein (Maharajan et al., 1998), an effect that also might be related to neuronal damage induced by opiate drugs. Recently, chronic administration of morphine or chronic self-administration of heroin has been shown to decrease neurogenesis in the adult rat hippocampus, without altering the normal number of apoptotic cells in this brain region (Eisch et al., 2000). All of this evidence combine to suggest that these structural, morphological and functional changes may reflect some form of neural injury induced by chronic opioid exposure in rats and humans (see Nestler, 1996; Ferrer-Alcón et al., 2000), and that this neuronal damage might be related, in part, to the ability of opiate drugs to markedly alter cytoskeletal NF proteins (Ferrer-Alcón et al., 2000). In turn, the disruption of the cytoskeleton in target neurones could induce cytotoxicity and apoptotic cell death (see above).
The current results together with previous findings (Singhal et al., 1999; Yin et al., 1999) clearly indicate that morphine and other opiate drugs, through the activation of opioid receptors, can promote in vitro and in vivo abnormal programmed cell death by enhancing the expression of pro-apoptotic Fas receptor (major effect) and damping the expression of anti-apoptotic Bcl-2 oncoprotein (minor effect). Therefore, the induction of aberrant apoptosis in specific types of neurones may be a major consequence of the neuronal damage induced by opiate drugs after long-term exposure (see Nestler, 1996; Ferrer-Alcón et al., 2000).
Acknowledgments
This study was supported by grant BFI2000-0306 (Fondo Nacional para el Desarrollo de la Investigación Científica y Técnica) from MCT (Madrid, Spain), and also in part by grant 32-57066.99 from FNSRS (Bern, Switzerland). M.A. Boronat was supported by a predoctoral fellowship from the University of the Balearic Islands (UIB) and M.J. García-Fuster by a predoctoral research contract from the UIB (Palma de Mallorca, Spain). J. A. García-Sevilla is a member of the Institut d'Estudis Catalans (Barcelona, Spain).
Abbreviations
- ANOVA
analysis of variance
- DAMGO
D-ALa2, Mephe4, Gly-OH5-enkephalin
- IOD
integrated optical density
- MDMA
3,4-methylenedioxymethamphetamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- NF
neurofilament
- SDS – PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
References
- ABBRACCHIO M.P., ONGINI E., MEMO M. Disclosing apoptosis in the CNS. Trends Pharmacol. Sci. 1999;20:129–131. doi: 10.1016/s0165-6147(99)01345-0. [DOI] [PubMed] [Google Scholar]
- ADAMS J.M., CORY S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- BEITNER-JOHNSON D., GUITART X., NESTLER E.J. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J. Neurosci. 1992;12:2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORONAT M.A., OLMOS G., GARCÍA-SEVILLA J.A. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br. J. Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON G., DAWSON S.A., GOSWAMI R. Chronic exposure to κ-opioids enhances the susceptibility of immortalized neurons (F-11 k7) to apoptosis-inducing drugs by a mechanism that may involve ceramide. J. Neurochem. 1997;68:2363–2370. doi: 10.1046/j.1471-4159.1997.68062363.x. [DOI] [PubMed] [Google Scholar]
- EISCH A.J., BARROT M., SCHAD C.A., SELF D.W., NESTLER E.J. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Nat. Acad. Sci. U.S.A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCRIBÁ P.V., SASTRE M., GARCÍA-SEVILLA J.A. Increased density of guanine nucleotide-binding proteins in the postmortem brain of heroin addicts. Arch. Gen Psychiatry. 1994;51:494–501. doi: 10.1001/archpsyc.1994.03950060058006. [DOI] [PubMed] [Google Scholar]
- FERRER-ALCÓN M., GARCÍA-SEVILLA J.A., JAQUET P.E., LA HARPE R., RIEDERER B.M., WALZER C., GUIMON J. Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J. Neurosci. Res. 2000;61:338–349. doi: 10.1002/1097-4547(20000801)61:3<338::AID-JNR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- GARCÍA-SEVILLA J.A., VENTAYOL P., BUSQUETS X., LA HARPE R., WALZER C., GUIMON J. Marked decrease of immunolabelelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. NeuroReport. 1997;8:1561–1570. doi: 10.1097/00001756-199705060-00003. [DOI] [PubMed] [Google Scholar]
- GOSWAMI R., DAWSON S.A., DAWSON G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis in both embryonic and immortalized (F-11 k7) neurons. J. Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- GOVITRAPONG P., SUTTITUM T., KOTCHABHAKDI N., UNEKLABH T. Alterations of immune functions in heroin adddicts and heroin withdrawal subjects. J. Pharmacol. Exp. Ther. 1998;286:883–889. [PubMed] [Google Scholar]
- HASSOUNA I., WICKERT H., ZIMMERMANN M., GILLARDON F. Increase in Bax expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci. Lett. 1996;204:85–88. doi: 10.1016/0304-3940(96)12323-5. [DOI] [PubMed] [Google Scholar]
- HOCKENBERY D., NUÑEZ G., MILLIMAN C., SCHREIBER R.D., KORSMEYER S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- JAQUET P.J., FERRER-ALCÓN M., VENTAYOL P., GUIMON J., GARCÍA-SEVILLA J.A. Acute and chronic effects of morphine and naloxone on the phosphorylation of neurofilament-H proteins in the rat brain. Neurosci. Lett. 2001;304:37–40. doi: 10.1016/s0304-3940(01)01729-3. [DOI] [PubMed] [Google Scholar]
- KINLOCH R.A., TREHERNE J.M., FURNESS L.M., HAJIMOHAMADREZA I. The pharmacology of apoptosis. Trends Pharmacol. Sci. 1999;20:35–42. doi: 10.1016/s0165-6147(98)01277-2. [DOI] [PubMed] [Google Scholar]
- KOVOOR A., CELVER J.P., WU A., CHAVKIN C. Agonist induced homologous desensitization of μ-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol. Pharmacol. 1998;54:704–711. [PubMed] [Google Scholar]
- KOTHAKOTA S., AZUMA T., REINHARD C., KLIPPEL A., TANG J., CHU K., MCGARRY T.J., KIRSCHNER M.W., KOTHS K., KWIATKOWSKI D.J., WILLIAMS L.T. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- KRAMMER P.H. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAHARAJAN P., PRENCIPE R., FALCHETTI R., DI FRANCESCO P., PAINO G., MAHARAJAN V. Chronic morphine alters calbindin D-28k immunostaining patterns in mouse forebrain. Neurosci. Lett. 1998;243:65–68. doi: 10.1016/s0304-3940(98)00065-2. [DOI] [PubMed] [Google Scholar]
- NAGATA S. Fas ligand-induced apoptosis. Annu. Rev. Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- NAGATA S., GOLSTEIN P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J. Under siege: the brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- ORLINICK J.R., VAISHNAW A.K., ELKON K.B. Structure and function of Fas/Fas ligand. Int. Rev. Immunol. 1999;18:293–308. doi: 10.3109/08830189909088485. [DOI] [PubMed] [Google Scholar]
- PEZAWAS L.M., FISCHER G., DIAMANT K., SCHEIDER C., SCHINDLER S.D., THURNHER M., PLOECHL W., EDER H., KASPER S. Cerebral CT findings in male opioid-dependent patients: stereological, planimetric and linear measurements. Psychiatry Res. (Neuroimaging Sect.) 1998;83:139–147. doi: 10.1016/s0925-4927(98)00028-6. [DOI] [PubMed] [Google Scholar]
- PIKE B.R., ZHAO X., NEWCOMB J.K., POSMANTUR R.M., WANG K.K., HAYES R.L. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. NeuroReport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- PREHN J.H.M., BINDOKAS V.P., MARCUCCILLI C.J., KRAJEWSKI S., REED J.C., MILLER R.J. Regulation of neuronal Bcl-2 protein expression and calcium homeostasis by transforming growth factor type β confers wide-ranging protection on rat hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON T.E., KOLB B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- SASTRY P.S., RAO K.S. Apoptosis and the nervous system. J. Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- SINGHAL P.C., SHARMA P., KAPASI A.A., REDDY K., FRANKI N., GIBBONS N. Morphine enhances macrophage apoptosis. J. Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- SINGHAL P.C., KAPASI A.A., REDDY K., FRANKI N., GIBBONS N., DING G. Morphine promotes apoptosis in Jurkat cells. J. Leukoc. Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- SKLAIR-TAVRON L., SHI W.-X., LANE S.B., HARRIS H.W., BUNNEY B.S., NESTLER E.J. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M.F., DE BOER-BROUWER M., WOOLVERIDGE I., TEERDS K.J., MORRIS I.D. Leyding cell apoptosis after the administration of ethane dimethanesulfonate to the adult male rat is a fas-mediated process. Endocrinology. 1999;140:3797–3804. doi: 10.1210/endo.140.8.6919. [DOI] [PubMed] [Google Scholar]
- ULIBARRI I., GARCÍA-SEVILLA J.A., UGEDO L. Modulation of brain α2-adrenoceptors and μ-opioid receptor densities during morphine dependence and spontaneous withdrawal in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:530–537. doi: 10.1007/BF00169310. [DOI] [PubMed] [Google Scholar]
- XIAO Y., HE J.H., GILBERT R.D., ZHANG L. Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. J. Pharmacol. Exp. Ther. 2000;292:8–14. [PubMed] [Google Scholar]
- YIN D.L., REN X.H., ZHENG Z.L., PU L., JIANG L.Z., MA L., PEI G. Etorphine inhibits cell growth and induces apoptosis in SK-N-SH cells: involvement of pertussis toxin-sensitive G proteins. Neurosci. Res. 1997;29:121–127. doi: 10.1016/s0168-0102(97)00080-1. [DOI] [PubMed] [Google Scholar]
- YIN D., MUFSON R.A., WANG R., SHI Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218. doi: 10.1038/16612. [DOI] [PubMed] [Google Scholar]
- YIN D., TUTHILL D., MUFSON R.A., SHI Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J. Exp. Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU Y., ZHANG L., YIN X., SUN H., UHL G.R., WANG J.B. μ Opioid receptor phosphorylation, desensitization, and ligand efficacy. J. Biol. Chem. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- YUAN J., YANKNER B.A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]


