Abstract
The regulation of glycine concentrations within excitatory synapses is poorly understood and it has been proposed that the GLYT1 subtypes of glycine transporters play a critical role in determining resting concentrations of glycine. Selective GLYT1 inhibitors may provide pharmacological tools to probe the dynamics of synaptic glycine concentrations, which may influence the activation properties of NMDA receptor activity.
We have characterized the selectivity and mechanism of action of the glycine transport inhibitor N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS). The glycine transporters, GLYT1a, b and c and GLYT2a were expressed in Xenopus laevis oocytes and two electrode voltage clamp techniques and radiolabelled 3H-glycine flux measurements were used to characterize the effects of NFPS on glycine transport.
NFPS inhibits glycine transport by the GLYT1a, b and c subtypes of glycine transporters, but has no effect on the GLYT2a subtype of transporter. We show that NFPS does not attain its specificity via an interaction with the Na+, Cl− or glycine site, nor does it act at an intracellular site. NFPS inhibition of glycine transport is time and concentration dependent and inhibition of transport by NFPS persists after washout of NFPS from the bath solution, which suggests that inhibition by NFPS is long lasting.
Keywords: GLYT1, GLYT2, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine, NFPS, long lasting inhibition, schizophrenia, glycine uptake, drug/lipid bilayer interactions
Introduction
The amino acid glycine is a co-agonist with glutamate at the NMDA subtype of glutamate receptor (McBain & Mayer, 1994). The GLYT1 subtype of glycine transporters are co-localized with NMDA receptors and are thought to regulate glycine concentrations at excitatory synapses. Glycine transporters are members of the Na+/Cl− dependent family of neurotransmitter transporters (Amara & Kuhar, 1993) and six glycine transporter subtypes derived from two distinct genes have been identified. There are four isoforms of GLYT1 and two isoforms of GLYT2 (Guastella et al., 1992; Hanley et al., 2000; Kim et al., 1994; Liu et al., 1992; 1993; Ponce et al., 1998; Smith et al., 1992). The GLYT1 subtypes have a stoichiometry of ion flux coupling of 2Na+:1Cl−:1 glycine (Aragon et al., 1987), from which the equilibrium glycine concentration can be calculated. Under standard ionic conditions, the equilibrium extracellular glycine concentration has been predicted to be 150 nM (Attwell et al., 1993). EC50 values for glycine binding to the glycine site of the NMDA receptor have been reported to range from 0.2 – 1.7 μM for the various NMDA receptor subtypes (Hollmann & Heinemann, 1994; McBain & Mayer, 1994). If resting glycine concentrations are as low as 150 nM, then changes in transporter activity would lead to fluctuations in extracellular glycine, and given the variation in EC50 values for glycine activation of NMDA receptors, subtype selective modulation of NMDA receptors would be expected. In a recent study employing a novel glycine transport blocker, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) (Figure 1) (Bergeron et al., 1998) inhibition of glycine uptake enhanced the amplitude of the NMDA component of glutamatergic EPSCs. This suggests that block of glycine transport leads to an elevation in extracellular glycine concentrations and enhancement of NMDA receptor activity, which implies that glycine transporters maintain glycine levels at subsaturating levels in the local area of NMDA receptor containing synapses.
Figure 1.
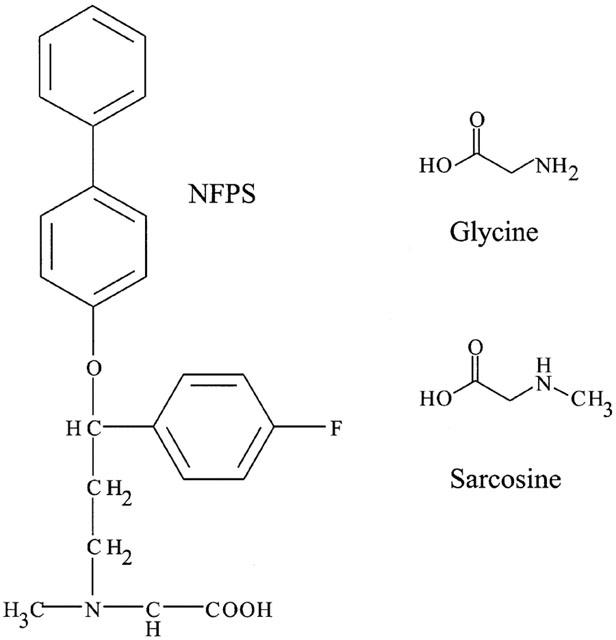
The structure of NFPS. Sarcosine is the N-methylated derivative of glycine and is a competitive substrate inhibitor of GLYT1 but not GLYT2 transporters. NFPS consists of a large lipophilic tail with a sarcosine head group.
In this study we have investigated the mechanism of action of NFPS on the GLYT1 subtypes of glycine transporters and also its degree of selectivity of action compared to the GLYT2a subtype of glycine transporter. NFPS consists of the N-methyl derivative of glycine, sarcosine, with a large bulky hydrophobic group attached. Sarcosine is a substrate of the GLYT1 subtypes of glycine transporters, but not the GLYT2 transporters (Supplisson & Bergman, 1997). It may be predicted that this difference in substrate selectivity between transporter subtypes is likely to provide the molecular basis for selectivity of NFPS for the GLYT1 subtypes over the GLYT2 subtypes of glycine transporters. NFPS also has a large hydrophobic group attached to the sarcosine moiety. For other neurotransmitter transport inhibitors, the size of the compound is a very important factor in determining whether or not the compound is a competitive substrate or non-transported inhibitor (Shimamoto et al., 1998). In this study we have investigated a number of aspects of the mechanism of action of NFPS on glycine transporters, which will provide the basis for understanding the pharmacological actions of this potentially therapeutic anti-psychotic agent and also allow a better understanding of the role of glycine transporters in regulation of synaptic NMDA receptor activity.
Methods
Materials
All chemicals were obtained from SIGMA Chemical Co. (Sydney, Australia) unless otherwise stated. Restriction enzymes were obtained from Bresatec (Adelaide, Australia) and Fermentas (Brisbane, Australia). Dr Marc Caron (Howard Hughes Medical Institute Research Laboratories, Department of Cell Biology and Medicine, Duke University Medical Center, Durham, NC, U.S.A.) kindly supplied the plasmids containing the human GLYT1a, b and c cDNAs and Dr John Marrow (Target Discovery Section, Organon Laboratories Limited, Newhouse, Lanarkshire, U.K.) kindly supplied the plasmid containing human GLYT2acDNA. N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) (also termed ALX-5407) was supplied by Allelix Neuroscience Inc.
Expression of GLYTs in Xenopus laevis oocytes and electrophysiological recordings
Complementary DNAs encoding human GLYT1a, b and c and GLYT2a were subcloned into (Oocyte Transcription Vector (pOTV) (Arriza et al., 1994)). The GLYT1a, b, c and 2a-OTV plasmids were linearized with SpeI and cRNA was transcribed from the cDNA constructs with T7 RNA polymerase and capped with 5′7-methyl guanosine using the mMESSAGE mMACHINE kit (Ambion Inc., Austin, TX, U.S.A.).
Xenopus laevis frogs were anaesthetized with 0.17% 3-aminobenzoic acid ethyl ester and a lobe of the ovaries removed. The lobe containing oocytes was then rinsed in OR2 buffer (mM: NaCl 82.5, KCl 2, MgCl2.6H2O 1, HEPES 5, pH 7.5) and treated with Collagenase A (2 mg ml−1 in OR2 buffer, Boehringer Mannheim) for 2 h. Released oocytes were rinsed in frog ringers solution (mM: NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, HEPES 5, pH 7.55), supplemented with 2.5 mM sodium-pyruvate, 0.5 mM theophylline, 50 μg ml−1 gentamicin and stage V oocytes isolated for use. This procedure followed a protocol ethically approved under the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Fifty nL of GLYT cRNA was injected into the defolliculated stage V Xenopus laevis oocytes and incubated at 16°C in supplemented standard frog ringers solution (above). Three to eight days later, current recordings were made using the two-electrode voltage clamp technique with a Geneclamp 500 (Axon Instruments, Foster City, CA, U.S.A.) interfaced with a MacLab 200 chart recorder (ADInstruments, Sydney, Australia).
Recordings were made using the standard frog ringers solution (ND96) and in experiments where sodium or chloride was omitted, equimolar choline base and gluconate was substituted and potassium hydroxide was used to adjust pH. NFPS was made into a stock solution of 1 mM in DMSO and diluted in frog ringers as appropriate. The maximum DMSO concentration applied to the oocytes was 0.1% (vol vol−1), which did not generate a measurable current in either nude oocytes or oocytes expressing GLYT1b, nor did it inhibit GLYT1b when applied with glycine.
3H-Glycine uptake studies
NFPS inhibition of 3H-glycine uptake was measured in nude oocytes and oocytes expressing GLYT1b. Oocytes (five per dish) were incubated with 30 μM 3H-glycine at room temperature either: in the absence of NFPS; after 10 min preincubation with 1 μM NFPS; or co-applied with 1 μM NFPS. After 10 min uptake the oocytes were washed three times with frog ringers buffer and then each oocyte dissolved in 50 mM NaOH and 3H-glycine measured by scintillation counting.
Data analysis
The Kruskal-Wallis test with the relevant post-hoc test or the student's t-test were preformed using Prism GraphPad (Motulsky, 1999) to assess difference in the means. P-values of less then 0.05 were taken to be significant and are indicated in figures as (*).
Results
NFPS is a selective blocker of GLYT1
The uptake of glycine by high affinity glycine transporters 1 (GLYT1) is accompanied by the co-transport of two Na+ ions and one Cl− ion (Aragon et al., 1987; Roux & Supplisson, 2000) resulting in the net transfer of one positive charge with each glycine molecule. Application of glycine to oocytes expressing the GLYT1b isoform of glycine transporter, voltage clamped at −60 mV, resulted in a dose dependent inward current with an EC50 of 20±4 μM (Aubrey et al., 2000). Co-application of 100 μM glycine with 300 nM NFPS resulted in a gradual reduction in the inward current with 69±4% (n=10) inhibition after 3 min (Figure 2a). Application of NFPS alone does not generate a current, which indicates that NFPS is not a transportable inhibitor of GLYT1b. Co-application of 300 nM NFPS with 30 μM glycine for 3 min to oocytes expressing GLYT1a or GLYT1c caused a 66±5% and 77±3% inhibition respectively, when compared to glycine currents before NFPS application. These results indicate that NFPS acts at a comparable rate on all the GLYT1 isoforms of glycine transporter (Figure 2c). In contrast to the GLYT1 subtypes, application of up to 1 μM NFPS to oocytes expressing GLYT2a had no effect on glycine transport currents (Figure 2b). Thus, NFPS appears to be a selective inhibitor of the GLYT1 glycine transporters. In the following experiments we have characterized in more detail the mechanism of action of NFPS on the GLYT1b glycine transporters.
Figure 2.
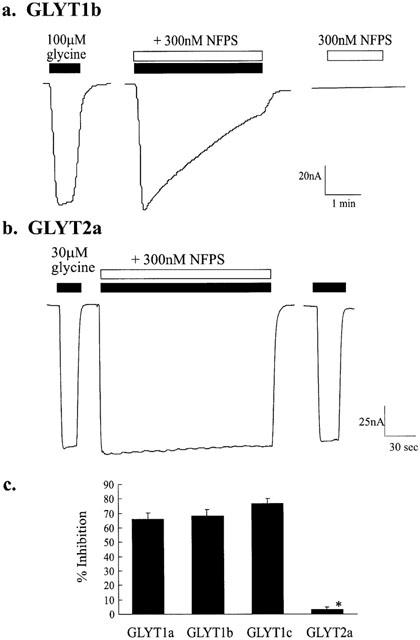
NFPS is a selective blocker of GLYT1 subtypes of glycine transporters. Representative traces from oocytes expressing GLYT1b (a) and GLYT2a (b) voltage clamped at −60 mV. One hundred μM glycine (solid bar) was applied first and after washout, 100 μM glycine (solid bar) and 300 nM NFPS (open bar) were co-applied for 3 min. After a further 10 min of superfusion in drug-free buffer, 300 nM NFPS alone (open bar) was applied. In the trace for an oocyte expressing GLYT2a (b) 30 μM glycine with 300 nM NFPS was applied together after a control dose of glycine (30 μM). In uninjected oocytes, application of glycine and NFPS, together or alone, did not generate a current. (c) NFPS similarly inhibited the other GLYT1 transporter subtypes. Thirty μM glycine and 300 nM NFPS were applied to oocytes expressing GLYT1a, b and c and GLYT2a as described above and the per cent inhibition measured after 3 min exposure to NFPS. Statistical analysis was carried out using the Kruskal-Wallis test. Significance (P⩽0.05) is indicated by *.
NFPS inhibition of glycine transport currents does not become apparent until after the glycine transport current has reached its peak value (see Figure 2a). This may indicate that NFPS requires an active state of the transporter before it inhibits transport. We investigated this possibility by first exposing oocytes expressing GLYT1b to 30 μM glycine to measure the control transport current, followed by washout of glycine. The oocytes were then exposed to 300, 100 or 30 nM NFPS in the absence of glycine for 3 min followed by a washout for 2 min. Subsequent application of glycine alone resulted in a transport current that was reduced by 76±6% (n=3) (Figure 3a); 41±6% (n=5) and 22±3% (n=3) respectively, compared to the glycine transport current measured before NFPS application. This suggests, first, that the glycine transporter does not have to be in an active state for the blocker to be effective, and second, that NFPS inhibition is still apparent after washout of NFPS from the bath solution. Glycine binding and transport by GLYT1b is dependent upon the presence of Na+ and Cl− ions (Aragon et al., 1987), and so we investigated the possibility that NFPS binding to GLYT1b requires Na+ or Cl−. Glycine was first applied to oocytes expressing GLYT1b in normal frog ringers buffer to measure the control response, and after washout of glycine the buffer was switched to either a Na+ free buffer (choline substitution) or a Cl− free buffer (gluconate substitution) and then 300, 100 or 30 nM NFPS applied for 3 min. After washout of NFPS from the bath solution and subsequent return to normal frog ringer's buffer, glycine was re-applied. Glycine transport currents were reduced by 87±10% (n=3); 40±1% (n=3) and 30±3% (n=4) respectively in choline substituted buffer and 92±11% (n=3); 45±3% (n=4) and 24±4% (n=6) respectively in gluconate substituted buffer, compared to currents observed before NFPS was applied. The level of inhibition does not significantly differ for each NFPS dose in Na+ or Cl− substituted buffers, compared to inhibition by the same dose of NFPS in normal frog ringers solution (Kruskal-Wallis test). This indicates that NFPS binding to GLYT1b does not require the presence of either Na+ or Cl− ions and further suggests that the glycine transporter does not need to be in an active conformation to bind NFPS.
Figure 3.
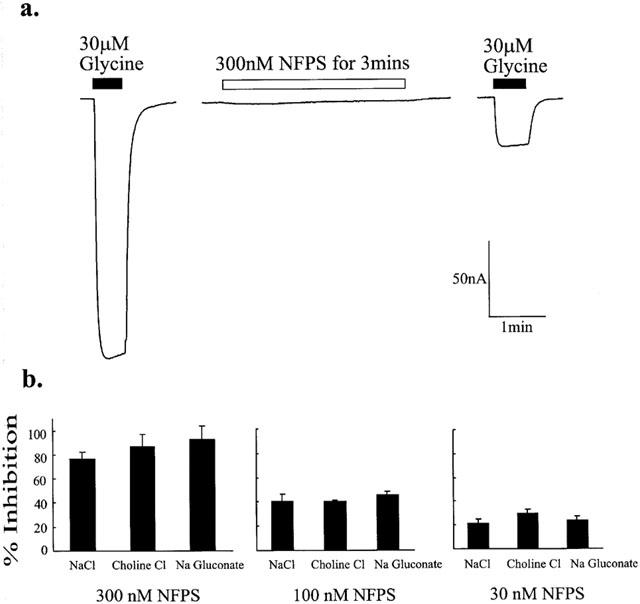
NFPS binding to GLYT1b does not require glycine, Na+ or Cl−. Current trace from representative oocytes expressing GLYT1b voltage clamped at −60 mV (a). In normal frog ringers buffer, 30 μM glycine was first applied (solid bar) followed by wash out in frog ringers. Three hundred nM NFPS was then applied alone for 3 min (open bar) followed by a washout period in drug-free frog ringers. Thirty μM glycine (solid bar) was then re-applied. (b) Experiments were carried out in choline substituted Na+ free buffer or gluconate substituted Cl− free buffer. After application of 30 μM glycine to measure control responses, the bath solution was switched and allowed to equilibrate for 2 min before 300, 100 or 30 nM NFPS was applied for 3 min. After 1 min wash out of NFPS in the ion substituted buffer, the buffer was switched back to standard frog ringers buffer and 30 μM glycine reapplied. The per cent inhibition of glycine transport currents after the three NFPS treatments are presented. The absence of Na+ or Cl− did not significantly change the level of inhibition for any of the treatments (Kruskal-Wallis test).
The effects of NFPS on 3H-glycine uptake by GLYT1b were measured to confirm that the reduction in glycine transport currents reflects a reduction in the rate of glycine transport. Uninjected oocytes (five per dish) and oocytes expressing GLYT1b (five per dish) were incubated with 30 μM 3H-glycine at room temperature for 10 min under three different conditions. First, after 10 min pre-incubation of the oocytes with 1 μM NFPS, second with addition of 1 μM NFPS at the same time as 3H-glycine, and third, in the absence of NFPS (Figure 4). The uninjected oocytes showed a low level of 3H-glycine uptake, which was not influenced by the presence of NFPS (either pre-incubated or co-applied). In oocytes expressing GLYT1b, 3H-glycine uptake was more then 20 fold increased compared to uninjected oocytes and with the oocytes pre-incubated with 1 μM NFPS for 10 min the level of uptake was significantly reduced (Kruskal-Wallis followed by Dunns test) to levels observed for uninjected oocytes. In the oocytes in which 1 μM NFPS and 30 μM 3H-glycine were co-applied, the extent of inhibition was reduced compared to the pre-incubated oocytes, however this reduction did not reach significance. These observations are consistent with the transport current measurements in which NFPS blocks the transport current and that the onset of inhibition is slow and gradual, reaching complete inhibition over the 10 min of this experiment.
Figure 4.
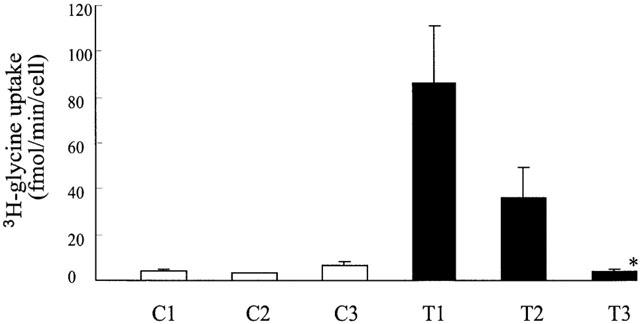
NFPS inhibits 3H-glycine uptake in oocytes expressing GLYT1b. 3H-glycine uptake by uninjected oocytes (C1, 2 and 3) and oocytes expressing GLYT1b (T1, 2 and 3) was measured as described in Methods. C1 and T1: 30 μM 3H-glycine alone. C2 and T2: 30 μM 3H-glycine and 1 μM NFPS. C3 and T3 30 μM 3H-glycine and 1 μM NFPS after 10 min pre-incubation with 1 μM NFPS. In each case the results are the mean±s.e.mean from five oocytes and Kruskal-Wallis followed by Dunns test was used to assess statistical significance. P⩽0.05 is indicated by *.
Sarcosine is an alternative substrate for the GLYT1 but not the GLYT2 glycine transporters (Guastella et al., 1992; Kim et al., 1994) and generates a dose dependent current when applied to oocytes expressing GLYT1 voltage clamped at −60 mV. Given that NFPS (Figure 1) consists of a sarcosine head group with a lipophilic tail we hypothesized that the selectivity of NFPS for GLYT1 was due to an interaction of the sarcosine moiety of NFPS with the glycine binding site on GLYT1. To test this hypothesis, oocytes expressing GLYT1 were exposed to a control dose of 30 μM glycine, and after a 2 min wash the cells were exposed to 30 or 300 nM NFPS with either 0, 30 or 3000 μM glycine for a period of 3 min (Figure 5) to investigate if increasing the concentration of substrate present can protect GLYT1 from inhibition by NFPS. NFPS and glycine were washed out for 5 min before 30 μM glycine was applied once more and compared with the control glycine current. In the presence of 30 or 300 nM NFPS alone, glycine currents were inhibited by 22±3% (n=8) and 76±6% (n=3) respectively, compared to control. After exposure to 30 nM NFPS together with 30 or 3000 μM glycine, transport currents were decreased by 28±2% (n=3) and 22±2% (n=3), and after exposure to 300 nM NFPS together with 30 or 3000 μM glycine, transport currents were decreased by 72±2% (n=3) and 71±1% (n=3) respectively. Clearly there is no significant alteration to the extent of inhibition by these two doses of NFPS in the presence of glycine (Kruskal-Wallis test), indicating that under these conditions glycine does not offer any significant protection to the effects of NFPS. Similar experiments using sarcosine, instead of glycine, to protect GLYT1b from NFPS binding did not alter the extent of inhibition by NFPS. The lack of protection by glycine may be due to the transporter being in an active state and after completing a transport cycle the substrate binding site may become accessible to NFPS and thereby remove the protective effect of glycine. In order to insure that GLYT1 transporters we not in an active state that may transiently expose the glycine binding site, further experiments following similar protocol were carried out in the absence of Na+ and Cl− (in choline or gluconate substituted buffer) to prevent transport. Cells were equilibrated in the relevant buffer and 300 nM NFPS was applied for 3 min in the presence of saturating sarcosine. Under these conditions, inhibition by NFPS was still not significantly altered (Kruskal-Wallis test, data not shown). These experiments indicate that glycine, or sarcosine, do not provide any protection against NFPS under these conditions.
Figure 5.
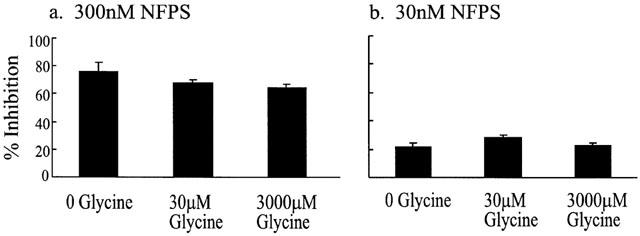
NFPS does not compete with glycine for the glycine binding site. A control current induced by 30 μM glycine was measured in oocytes expressing GLYT1b, voltage clamped at −60 mV. Three hundred or 30 nM NFPS was co-applied with 0, 30 or 3000 μM glycine for 3 min followed by a 5 min washout. Thirty μM glycine was applied again and compared to control currents. The extent of inhibition by 300 (a) or 30 nM (b) NFPS after 3 min application was not significantly altered in the presence of 0, 30 or 3000 μM glycine (Kruskal-Wallis test).
NFPS is a persistent inhibitor of GLYT1b
In Figure 2a, glycine transport currents were reduced by 69±4% after washout of NFPS from the bath solution compared to control values, which indicates that inhibition persists after removal of NFPS from the bath solution. This observation was investigated further (Figure 6a) by first applying a control dose of 10 μM glycine and when the transport current reached a peak (Figure 6a, point 1) 300 nM NFPS was added. After 3 min of glycine and NFPS exposure, NFPS was removed and the glycine transport current re-established (point 2). Following a 2 min washout of glycine with normal frog ringers solution, re-application of 10 μM glycine alone induced a similarly reduced current (point 3) when compared to control values (n=6, Figure 6b) (Kruskal-Wallis test). This reduced current remained stable for up to 90 min after wash out of NFPS (data not shown). We investigated whether NFPS inhibition of transport was reversible over a more extended period by incubating a batch of oocytes expressing GLYT1b with 1 μM NFPS for 10 min followed by repeated washing of the oocytes by changing the frog ringers buffer at least five times a day over the next 3 days. Glycine transport currents were measured for 3 days following the single NFPS application. After 3 days glycine transport currents recovered slightly, probably due to new transporter production, but did not return to control currents measured from the same batch of oocyte expressing GLYT1 but not treated with NFPS (Figure 7a) (Student's t-test).
Figure 6.
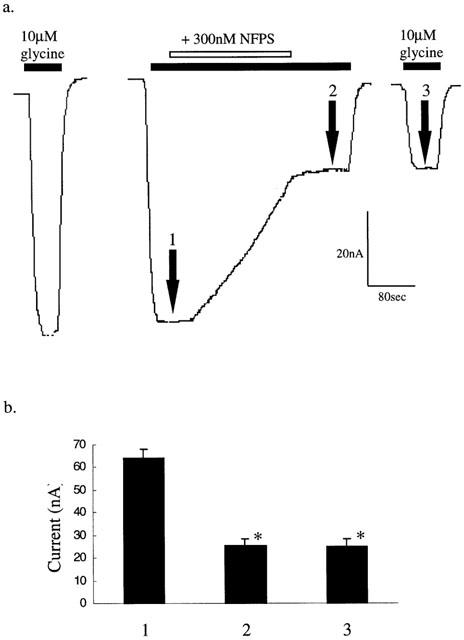
NFPS is an apparent irreversible inhibitor. (a) Representative current trace from an oocyte expressing GLYT1b voltage clamped at −60 mV. After a control (10 μM) glycine response, glycine (10 μM) was reapplied until the current reached a maximal response (point 1) followed by the co-application of 300 nM NFPS. Three minutes later NFPS was washed out of the bath, but in the continued presence of 10 μM glycine (point 2). After washout of glycine for a further 5 min, 10 μM glycine was reapplied (point 3). Currents induced by subsequent application of 10 μM glycine up to 90 min later remained equally and significantly inhibited (Kruskal-Wallis test). (b) Current responses to application of 10 μM glycine were measured at the indicated points (1 – 3) and represented in a bar graph (n=6). Significance (P⩽0.05) is indicated by *.
Figure 7.
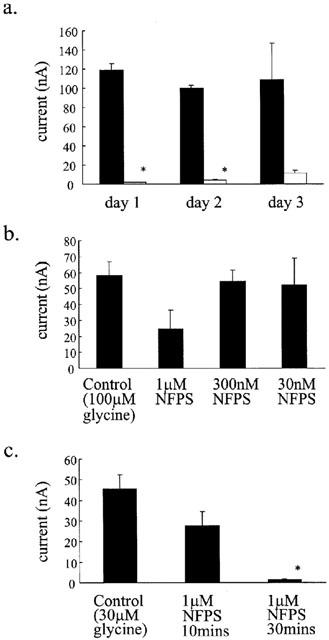
NFPS interacts with the oocyte membrane. (a) Oocytes expressing GLYT1b were exposed to 1 μM NFPS for 10 min and then washed at least three times daily for the next 3 days. Glycine transport currents were measured from oocytes 1, 2 and 3 days after NFPS exposure and compared to unexposed oocytes (Student's t-test). Currents were significantly inhibited on days 1 and 2, however on day 3, variability was high and although reduced this decrease did not reach significance. Data represents mean±s.e.mean, n⩾3 for each condition. (b) Immediately after injection of GLYT1b cRNA, NFPS was applied to the oocytes at the indicated doses for 10 min followed by extensive washing in standard buffer. Glycine transport currents were measured 3 days later and compared to unexposed oocytes. Data is mean±s.e.mean, n=3 for each condition. (c) A similar protocol to Figure 7b was used, except that 1 μM NFPS was applied for 10 min and 30 min immediately after cRNA injection. Data presented are from mean±s.e.mean, n=10 for each condition. Control experiments were also included where GLYT2a cRNA was injected instead of GLYT1b and in each case NFPS had no effect on the glycine transport currents. Data analysis for b and c was carried out using the Kruskal-Wallis test and Dunns test. Significance (P⩽0.05) is indicated by *.
Given that NFPS has a bulky lipophilic side chain, we investigated whether the irreversibility of inhibition arises through NFPS being incorporated into the lipid membrane. Immediately after injection of GLYT1b cRNA into the oocytes, the oocytes were exposed to 1 μM, 300 or 30 nM NFPS for 10 min. GLYT1b is not expressed on the cell surface of the oocyte until approximately 3 days after cRNA injection, so if NFPS does bind irreversibly to a component of the lipid membrane, the inhibition caused by pre-expression exposure to NFPS should be comparable to that observed in the conditions of Figure 7a. Exposure to 1 μM NFPS for 10 min did reduce GLYT1b-mediated glycine transport currents compared to control, but the reduction did not reach significance (Kruskal-Wallis test) (Figure 7b). GLYT1b-mediated glycine transport currents measured from cells exposed to 300 nM (n=3) or 30 nM (n=3) NFPS before GLYT1b expression were not altered when compared to control. However, exposure to high dose NFPS (1 μM) for longer periods (30 min) resulted in significantly reduced glycine transport currents (Figure 7c) (Kruskal-Wallis and Dunns test), which were more comparable to that observed following 3 min exposures to lower doses of NFPS (see Figure 2a). These results indicate that NFPS does have the capacity to be incorporated into the lipid membrane in the absence of transporter proteins, but the exposure times and doses required are significantly greater than that required for the essentially irreversible inhibition of GLYT1b seen previously. Therefore the NFPS/lipid bilayer interaction alone is not sufficient to keep NFPS present in the membrane of the oocyte permanently. In control experiments, oocytes were injected with GLYT2 cRNA and immediately exposed to 1 μM NFPS for 30 min. Three days later glycine transport currents were not significantly different compared to untreated cells, which indicates that NFPS reduction in glycine transport currents is selective for the GLYT1 subtypes and that NFPS does not interfere with the intracellular machinery required for expression of proteins at the cell surface.
Another possible mechanism for the long term inhibition by NFPS is that it may cross the membrane and act at an intracellular recognition site. This idea is also consistent with the observation that the onset of action of NFPS was relatively slow and may also explain the lack of effective washout of NFPS. After measuring a control glycine current, 50 nL of 20 μM NFPS was injected into the cytoplasm of oocytes expressing GLYT1b. Assuming that this generates an even distribution of NFPS within the cytoplasm and that the internal volume of an oocyte is approximately 1 μl, the intracellular concentration of NFPS would be approximately 1 μM. However, glycine transport currents, measured for up to 15 min later, were not affected by the presence of NFPS in the cytoplasm (Figure 8). Subsequent co-application of 30 μM glycine and 1 μM NFPS to the extracellular bath solution caused a slow onset and irreversible inhibition. These results suggest that the NFPS site of action is not accessible from the cytoplasm.
Figure 8.
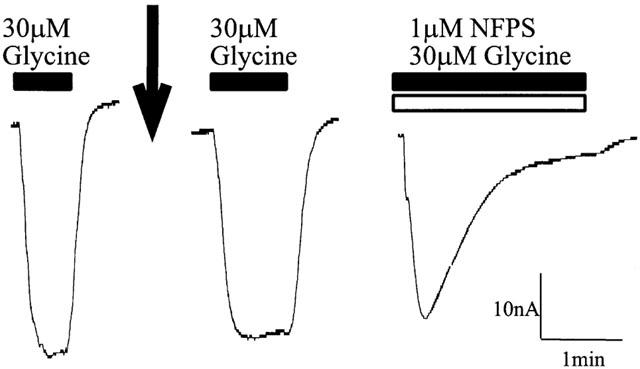
NFPS does not inhibit transport when injected into the cytoplasm of the oocyte. A representative current trace from an oocyte expressing GLYT1b voltage clamped at −60 mV. After insertion of the voltage clamp electrodes into the oocyte, a microinjection needle, containing 20 μM NFPS, was inserted into the oocyte. A control current response to 30 μM glycine was measured (solid bar) and then 50 nL of 20 μM NFPS was injected into the oocyte (indicated by the arrow). After stabilization of the baseline current, 30 μM glycine was re-applied (solid bar) and the current measured. After washout of glycine, 30 μM glycine (solid bar) and 1 μM NFPS (open bar) were co-applied to the bath solution.
A dose dependent time course of NFPS inhibition of GLYT1 is presented in Figure 9. The time required for 50% inhibition (t1/2) of the current generated by 30 μM glycine is plotted as a function of NFPS concentration. This figure demonstrates that the rate of inhibition at high doses (1 μM) is considerably faster (t1/2=58±10 s) then at lower doses (t1/2 (10 nM)=904±90 s). Although lower doses (<10 nM) are likely to be effective in inhibiting transport, the oocytes do not remain sufficiently stable for the times required to reach 50% inhibition. As we were unable to measure the off rate of the NFPS/GLYT1 interaction further kinetic analysis of this inhibition was not possible.
Figure 9.
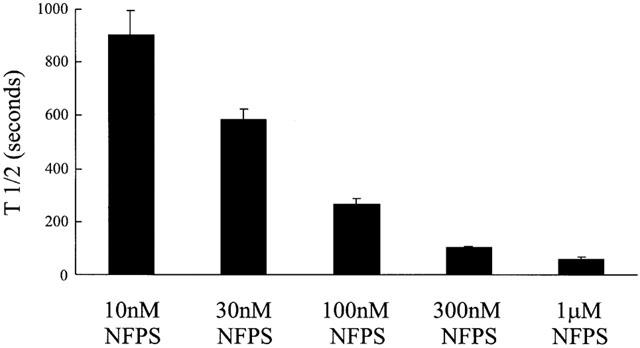
NFPS inhibits GLYT1b in a time and dose dependent manner. In oocytes voltage clamped at −60 mV a control glycine (30 μM) current was first established. Then NFPS, at doses from 10 to 1000 nM, was co-applied with 30 μM glycine. Inhibition was allowed to persist until currents were reduced to half the control glycine current and measured as time taken to reach half of complete inhibition (t1/2). Data represents mean±s.e.mean with n=5 for each NFPS dose.
Discussion
The GLYT1 and GLYT2 subtypes of glycine transporters differ in their substrate selectivity and on this basis it is tempting to speculate that the sarcosine moiety of NFPS is responsible for conferring selectivity between the GLYT1 and GLYT2a subtypes of glycine transporters. However, we found no evidence to support this idea. Glycine or sarcosine do not compete with NFPS, nor do they provide any protection against NFPS inhibition of transport (see later section for more discussion of this point). We have also shown that NFPS inhibition of transport persists after washout of NFPS from the bath solution. Therefore we must conclude that NFPS is a non-competitive, long lasting inhibitor of GLYT1b. These observations raise a number of issues such as how does NFPS bind to the transporter and what is the basis for the persistent inhibition of transporter function? Whilst our studies have focused primarily on the GLYT1b subtype it is envisaged that these results can be generalized to the other GLYT1 subtypes.
NFPS does not contain any reactive groups that are likely to form covalent bonds with the transporter protein under physiological conditions, but it does have the capacity to form multiple van der Walls interactions and hydrogen bonds with both the lipid bilayer and the protein to form a highly stable complex. At concentrations (10 nM – 1 μM) and exposure times that we used in these experiments NFPS does not appear to be irreversibly incorporated into the lipid membrane if glycine transporters are not present (Figure 7b), however at higher concentrations and longer exposure times in oocytes expressing transporter (1 μM for 30 min, Figure 7c) NFPS does appear to remain in the cell membrane for at least 3 days at concentrations sufficient to inhibit transport. These results suggest that NFPS does have the capacity to be incorporated into the membrane. The lower concentrations and shorter exposure times for inhibition of transport suggest that the incorporation of NFPS into the membrane may be stabilized in the presence of GLYT1. Thus, we propose the following interactions between NFPS and GLYT1. NFPS may have two modes of interaction, the first being a non-specific incorporation into the lipid bilayer and the second involving specific interactions with the GLYT1 protein. The lipid bilayer may provide a ‘sink' for NFPS and with prolonged exposure it may accumulate NFPS to significantly higher concentrations and as such effectively increase the concentration available to interact with membrane proteins. In the absence of GLYT1, NFPS may be washed out or be removed by membrane turnover, but in the presence of GLYT1 the readily available NFPS in the membrane will specifically bind via a lipid accessible site and with high affinity to GLYT1. The lack of protection of the NFPS site by glycine or sarcosine suggests that the NFPS site on GLYT1 is distinct from the substrate binding/translocation site. However, an alternative interpretation of this experiment is that application of 30 or 300 nM NFPS to cells for 3 min in the presence of a saturating glycine (or sarcosine) dose results in incorporation of NFPS only into the lipid bilayer without binding to the transporter. After washout of NFPS and glycine from the bath solution, the NFPS still in the membrane may diffuse through the membrane and bind to the glycine-binding site on the transporter and inhibit subsequent transporter activity. In order to test this idea more completely, a fast flow system is required where rapid switching of solutions may help to clarify the kinetics of the two processes and whether the presence of substrates may slow the rate of specific binding of NFPS to GLYT1. Irrespective of the nature of the molecular basis for specific inhibition of GLYT1, a high affinity specific interaction in combination with a readily available source of inhibitor that is only slowly washed out would result in an apparent irreversible interaction.
In this study we have utilized the Xenopus oocyte expression system, and in light of the proposed mechanism of action of NFPS inhibition of glycine transport, an important issue is whether the results obtained in this study can be extrapolated to other cell expression systems and also to in vivo preparations. Atkinson et al. (submitted) have reported that 50 nM NFPS causes a similarly long lasting inhibition of GLYT1c expressed in quail fibroblast cells. This group measured a very slow return of glycine transporter function, with 50% recovery after 13 h washout. This observation may reflect a very slow dissociation of NFPS from glycine transporter or alternatively the recovery of glycine uptake observed may also be due to sequestration of NFPS bound to GLYT1 into the cell and synthesis and expression of new transporter proteins on the cell surface. As observed in the control measurements of Figure 7a, the turnover rate of the GLYT1 protein in the membrane of Xenopus oocytes does not markedly increase over 3 days. In this aspect our results are likely to differ from GLYT1 expression in quail fibroblast cells. This difference could explain the apparent discrepancy between the results presented in this study and that of Atkinson et al. (submitted). Bergeron et al. (1998), reported that in the rat hippocampus, the effects of NFPS on glycine transport appear to be reversible over the period of their whole cell patch-clamp recordings. At this stage the reasons behind these differences are not clear, but in light of the results presented here and those by Atkinson et al. (submitted) the reversibility of NFPS action in slice preparations warrants further investigation
The proposed mechanism of action of NFPS inhibition of glycine transport has parallels with the mechanism of action of xanomeline on M1 muscarinic receptors (Christopoulos et al., 1998; 1999) and also salmeterol stimulation of β-adrenergic receptors (Anderson et al., 1994; Rhodes et al., 1992). These drugs display persistent receptor stimulation after extensive washout and models incorporating two modes of interaction have also been used to explain this phenomenon. The first mode involves reversible aqueous phase interactions with the classical agonist binding site and the second phase involves the development of persistent attachment to a secondary site. The nature of the secondary site is not well established and one possibility is that the lipid bilayer may provide a ‘sink' for the drug such that a slower rate of diffusion from the lipid membrane may reduce the effective off rate of the drug or alternatively the lipid bilayer may be the secondary site.
One of the postulated mechanisms of schizophrenia is reduced activity of NMDA receptors and, as glycine is an essential co-agonist of NMDA receptors, it has been suggested that elevation of glycine concentrations may provide a novel therapeutic strategy for treating this debilitating disorder (Heresco-Levy et al., 1999). If resting concentrations of glycine within excitatory synapses are not sufficient to saturate NMDA receptors (Berger et al., 1998; Danysz & Parsons, 1998) then pharmacological elevation of synaptic glycine concentrations may selectively stimulate NMDA receptors (Bergeron et al., 1998). NFPS has the potential to act in this manner and the results presented in this study provide the basic framework for understanding the pharmacological actions of NFPS.
Acknowledgments
Thanks to Allelix Neuroscience Inc. for providing the NFPS for this study and to Mark Klitenick, Mark Connor, Elena Bagley, Stephanie Borgland, Rhonda Pearlman and Renae Ryan for discussion and critical comments on this manuscript. This work was funded by the Australian NHMRC.
Abbreviations
- GLYT
Glycine Transporter
- NFPS
N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine
- pOTV
Oocyte Transcription Vector
References
- AMARA S.G., KUHAR M.J. Neurotransmitter Transporters : Recent Progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- ANDERSON G.P., LINDEN A., RABE K.F. Why are long-acting beta-adrenoceptor agonists long-acting. Eur. Respir. J. 1994;7:569–578. doi: 10.1183/09031936.94.07030569. [DOI] [PubMed] [Google Scholar]
- ARAGON M.C., GIMENEZ C., MAYOR F. Stoichiometry of sodium- and chloride-coupled glycine transport in synaptic plasma membrane vesicles derived from rat brain. FEBS Lett. 1987;212:87–90. doi: 10.1016/0014-5793(87)81562-4. [DOI] [PubMed] [Google Scholar]
- ARRIZA J.L., FAIRMAN W.A., WADICHE J.I., MURDOCH G.H., KAVANAUGH M.P., AMARA S.G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATTWELL D., BARBOUR B., SZATKOWSKI M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- AUBREY K.R., MITROVIC A.D., VANDENBERG R.J. Molecular Basis for Proton Regulation of Glycine Transport by GLYT1b. Mol. Pharmacol. 2000;58:129–135. doi: 10.1124/mol.58.1.129. [DOI] [PubMed] [Google Scholar]
- BERGER A.J., DIEUDONNE S., ASCHER P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J. Neurophys. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- BERGERON R., MEYER T.M., COYLE J.T., GREENE R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPOULOS A., PARSONS A.M., EL-FAKAHANY E.E. Pharmacological analysis of the novel mode of interaction between xanomeline and the M1 muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 1999;289:1220–1228. [PubMed] [Google Scholar]
- CHRISTOPOULOS A., PIERCE T.L., SORMAN J.L., EL-FAKAHANY E.E. On the unique binding and activating properties of xanomeline at the M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 1998;53:1120–1130. [PubMed] [Google Scholar]
- DANYSZ W., PARSONS A.C.G. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- GUASTELLA J., BRECHA N., WEIGMANN C., LESTER H.A., DAVIDSON N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7189–7193. doi: 10.1073/pnas.89.15.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANLEY J.G., JONES E.M., MOSS S.J. GABA Receptor rhol subunit interacts with a Novel Splice Variant of the Glycine Transporter, GLYT-1. J. Biol. Chem. 2000;275:840–846. doi: 10.1074/jbc.275.2.840. [DOI] [PubMed] [Google Scholar]
- HERESCO-LEVY U., JAVITT D.C., ERMILOV M., MORDEL C., SILIPO G., LICHTENSTEIN M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia [see comments] Arch. Gen. Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- HOLLMANN M., HEINEMANN S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- KIM K.M., KINGSMORE S.F., HAN H., YANG-FENG T.L., GODINOT N., SELDIN M.F., CARON M.G., GIROS B. Cloning of the human glycine transporter type 1: molecular and pharmacological characterization of novel isoform variants and chromosomal localization of the gene in the human and mouse genomes. Mol. Pharmacol. 1994;45:608–617. [PubMed] [Google Scholar]
- LIU Q.R., LOPEZ-CORCUERA B., MANDIYAN S., NELSON H., NELSON N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J. Biol. Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- LIU Q.R., NELSON H., MANDIYAN S., LOPEZ-CORCUERA B., NELSON N. Cloning and expression of a glycine transporter from mouse brain. FEBS Lett. 1992;305:110–114. doi: 10.1016/0014-5793(92)80875-h. [DOI] [PubMed] [Google Scholar]
- MCBAIN C.J., MAYER M.L. N-methyl-D-aspartic acid receptor structure and function. Physiol. Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- MOTULSKY H. GraphPad software Inc., San Diego, CA, U.S.A; 1999. Analysing data with GraphPad Pris. [Google Scholar]
- PONCE J., POYATOS I., ARAGON C., GIMENEZ C., ZAFRA F. Characterization of the 5′ region of the rat brain glycine transporter GLYT2 gene: identification of a novel isoform. Neurosci. Lett. 1998;242:25–28. doi: 10.1016/s0304-3940(98)00037-8. [DOI] [PubMed] [Google Scholar]
- RHODES D.G., NEWTON R., BUTLER R., HERBETTE L. Equilibrium and kinetic studies of the interactions of salmeterol with membrane bilayers. Mol. Pharmacol. 1992;42:596–602. [PubMed] [Google Scholar]
- ROUX M.J., SUPPLISSON S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- SHIMAMOTO K., LEBRUN B., YASUDA-KAMATANI Y., SAKAITANI M., SHIGERI Y., YUMOTO N., NAKAJIMA T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- SMITH K.E., BORDEN L.A., HARTIG P.R., BRANCHEK T., WEINSHANK R.L. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- SUPPLISSON S., BERGMAN C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J. Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


