Abstract
In some asthmatics, muscarinic receptor antagonists are effective in limiting bronchoconstrictor response, suggesting an abnormal cholinergic drive in these subjects. There is a growing body of evidences indicating that cholinergic neurotransmission is also enhanced by endothelin-1 (ET-1) in rabbit bronchi, mouse trachea and in human isolated airway preparations.
We investigated the role of secondary mediators in ET-1 induced potentiation of cholinergic nerve-mediated contraction in human bronchi, in particular the possible role of neuropeptides in this phenomenon.
Bronchial tissues after endothelin treatment were exposed to a standard electrical field stimulation (EFS) (30% of EFS 30Hz)-induced contraction. In addition, in some experiments, preparations were treated with a tachykinin NK2 receptor antagonist and subsequently exposed to the same protocol. HPLC and RIA were performed on organ bath fluid samples. Moreover, the human bronchi were used for the β-PPT (preprotachykinin) mRNA extraction and semiquantitative reverse transcription polymerase chain reaction (RT – PCR), prior to and 30 – 40 min following ET-1 challenge.
The selective tachykinin NK2 receptor antagonist, SR48968, was effective to reduce ET-1 potentiation of EFS mediated contraction. HPLC or RIA showed significant increased quantities of NKA in organ bath effluents after EFS stimulation in bronchi pretreated with ET-1. Finally, β-PPT mRNA level after stimulation of bronchi with ET-1 was increased about 2 fold respect to control untreated bronchi.
In conclusion, this study demonstrated that, at least in part, the ET-1 potentiation of cholinergic nerve-mediated contraction is mediated by tachykinin release, suggesting that in addition to nerves, several type of cells, such as airway smooth muscle cell, may participate to neuropeptide production.
Keywords: Endothelin-1, cholinergic transmission, tachykinin synthesis, human bronchi
Introduction
Airway inflammation and alterations in the neuronal function, central features of the pathogenesis of asthma (Hogg, 1988; Beasley et al., 1989; Barnes, 1996), are involved in the pathophysiology of airway hyper-responsiveness (Holzer, 1988). Human airways are innervated by efferent and afferent autonomic nerves which regulate many aspects of airway function (Barnes, 1986a, 1986b; Richardson, 1982). Vagal cholinergic nerves innervate airway smooth muscle in many mammalian species and airway regions, and play a predominant role in the control of airways tone. Stimulation of these nerves leads to the release of acetylcholine (ACh) from postganglionic cholinergic nerves which, via activation of M3 cholinoceptors, induces airway smooth muscle contraction (Henry et al., 1996). The release of ACh can be modulated by many endogenous substances. Indeed, various autacoids and inflammatory mediators including 5-hydroxytryptamine (Van Oosterhout et al., 1991), thromboxane A2 (Serio & Daniel, 1988) and neuropeptides (Chung et al., 1985; Tanaka & Grunstein, 1986) can enhance cholinergic nerve-mediated contractions, purportedly via a mechanism involving the increased release of ACh. In particular endogenous tachykinin release from afferent sensory nerves may normally facilitate cholinergic neurotransmission; in fact a capsaicin pretreatment which depletes sensory nerves of tachykinins, results in a significant reduction in cholinergic response both in vitro and in vivo in guinea-pig airways (Stretton et al., 1992). Moreover, capsaicin at a sub-threshold concentration, acutely releases tachykinins which enhance cholinergic neurotransmission in guinea-pig trachea in vitro (Aizawa et al., 1990).
In some asthmatics, muscarinic cholinoceptor antagonists such as ipratropium bromide and atropine produce bronchodilation (Ward et al., 1981) and are effective in limiting bronchoconstrictor response to cold-dry air (Sheppard et al., 1982), suggesting an abnormal cholinergic drive in some asthmatic subjects.
There is a growing body of evidence indicating that cholinergic neurotransmission is also enhanced by endothelin-1 (ET-1) in rabbit bronchi (Mckay et al., 1993; Yoneyama et al., 1995), mouse trachea (Henry & Goldie, 1995) and in human isolated airway preparations (Fernandes et al., 1996). Although the precise mechanisms underlying agonist-induced modulation of ACh release are not well understood, these processes appear to have an important role for the neurogenic control of airway smooth muscle tone (Barnes, 1992). Therefore, the aim of this study was to examine further the role of secondary mediators in ET-1 induced potentiation of cholinergic nerve-mediated contraction in human bronchi, in particular the possible role of neuropeptides in this phenomenon. We have studied the effects of the tachykinin NK2 receptor antagonist, SR 48968, on this phenomenon. We have also studied the effect of ET-1 on the synthesis and release of tachykinins.
Methods
Functional studies
Bronchial tissues were obtained immediately after surgery from 24 patients (10 females of 57±5 years and 14 males 62±4 years of age) undergoing lobectomies for respiratory tract tumour at the Second University Hospital of Naples, at the Ascalesi and Monaldi Hospitals of Naples. The study was approved by the local Ethic Committee, all patients were properly informed of the nature of the study and written informed consent was obtained. Bronchial rings (approx. 2 – 7 mm i.d.×5 – 6 mm wide) were mounted in Krebs bicarbonate solution (KBS) continuously aerated with 95% O2 and 5% CO2 and maintained at 37°C, on two L-shaped metal prongs (0.2 mm in diameter), one of which was connected to an isometric force transducer (Narco F-60 Austin, Texas U.S.A.) linked to a Linseis physiograph (Bioblock, Illkirch, France) for continuous recording of the change in tension. The rings were set up for tension measurement under a 1 g load and then were equilibrated for 90 min, with changes in KBS every 10 min. In order to assess tissue responsiveness, carbachol 10 μM was added to the organ baths, upon reaching a contraction plateau, preparations were repeatedly washed and rested for a further 20 min.
The cyclo-oxygenase inhibitor, indomethacin 3 μM to abolish prostanoid synthesis, propranolol 1 μM to abolish beta-adrenergic effects, Nω-nitro-L-arginine methyl ester 100 μM to abolish neuronal nitric oxide-mediated relaxation and, to abolish the intrinsic tone, a leukotriene receptor antagonist, LY 171883 10 μM and (except for histamine dose response curve) an H1 receptor antagonist, mepyramine 1 μM (Ellis & Undem, 1994) were present in the KBS during all studies.
A frequency-response curve was then constructed for each preparation (70 V, 0.5 ms, 10 s train; 0.3, 1, 3, 10 and 30 Hz at 2 min intervals). Contractile responses to electrical field stimulation (EFS) are expressed as a percentage of the response obtained to a maximally effective concentration of acetylcholine (ACh 1 mM, 100% Cmax) added to the bath at the beginning of the experiment. EFS was delivered by a Grass S44 stimulator connected to a stimulus isolation unit (SIU5, Grass Instruments, Quincy, MA, U.S.A.) and an automated timing device. Stimuli were applied across the bronchial ring preparation by means of two parallel platinum electrodes.
The contractile response of four bronchial rings were examined concurrently. One segment was used as a time control and the remaining three as test preparations.
To evaluate the role of cholinergic nerves in the contractile response to EFS (frequency-response curve, 0.3 – 30 Hz), we investigated the effects of the muscarinic receptor antagonist, atropine 0.1 μM, the neurotoxin, tetrodotoxin 10 μM and the ganglion blocker, hexamethonium 10 μM. The influence of these agents on contractile response to cumulatively applied acetylcholine (10 nM – 0.3 mM at 0.5 log concentration increments) was also tested.
Experimental protocol
Each of the three test preparations was exposed to two concentrations (0.1 and 1 nM) of endothelin-1 or [β Ala8] NKA 4-10, a tachykinin NK2 receptor agonist (3 and 30 nM) or histamine (0.1 and 1 μM). When the endothelin-1, [β Ala8] NKA 4-10 or histamine-induced contraction reached a plateau (about 30 – 40 min), a standard EFS (EFSst) was performed every 3 min. EFSst was defined as the frequency (0.5 – 1 Hz) that produced a response of 30% of the contraction observed for EFS 30 Hz.
In addition, in some experiments, preparations were exposed for 30 min to a tachykinin NK2 receptor antagonist, SR 48968 (0.01 – 1 μM) and subsequently exposed to a standard EFS or to a dose response curve of Ach or ET-1. The effects of two different concentrations of ET-1 (0.1 and 1 nM) on Ach dose response curve were also evaluated. In the time-control preparations, the bronchial rings were exposed only to EFSst. Responses were expressed as per cent of EFS 30 Hz. Samples of organ bath fluid (10 ml) were collected for the extraction and characterization of tachykinins in reverse phase HPLC. Release of tachykinins were determined just before, 30 and 40 min after the addition of ET-1. Basal release of tachykinins, expressed as pg ml−1 of organ bath fluid, was defined as the release of tachykinins during 10 min before the contractile challenge. All the samples were stored at −80°C until assayed.
In a separate set of experiments, the human bronchi were used for the RNA extraction and semiquantitative reverse transcription polymerase chain reaction (RT – PCR), prior to and 30 – 40 min after ET-1 challenge.
Extraction and assay
C18 Sep-pak cartridges (Waters Associates) were used to extract peptides from organ bath fluid. Organ bath fluid (10 ml) was passed four times through the cartridge to bind peptides to the hydrated gel in the cartridge. The peptides were eluted using 0.1% v v−1 trifluoroacetic acid (TFA) in acetonitrile and the eluent allowed to evaporate to dryness at room temperature. The samples were then reconstituted in assay buffer (40 mM sodium phosphate pH 7.4 with 2% horse serum) and subjected to each assay in serial dilution.
To examine peptide recovery, known concentrations of synthetic peptides were added to organ bath samples and extracted and assayed as above.
Reverse phase HPLC
After sep-pak extraction, organ bath samples were reconstituted in 0.1% TFA in water and added to a micro-Bondapak C-18 reverse phase column (Waters Associates). The column was eluted with a gradient from TFA/water (0.1 : 99.9) to TFA/water/acetonitrile (0.1 : 89.9 : 10) in 3 min and then to TFA/water/acetonitrile (0.1 : 54.9 : 45) over the next 45 minutes at a flow rate of 1.5 ml min−1. Fractions were collected each minute, lyophilized using a Univap evaporator attached to a refrigerated solvent trap (Uniscences) and a vacuum pump (Speed Vac Savant), reconstituted in assay buffer, and assayed by RIA. The gradient employed had previously been found to resolve synthetic SP, NKA and NKB (Shaw et al., 1989).
Neuropeptide assays
Substance P (SP)-like immunoreactivity was measured using an anti-serum (SP-152) highly specific for the whole molecule (Maule et al., 1989) which was raised in rabbit to synthetic human SP. It shows no significant cross reactivity with neurokinin A (NKA) and neurokinin B (NKB). NKA-like immunoreactivity was measured using a C-terminal specific anti-serum (SK-570) which was raised in rabbit to synthetic human NKA. It cross reacts fully with NKB, but less than 0.1% with SP. Using monoiodinated, reverse phase HPLC purified tracers the SP assay can detect 0.5 pg per assay tube and NKA assay 2 pg per assay tube (Maule et al., 1989). All assays were performed in duplicate and samples were counted in a β-scintillation counter (Packard, U.S.A.). Analysis of results was performed using the Securia 1 (Packard, U.S.A.) computer programme and finally expressed as pg×1000 neuropeptide ml−1 of organ bath fluid.
RNA extraction and semiquantitative (RT – PCR)
The total RNA was extracted after each human bronchus, then was disrupted in liquid nitrogen and homogenized with a TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, U.S.A.) according to the manufacturer's protocol. The RNA was treated with 5 u of DNase I (Rnase A free) (Amersham Pharmacia Biotech, U.K.) at 37°C for 30 min.
Primers used to amplify the PPT (preprotachykinin)-I mRNAs corresponded to β-PPT. Total RNA (2.0 μg) isolated from human bronchus, was reverse transcribed with random examers as primer and 10 units Avian Myeloblastosis Virus (AMV) reverse transcriptase (Promega Corporation, Madison, WI, U.S.A.) at 42°C for 60 min. The cDNA was subjected to 33 cycles of amplification with 1.25 units Taq-DNA polymerase (Promega Corporation, Madison, WI, U.S.A.) in 10 mM Tris HCl (pH 9.0), 50 mM KCl, 0.1% Triton, 1.5 mM MgCl2, 200 μM dNTP, and 0.3 μM each of the 5′- and 3′-primers. Each cycle consisted of denaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 1 min.
Sequences for the human HPRT (Hypoxanthine phosphoribosyltransferase) and PPT human mRNAs from GeneBank (DNASTAR Inc., Madison, WI, U.S.A.) were used to design primer pairs for RT – PCR experiments (OLIGO 4.05 software, National Biosciences Inc., Plymouth, MN, U.S.A.). Primers were 20/22 nucleotides long and contained 50 – 60% G/C. Appropriate regions of the HPRT cDNA were amplified as controls.
Each RT – PCR experiment was repeated at least three times. Amplification products were electrophoresed on 2% agarose gel in 1×TAE. A semiquantitative analysis of mRNA levels was carried out using the software associated with the GEL DOC 1000 u.v. Fluorescent Gel Documentation System (BioRad Company, Hercules, CA, U.S.A.).
Drugs
The drugs and the chemicals used were: endothelin-1 (Novabiochem, Laufelfingen, Switzerland); acetylcholine HCl, atropine HCl, tetrodotoxin, hexamethonium, indomethacin, propranolol HCl, carbachol HCl, N-nitro-L-arginine methyl-ester, LY 171883 {5-(4-[4-Acetyl-3-hydroxy-2-propylphenoxy]butyl)-1H-tetrazole}, mepyramine HCl, histamine HCl, [β Ala8] Neurokinin A (4-10), (Sigma Aldrich, Milan, Italy); SR 48968 {(S)-N-methyl-N-[4-acetyl-amino-4-phenilpiperidino-2-(3,4-dichlorophenyl)-butyl]-benzamide} (Sanofi Recherche, Montpellier, France).
Data analysis
In time control preparations, there were no apparent difference in the effect of time on EFS-induced cholinergic contractile responses, therefore data obtained from all time control experiments were pooled and used in comparisons with treatment groups. The time-related changes in EFS responses were subtracted to ET-1 related changes in EFS-response at each time-point. ET-1 induced changes were expressed as means±s.e.mean.
Differences between EFS treatment groups were assessed by analysis of variance, paired t-test was used for the EFS responses before and after ET-1 treatment.
For HPLC results, comparisons were made using non-parametric methods throughout. The Kruskal – Wallis of variance was used to examine for significant intergroup differences and, if significant, the Mann – Whitney U test was used for between group comparison.
Results
EFS-induced contractions in human bronchus
Electrical field stimulation (EFS; 70 V, 0.5 ms duration, 10 s train, 0.3 – 30 Hz) induced frequency-dependent contractions of human bronchial rings (Figure 1) which were prevented by prior incubation either with the muscarinic receptor antagonist atropine (0.1 μM) or with the neural sodium channel blocker, tetrodotoxin (10 μM) but not by the ganglion blocker, hexamethonium (10 μM) (data not shown). Additionally, responses to exogenously applied ACh were inhibited by atropine, but were unaffected by hexamethonium and tetrodotoxin (data not shown). EFS-induced contractions obtained at 0.5 – 1 Hz (standard EFS-induced contraction EFSst) were 28±5% of the response to stimulation at 30 Hz (EFSst=1265±115 mg; n=8).
Figure 1.
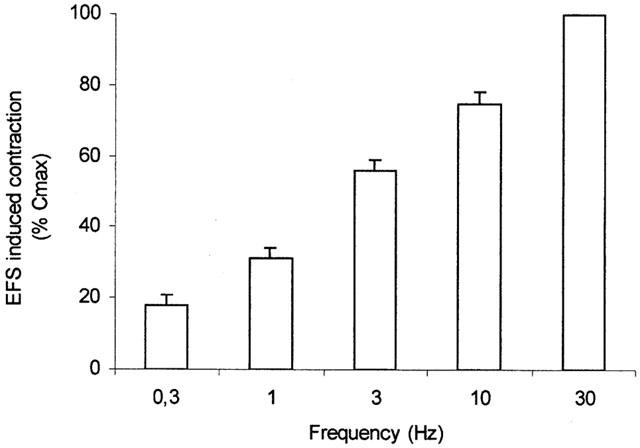
Means EFS frequency-response curves in human isolated bronchial rings. Data are expressed as percentage of the response to a maximally effective concentration of carbachol (10 μM, 100% Cmax). Values are means±s.e.mean of eight preparations.
Modulation of EFS-induced contractions
ET-1 1 nM induced a transient contraction that peaked within 5 – 8 min (19±2% Cmax), but which decreased towards baseline levels of tone over about 30 min period; ET-1 0.1 nM showed no significant contractile effect (data not shown). Both ET-1 0.1 and 1 nM increased the EFSst-induced contractions [40±4% of EFS30 (n=8, P<0.05); 56±2.5% of EFS30 (n=8, P<0.01), vs control 28±5% of EFS30 respectively] (Figure 2).
Figure 2.
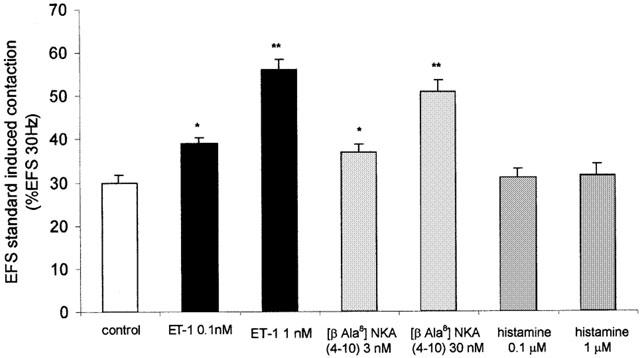
Effect of endothelin-1 (ET-1 0.1 and 1 nM), [βAla8] NKA 4-10 (3 and 30 nM) and histamine (0.1 and 1 μM) on EFS standard induced contraction (a stimulus frequency that induced a 30% of contraction induced by an EFS on 30 Hz) in human isolated bronchial rings. Values are per cent of contraction induced by an EFS of 30 Hz. *P<0.05, **P<0.01 compared to control values. Data are expressed as the means±s.e.mean from eight preparations.
[β Ala8] NKA 4-10 (30 nM) induced a small contraction (16±3% Cmax); [β Ala8] NKA 4-10 (3 nM) showed no significant contractile effect (data not shown). Both concentrations of [β Ala8] NKA 4-10 (3 and 30 nM) increased the EFSst-induced contractions [37±1.73% of EFS30 (n=8, P<0.05); 51±2.5% of EFS30 (n=8, P<0.01) respectively] (Figure 2). The latter was unchanged by a previous exposition to histamine (0.1 and 1 μM) (Figure 2) that only at 1 μM induced a transient contraction (22±3% Cmax) (data not shown).
Endothelin-1 effect on exogenously applied Ach
The response of human isolated bronchi to exogenously applied ACh was also assessed in the presence and in absence of 0.1 or 1 nM ET-1. Cumulative concentration-effect curves to ACh were unchanged in the presence of 0.1 or 1 nM ET-1 (Figure 3).
Figure 3.
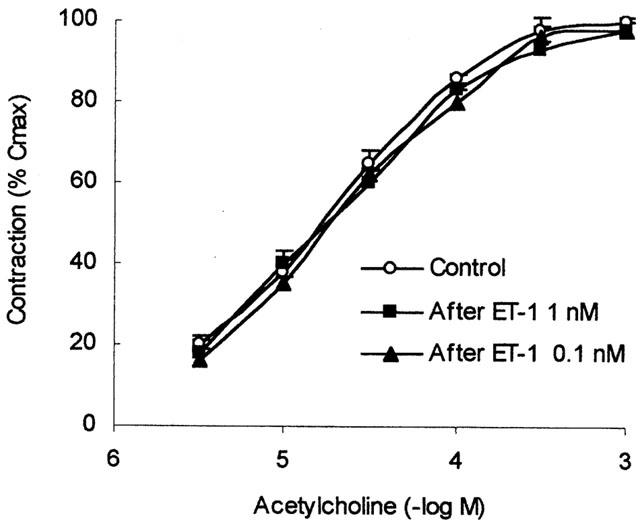
Cumulative concentration-response curves to acetylcholine (ACh) in human isolated bronchial preparations, obtained in the absence and presence of 0.1 nM ET-1 or 1 nM ET-1. Data are expressed as the means±s.e.mean of response from five preparations. Response are shown as a percentage of the response to a maximally effective concentration of carbachol (10 μM, 100% Cmax).
Maximal contraction was also unmodified by ET-1 (ACh control: 3.1±0.3 g, n=5; ACh after ET-1 0.1 nM 2.9±0.4 g, n=5; ACh after ET-1 1 nM 3.0±0.3 g, n=5).
Inhibition of ET-1 effects by SR 48968
In presence of SR 48968 (0.1 μM), a tachykinin NK2 receptor antagonist, the ET-1 1 nM potentation of the EFSst-induced contractions, was attenuated from 56±2.5% of EFSst to 38±4% of EFSst (n=8; P<0.05) (Figure 4).
Figure 4.
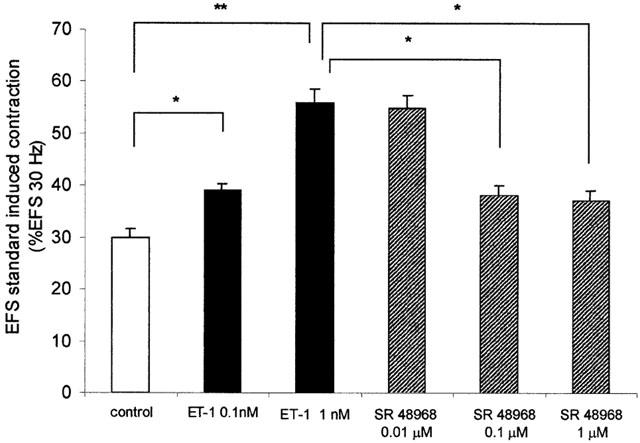
Effect of endothelin-1 (ET-1; 1 nM) on EFS standard induced contraction (a stimulus frequency that induced a 30% of contraction induced by an EFS of 30 Hz) prior to and 30 min after pretreatment with tachykinin NK2 receptor antagonist (SR 48968: 0.01 – 1 μM). Values are per cent of contraction induced by an EFS of 30 Hz. *P<0.05, **P<0.01. Data are expressed as the means±s.e.mean from eight preparations.
The highest dose (1 μM) of the NK2 receptor antagonist did not enhance the inhibitory effect of SR 48968 (0.1 μM) on ET-1 potentation of the EFSst-induced contractions (Figure 4), while the lowest dose (0.01 μM) did not show significant effects (Figure 4).
SR 48968 (0.1 μM) had no effect on both ACh and ET-1 concentration response curves and on EFS alone (Figure 5).
Figure 5.
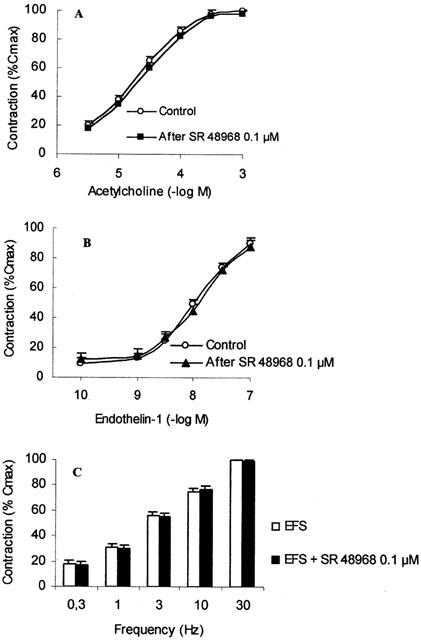
Effect of SR 48968 (0.1 μM) pre-treatment on acetylcholine (ACh; A), endothelin-1 (ET-1; B), and EFS (C) induced bronchial smooth muscle contraction. Data are expressed as per cent of the response to a maximally effective concentration of carbachol (10 μM; 100% Cmax). Values are the means±s.e.mean from eight preparations.
Reverse phase HPLC for tachykinins
SP, NKA and NKB immunoreactive peptides were resolved in reverse phase chromatographic fractions using organ bath fluid specimens (10 ml). Figure 6 shows the retention times for synthetic human tachykinin peptides previously calibrated using Shaw et al. (1989) system.
Figure 6.
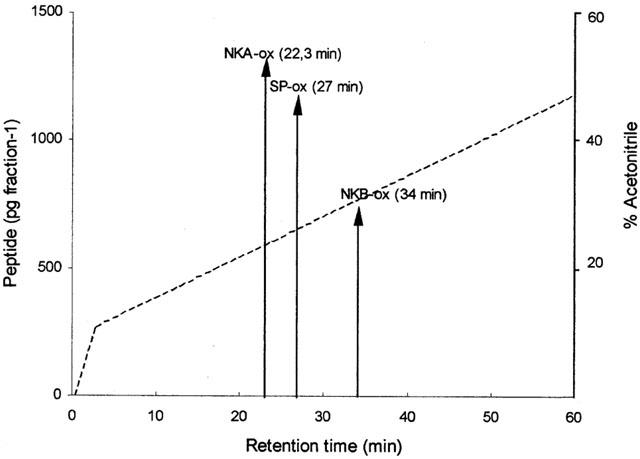
Reverse phase HPLC chromatogram showing retention times for synthetic neurokinin A (NKA), neurokinin B (NKB), and substance P (SP). (- - - - -) gradient of elution.
Radioimmunoassay quantitation of NKA and SP
The concentrations of NKA and SP in the different samples are expressed as pg×1000/ml of organ bath fluid and are shown in Figures 7A,B. Significantly highest concentrations were found in all bronchial preparations treated with ET-1.
Figure 7.
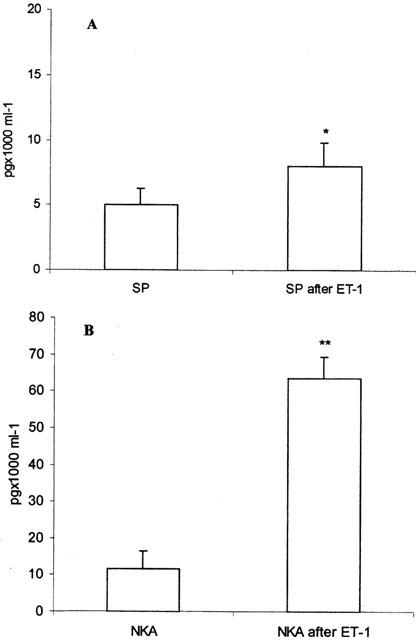
SP (A) and NKA (B) concentrations in organ bath fluid prior to and 40 min after ET-1 treatment. *P<0.05 compared with pretreatment; **P<0.01 compared with pre-treatment. Data are expressed as the means±s.e.mean from eight preparations.
Reverse transcription (RT)-polymerase chain reaction (PCR)
PPT mRNA level 40 min after stimulation of bronchi with ET-1 (1 nM) was increased about 2 fold respect to control untreated bronchi (Figures 8 and 9), while PPT mRNA level 30 min after ET-1 (1 nM) treatment, was not significantly different from control untreated bronchi (Figures 8 and 9). The mRNA levels were measured by a GELDOC instrument and normalized with respect to HPRT mRNA, which was chosen as the RT – PCR control.
Figure 8.
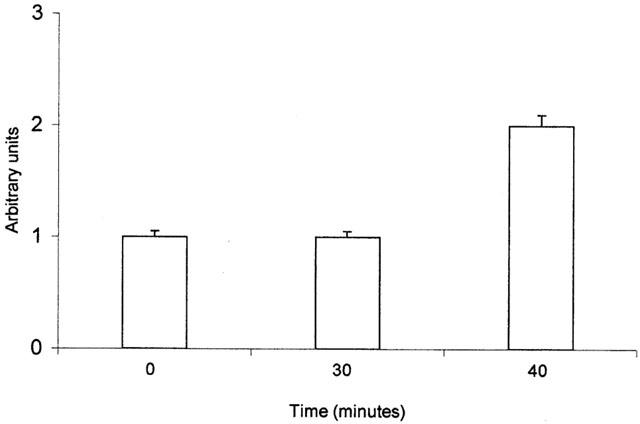
mRNA expression levels of PPT gene in untreated bronchi and in bronchi stimulated for 30 and 40 min with ET-1 respectively. The mRNA levels were measured by GELDOC instrument and normalized with respect to HPRT mRNA, which was chosen as the RT – PCR control. Each value is the mean of at least three different experiments.
Figure 9.
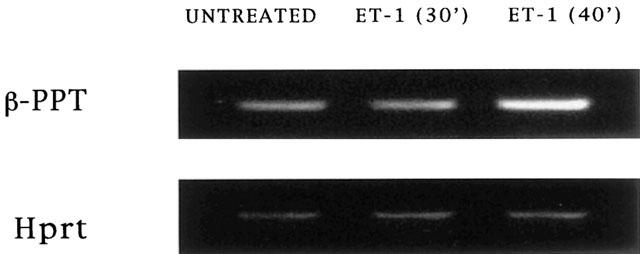
Agarose gel electrophoresis analysis of RT – PCR products of β-PPT and HPRT mRNAs in untreated bronchi and in bronchi stimulated for 30 and 40 min with ET-1 respectively.
Discussion
The first report that suggest a neuromodulatory role of endothelin-1 in the peripheral nervous system is the paper of Henry & Goldie (1995) where in mouse trachea, ET-1 was found to evoke the release of ACh in a concentration dependent manner. More recently, it has been suggested that ET-1 may play an important role in modulating cholinergic neurotransmission in human airway smooth muscle on the basis of functional studies (Fernandes et al., 1996; 1999). The present results demonstrate that ET-1 can produce an enhancement of contractile responses evoked by EFS of human bronchi in vitro, confirming the latter study. This effect was observed with low concentrations of ET-1 (0.1 and 1 nM) which induced only a slight contraction. Under similar conditions and for concentrations which induced a similar level of contraction, histamine was unable to increase the EFS responses. This shows that the effect of ET-1 is not related to the contraction itself. Moreover, this effect was specific for EFS-induced contractions, since ET-1 did not modify exogenous Ach concentration-response curves. In order to evaluate the role of tachykinins in the effect of ET-1, we performed experiments with SR 48968, a selective tachykinin NK2 receptor antagonists (Emonds-Alt et al., 1992) on ET-1 evoked potentiation of cholinergic nerve-mediated contraction of the human isolated bronchus. We showed that SR 48968 was effective to reducing ET-1 potentiation effect suggesting that tachykinin activation may be important in this phenomenon. Under our experimental conditions, SR 48968 was unable to modify (1) the ACh and ET-1 concentration-response curves and (2) the EFS-induced contractions without ET-1 pretreatment. These results demonstrate that the effect of SR 48968 is restricted to the enhancement of EFS response by ET-1.
The role of tachykinin and the involvement of tachykinin NK2 receptors were further confirmed by the ability of [β Ala8] NKA 4-10, a tachykinin NK2 receptor agonist (Regoli et al., 1994), to increase the EFS response in a similar manner to ET-1.
Previous data from this laboratory (D'agostino et al., 1998) have demonstrated that ET-1, acting via a tachykinin release, was able to evoke an airway hyperresponsiveness to inhaled histamine in the rabbit. Moreover, other papers in the literature documented that some ET-1 actions are mediated in part, through release of secondary mediators. In fact, PAF and TXA2 have been implicated in ET-1 -induced mobilization of intracellular Ca2+ in cultured vascular smooth muscle cells (Takayasu et al., 1989; Filep et al., 1991; Battistini et al., 1994).
In the current experiments and to confirm results of functional studies, we have first studied the effect of ET-1 on the synthesis of tachykinin and we have determined the production of the mRNA of β-PPT. Indeed, mammalian tachykinin peptides are the products of two distinct genes: the SP/NKA gene, also called preprotachykinin (PPT) gene I and the NKB gene (PPT gene II). (Carter & Krause, 1990; Nawa et al., 1984; Kotani et al., 1986) The neuropeptides SP and NKA, are encoded by mRNAs resulting from SP/NKA gene transcription (Nawa et al., 1983; Krause et al., 1986). Using primers corresponding to sequences that span a region of β-PPT mRNA in agreement with the method used by other authors for RNA extraction from carotid arteries (Forte et al., 2001), we demonstrated that β-PPT mRNA level after stimulation of bronchi with ET-1 was increased about 2 fold in respect to control untreated bronchi. We have then shown, using HPLC and RIA after sep-pak, that ET-1 was able to induce the generation of significant quantities of neurokinins that acting via TK receptors, appeared to amplify the ET-1 induced potentiation of EFS-induced contractions. The predominant tachykinin retrieved in organ bath effluents was NKA, which was present in significant quantities in respect to SP. All bronchial preparations released detectable amounts of neurokinins at rest which were increased after stimulation with ET-1.
The fact, that there are significant quantities of NKA in organ bath effluents raises an important issue: what is the exact source of tachykinins? In fact, such large quantities are unlikely to be derived solely from neural store, as previously thought, and may be derived from another cellular source.
Pro- and anti-inflammatory mediators are now being isolated from cells that were not previously thought to play a part in the regulation of inflammation. For example, over the past decade, the respiratory ephithelium has been shown as an important source of both pro- and anti-inflammatory factors, in addition to its physical protective function (Thompson et al., 1995; Raeburn & Webber, 1994; Churchill et al., 1989).
Airway smooth muscle has traditionally been thought of as a passive player in airway inflammation, responding only to the release of bronchoconstrictor mediators from other neighbouring cells by contraction, leading to narrowing of the airways and airways obstruction (Stephens et al., 1998). Recently, an increasing number of studies, derived mainly from isolated airway smooth muscle cells in culture, indicate that the airway smooth muscle can also exhibit a synthetic potential with the elaboration of inflammatory mediators (see review of Chung, 2000). In vitro airway smooth muscle cells have been shown to express immunoglobulin receptors (Hakonarson & Grunstein, 1988), HLA-DR (Lazaar et al., 1997), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1) (Lazaar et al., 1994), cytokines including RANTES (John et al., 1997; Hallsworth et al., 2001), IL-1 (Hakonarson et al., 1997), IL-6 (Elias et al., 1997), IL-8 (Hallsworth et al., 2001), eotaxin (Ghaffar et al., 1999; Hallsworth et al., 2001), and nerve growth factor (NGF) (Olgart et al., 1999). Our observation suggest that ET-1 might also be able to induce in ASM the synthesis and release of tachykinins such as NKA and to a lesser degree, SP.
In conclusion, these results support recent studies which demonstrate that ET-1 potentiated cholinergic nerve-mediated contraction in human bronchi; however, our data further demonstrated that, at least in part, the ET-1 potentiation of cholinergic nerve-induced contraction is mediated by tachykinin release. Moreover, these data contribute to the growing body of evidence suggesting that in addition to nerves and human inflammatory cells (macrophages (Germonpre et al., 1999) and T- and B-cells (Braun et al., 1999)), airway smooth muscle cells might be able to synthesize and release tachykinins under inflammatory conditions. However, further experiments are needed to establish in human bronchi the exact source of the neuropeptides.
Acknowledgments
The authors thank Dr Raffaele Sessa, Chirurgia Toracica Ospedale Monaldi; Dr Gaetano Liguori, Chirurgia Toracica Ospedale Ascalesi; Prof Vincenzo Pastore, Chirurgia Toracica Policlinico Seconda Università di Napoli for the supply of human lung tissues. They also thank Dr Prospero Di Pierro for technical assistance.
Abbreviations
- ACh
acetylcholine
- Cmax
maximally effective concentration
- EFS
electrical field stimulation
- EFSst
standard electrical field stimulation
- ET-1
endothelin-1
- HPRT
Hypoxanthine phosphoribosyltransferase
- KBS
Krebs bicarbonate solution
- NKA
Neurokinin A
- NKB
Neurokinin B
- PPT
preprotachykinin
- RT – PCR
semiquantitative reverse transcription polymerase chain reaction
- SP
Substance P
- TFA
trifluoroacetic acid
References
- AIZAWA H., MIYAZAKI N., INOUE H., IKEDA T., SHIGEMATSU N. Effect of endogenous tachykinins on neuro-effector transmission of vagal nerve in guinea-pig tracheal tissue. Respiration. 1990;57:338–342. doi: 10.1159/000195867. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Neuronal control of human airways in health and disease. Am. Rev. Respir. Dis. 1986a;134:1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Asthma as an axon reflex. Lancet. 1986b;1:242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Modulation of neurotransmission in airways. Physiol. Rev. 1992;72:699–729. doi: 10.1152/physrev.1992.72.3.699. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Pathophysiology of asthma. Br. J. Clin. Pharmacol. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTISTINI B., WARNER T.D., FOURNIER A., VANE J.R. Characterization of ETB receptors mediating contractions induced by endothelin-1 or IRL 1620 in guinea-pig isolated airways: effects of BQ-123, FR139317 or PD145065. Br. J. Pharmacol. 1994;111:1009–1016. doi: 10.1111/j.1476-5381.1994.tb14844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEASLEY R., ROCHE R.W., ROBERTS J.A., HOLGATE S.T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am. Rev. Respir. Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- BRAUN A., WIEBE P., PFEUFER A., GESSNER R., RENZ H. Differential modulation of human immunoglobulin isotype production by the neuropeptides substance P, NKA and NKB. J. Neuroimmunol. 1999;97:43–50. doi: 10.1016/s0165-5728(99)00051-x. [DOI] [PubMed] [Google Scholar]
- CARTER M.S., KRAUSE J.E. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J. Neurosci. 1990;10:2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K.F. Airway smooth muscle cells: contributing to and regulating airway mucosal inflammation. Eur. Respir. J. 2000;15:961–968. doi: 10.1034/j.1399-3003.2000.15e26.x. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F., EVANS T.W., GRAF P.D., NADEL J.A. Modulation of cholinergic neurotransmission in canine airways by thromboxane mimetic U46619. Eur. J. Pharmacol. 1985;117:373–375. doi: 10.1016/0014-2999(85)90012-3. [DOI] [PubMed] [Google Scholar]
- CHURCHILL L., CHILTON F.H., RESAU J.H., BASCOM R., HUBBARD W.C., PROUD D. Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am. Rev. Respir. Dis. 1989;140:449–459. doi: 10.1164/ajrccm/140.2.449. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO B., FILIPPELLI A., FALCIANI M., ROSSI F.SCA, ROSSI F. Endothelin-1 and bronchial hyperresponsiveness in the rabbit. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:561–566. doi: 10.1007/pl00005293. [DOI] [PubMed] [Google Scholar]
- ELIAS J.A., WU Y., ZHENG T., PANETTIERI R.A. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am. J. Physiol. (Lung Cell. Mol. Physiol. 17) 1997;273:L648–L655. doi: 10.1152/ajplung.1997.273.3.L648. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Role of cysteinyl leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. Am. J. Respir. Crit. Care Med. 1994;149:118–122. doi: 10.1164/ajrccm.149.1.8111568. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., BRELIERE J.-C., LE FUR G. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sciences. 1992;50:PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- FERNANDES L.B., HENRY P.J., GOLDIE R.G. Endothelin-1 potentiates cholinergic nerve-mediated contraction in human isolated bronchus. Eur. Respir. J. 1999;14:439–442. doi: 10.1034/j.1399-3003.1999.14b33.x. [DOI] [PubMed] [Google Scholar]
- FERNANDES L.B., HENRY P.J., RIGBY P.J., GOLDIE R.G. EndothelinB (ETB) receptor-activated potentiation of cholinergic nerve-mediated contraction in human bronchus. Br. J. Pharmacol. 1996;118:1873–1874. doi: 10.1111/j.1476-5381.1996.tb15617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILEP J.G., SIROIS M.G., ROUSSEAU A., FOURNIER A., SIROIS P. Effects of endothelin-1 on vascular permeability in the conscious rat: interactions with platelet-activating factor. Br. J. Pharmacol. 1991;104:797–804. doi: 10.1111/j.1476-5381.1991.tb12509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTE A., DI MICCO G., GALDERISI U., GUARINO M.F., CIPOLLARO M., DE FEO M., GREGORIO R., BIANCO M.R., VOLLONO C., ESPOSITO F., BERRINO L., ANGELINI F., RENZULLI A., COTRUFO M., ROSSI F., CASCINO A. Molecular analysis of arterial stenosis in rat carotids. J. Cell Physiol. 2001;186:307–313. doi: 10.1002/1097-4652(200002)186:2<307::AID-JCP1029>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- GERMONPRE P.R., BULLOCK G.R., LAMBRECHT B.N., VAN DE VELDE V., LUYTEN W.H.M.L., JOOS G.F., PAUWELS R.A. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur. Respir. J. 1999;14:776–782. doi: 10.1034/j.1399-3003.1999.14d08.x. [DOI] [PubMed] [Google Scholar]
- GHAFFAR O., HAMID Q., RENZI P.M., ALLAKHVERDI Z., MOLET S., HOGG J.C., SHORE S.A., LUSTER A.D., LAMKHIOUED B. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am. J. Respir. Crit. Care. Med. 1999;159:1933–1942. doi: 10.1164/ajrccm.159.6.9805039. [DOI] [PubMed] [Google Scholar]
- HAKONARSON H., GRUNSTEIN M. Autologously up-regulated Fc receptor expression and action in airway smooth muscle mediates its altered responsiveness in the atopic asthmatic sensitised state. Proc. Natl. Acad. Sci. U.S.A. 1988;95:5257–5262. doi: 10.1073/pnas.95.9.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKONARSON H., HERRICK D.J., GONZALEX SERRANO P., GRUNSTEIN M.M. Autocrine role of interleukin-1b in altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J. Clin. Invest. 1997;99:117–124. doi: 10.1172/JCI119122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLSWORTH M.P., TWORTH C.H.C., LEE T.H., HIRST S.J. β2-Adrenoceptor agonists inhibit release of eosinophil-activating cytokines from human airway smooth muscle cells. Br. J. Pharmacol. 2001;132:729–741. doi: 10.1038/sj.bjp.0703866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY P.J., GOLDIE R.G. Potentiation by endothelin-1 of cholinergic nerve-mediated contractions in mouse trachea via activation of ETB receptors. Br. J. Pharmacol. 1995;114:563–569. doi: 10.1111/j.1476-5381.1995.tb17176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY P.J., SHEN A., MITCHELSON F., GOLDIE R.G. Inhibition by endothelin of cholinergic nerve-mediated acetylcholine release and contraction in sheep isolated trachea. Br. J. Pharmacol. 1996;118:762–768. doi: 10.1111/j.1476-5381.1996.tb15465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGG J.C. Asthma: Basic Mechanism and Clinical Management 1988London: Academic Press; 1–9.ed. Barnes, P. & Rodger, I. pp [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- JOHN M., HIRST S.J., JOSE P.J., ROBICHAUD A., BERKMAN N., WITT C., TWORT C.H.C., BARNES P.J., CHUNG K.F. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: regulation by T helper 2 cytokines and corticosteroids. J. Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- KOTANI H., HOSHIMARU M., NAWA H., NAKANISHI S. Sequence analysis of cloned cDNA for rat substance P precursor: existence of a third substance P precursor. Biochem. Biophys. Res. Commun. 1986;139:1040–1046. doi: 10.1016/s0006-291x(86)80282-0. [DOI] [PubMed] [Google Scholar]
- KRAUSE J.E., CHIRGWIN J.M., CARTER M.S., XU Z.S., HERSHEY A.D. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc. Natl. Acad. Sci. U.S.A. 1986;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAAR A.L., ALBELDA S.M., PILEWSKI J.M., BRENNAN B., PURE E., PANETTIERI N.A. T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cells DNA synthesis. J. Exp. Med. 1994;180:807–816. doi: 10.1084/jem.180.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAAR A.L., REITZ H.E., PANETTIERI R.A., PETERS S.P., PURE E. Antigen receptor-stimulated peripheral blood and bronchoalveolar lavage-derived T cells induce MHC class II and ICAM-1 expression on human airway smooth muscle. Am. J. Respir. Cell. Mol. Biol. 1997;16:38–45. doi: 10.1165/ajrcmb.16.1.8998077. [DOI] [PubMed] [Google Scholar]
- MAULE A.G., SHAW C., HALTON D.W., JOHNSTON C.F., FAIRWEATHER I., BUCHANAN K.D. Tachykinin immunoreactivity in the parasitic flatworm Diclidophora merlangi and its fish host the whiting (Merlangius merlangus): radioimmunoassay and chromatographic characterisation using region-specific substance P and neurokinin A antisera. Comp. Biochem. Physiol. C. 1989;94:533–541. doi: 10.1016/0742-8413(89)90109-6. [DOI] [PubMed] [Google Scholar]
- MCKAY K.O., ARMOUR C.L., BLACK J.L. Endothelin-3 increases transmission in the rabbit pulmonary parasympathetic nervous system. J. Cardiovasc. Pharmacol. 1993;22 Supp. 8:S181–S184. doi: 10.1097/00005344-199322008-00049. [DOI] [PubMed] [Google Scholar]
- NAWA H., HIROSE T., TAKASHIMA H., INAYAMA S., NAKANISHI S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- NAWA H., KOTANI H., NAKANISHI S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984;312:729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- OLGART C., KASSEL O., BEHRA M.E., FROSSARD N. Secretion of nerve growth factor (NGF) from human lung fibroblasts in culture effect of inflammatory cytokines. Am. J. Respir. Crit. Care. Med. 1999;A336 [Google Scholar]
- REGOLI D., BOUDON A., FAUCHERE J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–559. [PubMed] [Google Scholar]
- RAEBURN D., WEBBER S.E. Proinflammatory potential of the airway epithelium in bronchial asthma. Eur. Respir. J. 1994;7:2226–2233. doi: 10.1183/09031936.94.07122226. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.B. The innervation of the lung. Eur. J. Respir. Dis. Suppl. 1982;117:13–31. [PubMed] [Google Scholar]
- SERIO R., DANIEL E.E. Thromboxane effects on canine trachealis neuromuscular function. J. Appl. Physiol. 1988;64:1979–1988. doi: 10.1152/jappl.1988.64.5.1979. [DOI] [PubMed] [Google Scholar]
- SHAW C., FOY W.L., JOHNSTON C.F., BUCHANAN K.D. Identification and characterisation of multiple tachykinin immunoreactivities in bovine retina: evidence for the presence of a putative oxidative inactivation system for the substance P. J. Neurochem. 1989;53:1547–1554. doi: 10.1111/j.1471-4159.1989.tb08551.x. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D., EPSTEIN J., HOLTZMAN M.J., NADEL J.A., BOUSHEY H.A. Dose-dependent inhibition of cold air-induced bronchoconstriction by atropine. J. Appl. Physiol. 1982;53:169–174. doi: 10.1152/jappl.1982.53.1.169. [DOI] [PubMed] [Google Scholar]
- STEPHENS N.L., LI W., WANG Y., MA X. The contractile apparatus of airways smooth muscle. Biophysics and biochemistry. Am. J. Respir. Crit. Care Med. 1998;158:S80–S94. doi: 10.1164/ajrccm.158.supplement_2.13tac300. [DOI] [PubMed] [Google Scholar]
- STRETTON C.D., BELVISI M.G., BARNES P.J. The effect of sensory nerve depletion on cholinergic neurotransmission in guinea-pig airways. J. Pharmacol. Exp. Ther. 1992;260:1073–1080. [PubMed] [Google Scholar]
- TAKAYASU M., KONDO K., TERAO S. Endothelin-induced mobilization of Ca2+ and the possible involvement of platelet activating factor and thromboxane A2. Biochem. Biophys Res. Commun. 1989;160:751–757. doi: 10.1016/0006-291x(89)92497-2. [DOI] [PubMed] [Google Scholar]
- TANAKA D.T., GRUNSTEIN M.M. Effect of substance P on neurally mediated contraction of rabbit airway smooth muscle. J. Appl. Physiol. 1986;60:458–463. doi: 10.1152/jappl.1986.60.2.458. [DOI] [PubMed] [Google Scholar]
- THOMPSON A.B., ROBBINS R.A., ROMBERGER D.J., SISSON J.H., SPURZEM J.R., TESCHLER H., RENNARD S.I. Immunological functions of the pulmonary epithelium. Eur. Respir. J. 1995;8:127–149. doi: 10.1183/09031936.95.08010127. [DOI] [PubMed] [Google Scholar]
- VAN OOSTERHOUT A.J., HOFMAN G., WOUTERSEN VAN NIJNANTEN F.M., NIJKAMP F.P. 5-HT1-like receptors mediate potentiation of cholinergic nerve-mediated contraction of isolated mouse trachea. Eur. J. Pharmacol. 1991;209:237–244. doi: 10.1016/0014-2999(91)90175-p. [DOI] [PubMed] [Google Scholar]
- WARD M.J., FENTEM P.H., SMITH W.H., DAVIES D. Ipratropium bromide in acute asthma. Br. Med. J. 1981;282:598–600. doi: 10.1136/bmj.282.6264.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEYAMA T., HORI M., TANAKA T., MATSUDA Y., KARAKI H. Endothelin ETA and ETB receptors facilitating parasympathetic neurotransmission in the rabbit trachea. J. Pharmacol. Exp. Ther. 1995;275:1084–1089. [PubMed] [Google Scholar]


